Review Article Open Access
Fibroblast Growth Factor 19: An Overview of its Diverse Physiological Functions
Ayantika Ghosh*
Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, PA 15224, USA
- *Corresponding Author:
- Ayantika Ghosh
Department of Pediatrics
University of Pittsburgh School of Medicine
Pittsburgh, PA 15224, USA
Tel: 4125137678
E-mail: ayantika.gb@gmail.com
Received date: March 24, 2015; Accepted date: May 1, 2015; Published date: May 10, 2015
Citation: Ghosh A (2015) Fibroblast Growth Factor 19: An Overview of its Diverse Physiological Functions. J Gastrointest Dig Syst 5:285. doi:10.4172/2161-069X.1000285
Copyright: ©2015 Ghosh A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Fibroblast Growth Factor 19 (FGF19) is an intestinally derived member of the Fibroblast Growth Factor (FGF) family that governs embryonic development, tissue morphogenesis, tumor growth and invasion and nutrient metabolism. The most important function of FGF19 is to negatively regulate the first and rate-limiting enzyme Cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis acting by means of its highly specific receptor, Fibroblast Growth Factor 4 (FGFR4). It also stimulates gall bladder refilling thus serving as a feed forward signal for bile acid excretion and storage. Furthermore, FGF19 has been shown to participate in glucose and lipid metabolism via mechanisms that are comparable to insulin signaling. Currently, there is no detailed theory to explain the multifaceted nature of FGF19 but future studies might shed light on this important phenomenon. FGF19 is also highly expressed in Hepatocellular Carcinoma (HCC) and is responsible for growth and invasion of tumors through its interactions with FGFR4. Consequently, researchers are exploiting this ligand-receptor interaction to target cancer treatments. This review will highlight the current state of knowledge about the roles of FGF19 and its potential implications in clinical research and therapeutics.
Keywords
FGF19; CYP7A1; Bile acid metabolism, Glucose metabolism; Cancer; Retinal development
Introduction
Fibroblast growth factor (FGF) are a family of growth factors, involved in variety of biological processes such as embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion. They possess metabolic, mitogenic, cell survival and angiogenic activities. FGFs typically bind to cell surface receptors, Fibroblast Growth Factor Receptors (FGFRs). Additionally, to bolster their interactions with FGFRs, FGFs show high affinity for cell surface polysaccharides-glycosaminoglycan heparan sulphate. The interactions of FGF with FGFR and heparan sulphate proteoglycans are essential for receptor dimerization, phosphorylation and subsequent signal transduction [1,2]. FGF19, (mouse ortholog-FGF15) an atypical member of the FGF family was initially characterized by its reduced affinity towards heparan sulphate mediated binding and unique and exclusive specificity towards FGFR4 and the trans-membrane receptor, b-klotho. To compensate for the loss of heparan sulfate-mediated high-affinity interactions observed with the receptors of other FGFs, the FGF19 subfamily members instead use single-transmembrane-containing Klotho proteins to facilitate their interactions with and activations of FGFR4. Reduced affinity of FGF19 towards heparin sulphate allows it to be poorly tethered to the pericellular proteoglycan and ultimately makes it free to diffuse from the extracellular matrix (ECM). This diffusion and escape from the ECM helps to explain why FGF19 functions as an endocrine hormone in addition to performing paracrine or autocrine functions. As an endocrine hormone, FGF19 play regulatory roles in bile acid synthesis and homeostasis and also in glucose and lipid metabolism [3,4]. As a paracrine effector, FGF19 is involved in embryogenesis, growth, differentiation and angiogenesis [5] while as a mediator of autocrine functions it promotes invasion and proliferation of metastatic tissues [6]. Although FGF19 transcripts are found in brain, cartilage, skin, kidney, gall bladder and intestine, its expression is primarily found in ileum. FGF19 is secreted into the circulation and transported back to the liver to repress the bile acid biosynthetic pathway. The endocrine regulation of hepatic bile acid metabolism by intestinal FGF19 is based on the fact that even though FGF19 is expressed in the ileum, its receptor FGFR4 is highly expressed in the liver. Interest into FGF19 as a metabolic regulator sparked from the observation that transgenic mice expressing FGF19 showed reduced adiposity, liver triglycerides and glucose levels and increased fatty acid oxidation and improved insulin profile [4]. In the light of the roles played by FGF19 in various physiological events, this review aims to present an insight of the recent developments taking place in the field of FGF19 biology.
