Fetal Movement in Actocardiogram and Prevention of Cerebral Palsy with Hypoxia Index
Received: 17-May-2018 / Accepted Date: 05-Jun-2018 / Published Date: 12-Jun-2018 DOI: 10.4172/2376-127X.1000380
Abstract
Aim: To analyze the relation of fetal movement and heart rate.
Method: Fetal movement and heart rate (FHR) was analysed by actodardiogram. Novel hypoxia index was calculated by the sum of FHR deceleration duration and the lowest FHR.
Results: As FHR increasesd when the fetus moved, FHR was studied in the relation to fetal movements, e,g, benign sinusoidal FHR was evoked by periodic fetal respiratory movement. No FHR acceleration was found in fetal hiccupping due to no formation of fetal movement burst. Hypoxic loss of FHR acceleration and variability were caused by no response of damaged fetal brain to fetal movement.
Discussion: The fetus should be cured from cerebral palsy by early delivery before the loss of variability, where the hypoxia index was 25 or more in the loss of variability followed by cerebral palsy, while normal variability and no cerebral palsy was preceded by 24 or less hypoxia index. Thus, the HI should be 24 or less to prevent cerebral palsy.
Conclusion: FHR changes, which had not solved by CTG, was solved by the application of fetal movement. Novel hypoxia index is usefull for the prevention of cerebral palsy by the 24 or less of hypoxia index.
Keywords: Fetus; Heart Rate; Brain Damage; Cerebral Palsy; Hypoxia Index; Ultrasound; Doppler Signal
Introduction
Although pregnant woman feels fetal movement as kicking, that is subjective and unsuitable to scientific study, Thus, there had been several technical methods to detect fetal movement, e,g, piezo-electric element, microphone, external uterine contractin detector, photo-diode technique, etc [1]. However, they detected the movement at maternal abdominal surface, but it was not direct detection of fetal movement in the amniotic fluid. The author intended to record fetal motion directly in the amniotic fluid with ultrasonic Doppler signal developed by fetal chest motion in amniotic fluid.
Methods
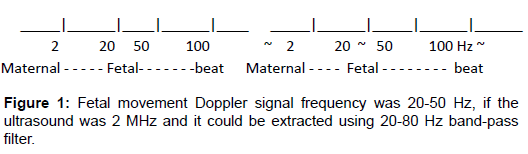
It was usual to detect ultrasonic Doppler vibration developed by fetal heart beat to record fetal heart rate (FHR) with ultrasonic probe in commercial ultrasonic Doppler fetal heart rate monitor, where the direction of ultrasound beam was fixed at fetal chest, therefore, the Doppler signals contained various movements including maternal and fetal movements and fetal heart beats. The author extracted the fetal movement Doppler signals using various frequecy filters in the remodeling of TOITU TN-400 fetal monitor (Tokyo) by himself, detecting the ultrasonic Doppler signal at fetal chest, which contained various movement Doppler signals. The author sit at the bed side observing oscilloscopic images measuring their frequencies, where maternal motion was 2 Hz or low, fetal heart beat was 100 Hz or more, then fetal movement Doppler frequencty was 20-50 Hz, checking the monitor sound of the ultrasonic fetal monitor and measuring Doppler signal frequency with real-time frequency analyzer.
The author cut off maternal motion Doppler signal by 8Hz highpass filter observing the oscilloscope, then cut-off the Doppler signal of fetal heart beat by 100 Hz high-pass filter, then obtained pure fetal movement Doppler signal, which was 20-50 Hz when ultrasound was 2 MHz and it was extracted using available 20-80 Hz band-pass filter, which was adjusted to fetal motion spikes to record in the fetal monitor recorder, where the prototype actocardiograph was completed [2,3] (Figure 1).
Results
Fetal movement spike amplitude
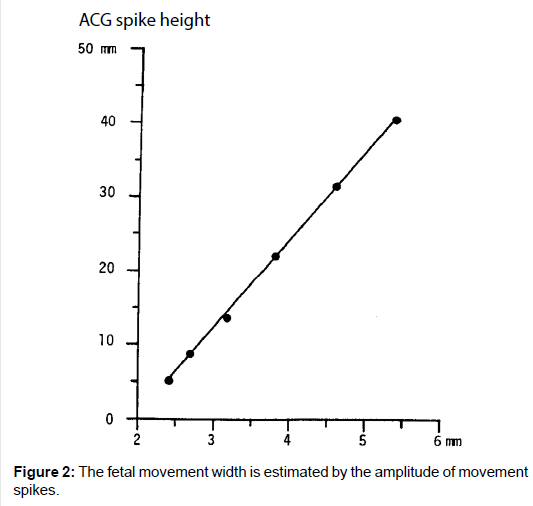
As the movement spike amplitude was parallel to the movement of the steel ball in the water set against the ultrasonic probe, fetal motion amplitude was able to be estimated with the height of actographic spike, namely, fetal chest movement width was estimated by the amplitude of recorded spike [2,3] (Figure 2