Metabolic Roles of FGF19
FGF19 in Bile acid metabolism
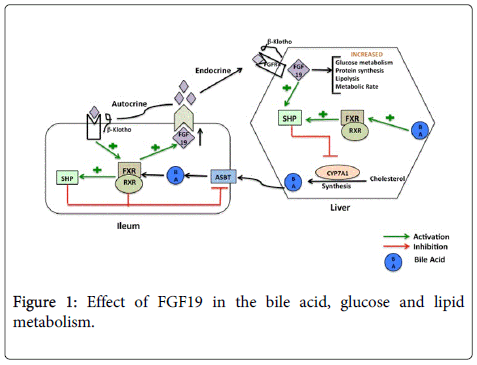
The synthesis of bile acids is a major pathway of cholesterol catabolism in mammals. Bile acid synthesis is initiated via hydroxylation of the cholesterol molecule at the 7th position, by the action of cholesterol 7α-hydroxylase (CYP7A1), which is an Endoplasmic Reticulum (ER) localized enzyme. As in a typical feedback mediated regulation, bile acids repress their own synthesis and CYP7A1 is the enzyme in the bile acid metabolic pathway that is repressed to prevent a potentially harmful expansion of the bile acid pool. Bile acids activate the nuclear receptor, Farnesoid X Receptor (FXR). Upon activation, FXR initiates transcription of a cohort of genes that function to decrease the concentration of bile acids within the liver, where bile acids are produced. In liver or ileum FXR also induces expression of Small Heterodimer Partner-1 (SHP-1), an atypical member of the nuclear receptor family that lacks a DNA-binding domain [7,8]. SHP-1 in turn inhibits expression of Cyp7a1 by reducing the activity of Liver Receptor Homolog 1 (LRH-1), an orphan nuclear receptor that is known to regulate CYP7A1 and the consequent bile acid biosynthetic pathway positively. Targeted disruption of the fxr gene in mice impaired bile acid and lipid homeostasis indicating the critical role of FXR in bile acid metabolism [9]. However, deletion of the SHP gene in mice reduced but did not completely eliminate the feedback repression of bile acid synthesis. This observation suggested existence of a SHP independent mechanism of bile acid regulation by FXR [10]. For years, there had been discrepancy as to why Cyp7a1 expression in the liver was inhibited in response to enteral but not intravenously administered bile acids. The puzzle was solved by the discovery of the FGF15/19 [11], when it was realized that in response to enteral bile acids, the ileum secretes FGF15/19 that binds to FGFR4 in the liver and mediates repression of bile acid synthesis in liver via inhibition of Cyp7a1 gene transcription. Evidence supporting the role of FGF15/19 in maintaining proper bile acid homeostasis came from analysis of mice models harboring deletions on the FGF15 signaling axis. Studies of these mice having deletion in the FGFR4 receptor gene (Fgfr4-/-), or β-Klotho gene (Klb-/-) or FGF 15 (Fgf15-/-) showed increased levels of Cyp7a1 expression in the liver, increased excretion of bile acids and an elevated bile acid pool size compared to wild type littermates [12-14]. In contrast, transgenic mice overexpressing a constitutively active FGFR4 receptor, showed repressed levels of CYP7A1 and decreased expression of bile acids. Moreover, administration of FXR agonists, GW4064 or cholic acid failed to repress Cyp7a1 in Fgfr4-/- or Fgf15-/- mice. Taken together, these findings confirm that FXR-mediated negative feedback repression of bile acids is not only dependent on FXR-SHP pathway but also on a novel enterohepatic signaling pathway mediated by FGF15/19.
The importance of the role of FGF19 in maintaining proper bile acid homeostasis has been highlighted further by clinical studies. In humans, serum FGF19 levels and bile acid synthesis is diurnally regulated [15]. After a meal, bile acids released into the intestine bind to and activate FXR, which then induces the expression of FGF19. The postprandial rise in serum bile acids is followed by a synchronous serum FGF19 peak that occurs at a delay of 90-180 minutes after a meal. The study supports the view that FGF19 is secreted in the ileum in response to postprandial increase in bile acid flux. In addition, patients with primary bile acid malabsorption syndrome have reduced FGF19 production by the ileum, which is associated with increased bile acid synthesis that spills into the colon to stimulate electrolyte and water secretion. The typical symptom of bile acid malabsorption is therefore chronic watery diarrhea, also known as Bile Acid Diarrhea (BAD) [16]. In response to bile acids FXR induces the expression of the apical sodium dependent bile acid transporter (ASBT) in the ileum, which is involved in reclamation of bile salts during the enterohepatic circulation of bile acids. In Crohn’s disease and in other intestinal bowel disease (IBD), bile acid malabsorption occurs via FGF19 mediated repression of ASBT [17]. The mechanism by which FGF19 represses ASBT is not known in details. In our recent study [18], we have shown that FGF19 mediated repression of ASBT occurs via activation of the MEK-ERK pathway which in turn phosphorylates and activates the AP-1 complex proteins, C-Jun and C-Fos. The ASBT promoter in rats, mice and human contains two distinct AP-1 binding sites. The upstream element, uAP1 binds a C-Jun homodimer and mediates transcriptional activation, while the downstream dAP-1 site binds a C-Jun/C-Fos heterodimer and mediates transcriptional repression. Binding of FGF19 to its receptor complex, FGFR4/b-klotho is associated with up-regulation and phosphorylation of C-Fos, which then represses ASBT promoter via binding of the dAP-1 element by a C-Jun/C-Fos heterodimer.
Patients with bile acid malabsorption are usually treated with bile acid sequestrants such as colestyramine, colestipol or colesevalam. As an alternative treatment measure, stimulation of FGF19 by FXR agonists can be used to reverse FGF19 deficiency, which is considered one of the factors of bile acid malabsorption and the resulting BAD [19]. However, treatment methods involving supraphysiological levels of FGF19 has always been held with skepticism because of FGF19’s reputation of promoting tumors. A recent study [20] has described an engineered non-tumorigenic variant of FGF19, M70 that has lost the tumor inducing property of FGF19 while has fully retained the physiological bile acid regulatory activity; spurring hopes of potent therapeutic applications in bile acid malabsorption.
FGF19 in glucose and lipid metabolism
In addition to its role in hepatic and ileal bile acid homeostasis, FGF19 has been suggested to be useful in lowering serum glucose and triglyceride levels in diabetic mice. Type 2 Diabetes mellitus occurs when the pancreas produces insufficient amount of the hormone insulin or the body’s tissues and organs (such as liver) become resistant to high or normal levels of insulin. One of the key problems in insulin signaling pathway is insufficient drug target owing to either lack of suitable ligand binding domains in the target or shared partners in other signaling pathways that regulate cell growth and differentiation. Studies undertaken to circumvent this problem led researchers to propose alternative pathways to insulin signaling for glucose metabolism. Kir et al demonstrated that FGF19 (in an alternative but overlapping signaling pathway from Insulin) could govern postprandial glucose metabolism in liver, raising interest and hopes about possible therapies involving this molecule [21]. Apart from human subjects, FGF19 can also improve glucose tolerance when expressed in mice with diabetes. Transgenic mice expressing fgf19 under the control of the myosin light-chain promoter have been reported to have increased metabolic rate, decreased adiposity, and increased insulin sensitivity compared to control littermates [4].
So how does the function of FGF19 in regulating glucose levels in the body compare to that of Insulin? Similar to Insulin, FGF19 can promote protein and glycogen synthesis in liver. Gingras et al showed that FGF19 increased phosphorylation of eukaryotic Initiation Factor 4B (eIF4B) and eIF4E proteins, which are components of the eIF4F complex [22]. The eIF4F complex mediates binding of mRNA to the ribosome and phosphorylation of these proteins (eIF4B and eIF4E) promotes initiation of translation. FGF19 also increased phosphorylation of ribosomal subunit protein S6 (rpS6). Phosphorylation of rpS6 enhances global protein synthesis [23]. In addition, as measured by in vivo 2H2O labeling, FGF19 stimulated total protein synthesis as well as albumin synthesis in mouse liver [21]. Thus, by inducing phosphorylation of eIF4B, eIF4E and rpS6, FGF19 stimulated hepatic protein synthesis. Kir et al further showed that insulin also functions in a similar manner as FGF19 in phosphorylating eukaryotic initiation factors or ribosomal protein S6 kinase. While insulin increased phosphorylation of further downstream protein kinases AKT and p70 S6 kinase, which are known to stimulate the mTOR pathway, FGF19 increased phosphorylation of p90 ribosomal S6 kinase via activation of ERK1 and ERK2. Hence they concluded that FGF19 signaling pathway acts in parallel but independent of the insulin pathway to govern glucose metabolism in liver.
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by negatively regulating glycogen synthase kinase 3α (GSK3α) and GSK3β, which phosphorylate and inhibit the enzyme glycogen synthase (GS), the rate-limiting enzyme in the pathway of glycogen synthesis. In a fashion similar to that of insulin, FGF19 induced phosphorylation of GSK3α and GSK3β and increased glycogen synthase activity [21].
In the diabetic liver, there is a overproduction of glucose and atherogenic lipoproteins such as very-low-density lipoproteins (VLDLs) and small dense LDLs probably from impaired secretion of insulin, which promotes fatty acid synthesis (lipogenesis) [24]. However, unlike insulin, recombinant FGF19 suppressed the ability of insulin to stimulate fatty acid synthesis [25] Not only that, it also suppressed the insulin induced expression of Sterol Regulatory Element Binding Protein-1c (SREBP-1c), a key transcriptional activator of lipogenic genes-the suppression being brought about without any alterations in the insulin signaling pathway.
The effect of FGF19 on lipid metabolism is somewhat controversial. Wu et al. [26] showed that treatment with FGF19 for 7 days in obese (ob/ob-mutant for leptin ) mouse models caused a reduction in the bile acid and plasma glucose levels but an increase in triglyceride and total cholesterol levels. This is in contrast to many literature reports that supported the hypothesis that FGF19 led to significant improvements in hyperglycemia and hyperlipidemia with increased fatty acid oxidation in models of genetic and acquired insulin resistance. The study by Fu et al. [27] showed that transgenic mice expressing FGF19 have increased metabolic rate and are resistant to diet induced obesity and diabetes. Furthermore, Wu et al. [28] mentioned that the FGF receptor, FGFR4 is required for bile acid regulation but not for improvement of glucose tolerance by FGF19 at a pharmacological dose. In Fgfr4 knock out mice FGF19 improved glucose tolerance in high fat fed diet. Similarly, FGF19v, a protein specifically impaired of FGFR4 binding and activation, ameliorated hyperglycemia in ob/ob mice indicating the dispensability of FGFR4 in regulating glucose metabolism. The role of FGF19 in improving insulin resistance and hyperglycemia in obese and diabetic mice is shared by a related endocrine hormone FGF21 [29-31].
Pharmacologically, FGF19 might be an attractive candidate in the management of diabetes; its future as a drug is currently ridden with holes. First of all, peptide-based diabetic medication was never popular owing to the complexity of measuring the correct dose as well as patient aversion to needles based approach to deliver the protein therapeutic. It was with the discovery of the incretin peptide hormone-an intestinal factor that stimulates insulin secretion in response to glucose that a renewed interest has born for injectable peptide based drugs. Secondly, FGF19 production is normal in diabetics, raising doubts about the benefits of boosting its actions. This stems from the concern that overexpression of FGF19 in transgenic mice correlate well with development of hepatocellular carcinoma in these models. Finally, FGF19 production falls in response to administration of bile acid absorption inhibitors like colesevelam, which increases circulating incretins and improves tissue glucose metabolism in both the fasting and postprandial states in a manner different from other approved agents [32]. Nevertheless, it might still be useful to find a therapeutic dose of FGF19, which is effective for treating metabolic disorders but is not in the tumorigenic range.
Mitogenic Roles of FGF19
FGF19 in tumor and cancer induction
FGF19 share some of its ability to regulate glucose, lipid, and energy homeostasis, with another related fibroblast growth factor, FGF 21; however it is only FGF19 that has potential mitogenic and proliferative activity. The FGFR4-FGF19 signaling axis is important in the development and progression of hepatocellular carcinoma (HCC) in mice and humans [33]. An oncogenomic cDNA screen developed by Sawey et al to identify oncogenes that drive HCC led them to identify FGF19 as a major driver gene along with the widely known gene cyclinD1 (CCND1). This group also showed that FGF19 co-amplified with CCND1 at 15% frequency specifically in HCCs harboring the highly amplified 11q13.3 gene locus [34]. Clonal growth and tumorigenicity of HCC cells harboring the 11q13.3 amplicon could be inhibited by RNAi-mediated knockdown of FGF19. FGF19 is significantly overexpressed in HCCs compared to non-cancerous liver tissue. In addition, administration of recombinant FGF19 protein increased the proliferation and invasion of HCC cell lines and inhibited the natural tendency of the cells to follow a programmed death pattern or apoptosis [35]. Knocking down FGF19 or FGFR4 via si-RNA in these cell lines evoked a reverse effect on HCC cellular proliferation and invasion. Examining the role of endogenous FGF15 (the mouse homolog of FGF19) in mouse models of hepatocarcinogenesis also showed that FGF15 knock out mice (Fgf15-/-) showed less tumors and histological neoplastic lesions compared to Fgf15+/+ mouse. Moreover, hepatocellular proliferation was reduced in Fgf15-/- mice, which also expressed lower levels of the HCC marker alpha-fetoprotein (AFP) [36].
Nicholes et al observed increased hepatocyte proliferation in normal mice injected with recombinant FGF19 in skeletal tissue for 6 days. Increased proliferation was observed as early as 2-4 months of age, and the mice finally developed HCC within 12 months [37]. It is believed that the highly specific interaction of FGFR4 with FGF19 and not other FGFs (such as FGF21) is responsible for its proliferative effects. Investigating the structural differences between FGF19 and FGF21 showed that abolishing a N-terminal five-amino acid region (residues 38-42) in FGF19 that is important for FGFR4 activation and heparan binding completely prevented FGF19 mediated hepatocyte proliferation [38]. Desnoyers group developed an anti-FGF19 monoclonal antibody (IA6) that selectively blocked the interaction of FGF19 with FGFR4. The blocked interaction effectively prevented HCC in FGF19 transgenic mice. This antibody has also been proven to be effective in all models of cancer, where FGF19 was overexpressed along with FGFR4. For example, the antibody was effective in preventing tumor formation in colon cancer xenografts models in vivo [39]. FGF19 is over expressed in prostrate cancer [6] or breast cancer [40] and treatment with the antibody suppressed tumorigenesis and cancer progression in these cancer types. The efficacy of the antibody was linked to inhibition of FGF19-dependent activation of FGFR4 and downstream targets Fibroblast Growth factor Receptor Substrate 2 (FRS2), Extracellular Signal Regulated Kinase (ERK), and β-catenin [41]. Several studies have shown that it is not FGF19 but the overexpression of FGFR4 that is associated with poor clinical prognosis in HCC [42] and in prostrate cancer [43-45]. When Ho et al [46] performed a comprehensive sequencing of the full length FGFR4 transcript and quantified their mRNA expressions they identified a highly frequent, G338R single nucleotide polymorphisms in FGFR4. The arginine substitution increases receptor stability and induces a migratory phenotype resulting in a more aggressive behavior and reduced survival in multiple cancer types, including breast cancer, head and neck carcinoma, colon cancer, and lung adenocarcinoma.
Angiogenic Roles of FGF19
FGF19 is a major growth factor expressed during retinal development and lens differentiation. FGF19 expressed in lens cells is necessary for lens fiber differentiation and survival. Knockdown of FGF19 in zebrafish embryos affects lens growth and differentiation of primary fiber cells thus reducing cell viability and increasing degeneration of lens [47]. In addition, FGF19 is implicated in the nasal-temporal patterning of the retina and guidance of retinal ganglion cell axons but not neuronal differentiation and lamination in the retina. The mouse homolog of FGF19, FGF15 is expressed in the early stage of the neural epithelium development and in later stages in specific groups of neural cells.
The role of FGF19 in retinal development stems from the fact that it plays a role in forebrain development. Human Fgf19 is expressed preferentially in fetal brain [48,49]. Chick Fgf19 is expressed in the embryonic brain, optic vesicles, lens primordial cells, and retinal horizontal cells [5] while in zebrafish it is expressed in the forebrain, midbrain and hindbrain. Expression of FGF19/15 in various species and in various stages of the developing nervous system suggests that it plays important roles in regulating cell division and patterning of the embryonic brain, spinal cord and the sensory organs. FGF19 is also critical for the development of the ventral region of the telencephalon and diencephalon and is involved in the specification of Gamma-Amino Butyric Acid-GABA ergic interneurons and oligodendrocytes generated in the ventral telencephalon and diencephalon. It is believed that FGF19 doesnot function alone in this process. A signaling cross talk exists between FGF19 pathway and the Hedgehog (Hh) signaling pathway, which is critical for the specification of the ventral neurons in the forebrain [50]. Inhibition of FGF19 functions affected cell proliferation and cell survival in the brain during mid-segmentation stages and led to a reduction in the size of the forebrain, midbrain and cerebellum at 24 hours post fertilization. FGF19 has also been implicated in inner ear development, acting in part by patterning the neuroectoderm. The inner ear develops from the otic placode, which is a patch of thickened cranial ectoderm next to the hindbrain. Lateral expression of FGF19 in the mesoderm and neural tube with the presumptive otic placode indicates the direct involvement of FGF19 in otic development. An initiating neural signal activates the otic signaling cascade that finally leads to complex synergistic interactions between FGF19 and wnt-8c [51].
Conclusion
Although essentially an endocrine hormone, FGF19 also exerts its autocrine and paracrine capabilities to act as a multifunctional regulator in a wide variety of physiological events. FGF19 runs almost a ‘one man show’ (Figure 1) and therefore befitting any such show an intact FGF19 signaling pathway is essential for maintaining bile acid synthesis and feedback regulation or glucose and lipid metabolism, tumor and cancer biology or even the neural developmental process. It is still not clear how and to which extent FGF19 mediates such diverse effects. Developing therapies with FGF19 is still in stages of infancy, owing to its involvement in various types of cancer. There is still much to be achieved before FGF19 can be used to treat diabetes or bile acid metabolic disorders. We will have to wait for future research to understand more about FGF19 and answer questions about its biology, pathophysiology and therapeutic applications.
References
- Ornitz DM (2000) FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22: 108-112.
- Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8: 235-253.
- Song KH, Li T, Owsley E, Strom S, Chiang JY (2009) Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha hydroxylase gene expression. Hepatology 49: 297-305.
- Tomlinson E, Fu L, John L, Hultgren B, Huang X, et al. (2002) Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143: 1741-1747.
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, et al. (2004) Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expr Patterns 4: 687-693.
- Feng S, Dakhova O, Creighton CJ, Ittmann M (2013) Endocrine fibroblast growth factor FGF19 promotes prostate cancer progression. Cancer Res 73: 2551-2562.
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, et al. (1999) Identification of a nuclear receptor for bile acids. Science 284: 1362-1365.
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517-526.
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, et al. (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731-744.
- Kerr TA, Saeki S, Schneider M, Schaefer K, Berdy S, et al. (2002) Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Developmental Cell 2: 713-720.
- Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, et al. (2003) Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17: 1581-1591.
- Yu C, Wang F, Kan M, Jin C, Jones RB, et al. (2000) Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275: 15482-15489.
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, et al. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217-225.
- Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, et al. (2005) Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J Clin Invest 115: 2202-2208.
- Lundasen T, Galman C, Angelin B, Rudling M (2006) Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 260: 530-536.
- Walters JR (2014) Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol 11: 426-434.
- Lenicek M, Duricova D, Komarek V, Gabrysova B, Lukas M, et al. (2011) Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis 17: 1322-1327.
- Ghosh A, Chen F, Banerjee S, Xu M, Shneider BL (2014) c-Fos mediates repression of the apical sodium-dependent bile acid transporter by fibroblast growth factor 19 in mice. Am J Physiol Gastrointest Liver Physiol 306: G163-171.
- Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, et al. (2013) Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol 304: G940-948.
- Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, et al. (2014) A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med 6: 247ra100.
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, et al. (2011) FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331: 1621-1624.
- Gingras AC, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913-963.
- Fumagalli S, Thomas G (2000) In Translational control of gene expression (eds.Sonenberg N, Hershey JWB, Mathews MB), 695-717. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Haeusler RA, Accili D (2008) The double life of Irs. Cell Metab 8: 7-9.
- Bhatnagar S, Damron HA, Hillgartner FB (2009) Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem 284: 10023-10033.
- Wu X, Ge H, Baribault H, Gupte J, Weiszmann J, et al. (2013) Dual actions of fibroblast growth factor 19 on lipid metabolism. J Lipid Res 54: 325-332.
- Fu L, John LM, Adams SH, Yu XX, Tomlinson E, et al. (2004) Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594-2603.
- Wu AL, Coulter S, Liddle C, Wong A, Eastham-Anderson J, et al. (2011) FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One 6: e17868.
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, et al. (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018-6027.
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, et al. (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627-1635.
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, et al. (2009) Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250-259.
- Beysen C, Murphy EJ, Deines K, Chan M, Tsang E, et al. (2012) Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis and cholesterol and bile acid kinetics in type 2 diabetes: a randomized controlled study. Diabetologia 55: 432-442.
- Zhou M, Wang X, Phung 1, Lindhout DA, Mondal K, et al. (2014) Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res 74: 3306-3316.
- Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, et al. (2011) Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening Cancer Cell 19: 347-358.
- Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, et al. (2012) Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 12: 56.
- Uriarte I, Latasa MU, Carotti S, Fernandez-Barrena MG, Garcia-Irigoyen O, et al. (2014) Ileal FGF15 contribute to fibrosis-associated hepatocellular carcinoma development. Int J Cancer 136: 2469-2475.
- Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, et al. (2002) A mouse model of hepatocellular carcinoma: Ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol 160: 2295-2307.
- Wu X, Ge H, Lemon B, Vonderfecht S, Baribault H, et al. (2010) Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc Natl Acad Sci U S A 107: 14158-14163.
- Desnoyers LR, Pai R, Ferrando RE, Hatzel K, Le T, et al. (2008) Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene. 27: 85-97.
- Buhmeida A, Dallol A, Merdad A, Al-Maghrabi J, Gari MA, et al. (2014) High fibroblast growth factor 19 (FGF19) expression predicts worse prognosis in invasive ductal carcinoma of breast. Tumour Biol 35: 2817-2824.
- Pai R, Dunlap D, Qing J, Mohtashemi I, Hotzel K, et al. (2008) Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating beta-catenin signaling. Cancer Res 68: 5086-5095.
- French DM, Lin BC, Wang M, Adams C, Shek T, et al. (2012) Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One 7: e36713.
- Wang J, Stockton DW, Ittmann M (2004) The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin Cancer Res 10: 6169-6178.
- Sahadevan K, Darby S, Leung HY, Mathers ME, Robson CN, et al. (2007) Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J Pathol 213: 82-90.
- Murphy T, Darby S, Mathers ME, Gnanapragasam VJ (2010) Evidence for distinct alterations in the FGF axis in prostate cancer progression to an aggressive clinical phenotype. J Pathol 220: 452-460.
- Ho HK, Pok S, Streit S, Ruhe JE, Hart S, et al. (2009) Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J Hepatol 50: 118-127.
- Nakayama Y, Miyake A, Nakagawa Y, Mido T, Yoshikawa M, et al. (2008) Fgf19 is required for zebrafish lens and retina development. Dev Biol 313: 752-766.
- Nishimura T, Utsunomiya Y, Hoshikawa M, Ohuchi H, Itoh N (1999) Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim Biophys Acta 1444: 148-151.
- Itoh N, Ornitz DM (2004) Evolution of the Fgf and Fgfr gene families. Trends Genet 20: 563-569.
- Miyake A, Nakayama Y, Konishi M, Itoh N (2005) Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev Biol 288: 259-275.
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH (2000) Identification of synergistic signals initiating inner ear development. Science 290: 1965-1967.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 17987
- [From(publication date):
June-2015 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 13213
- PDF downloads : 4774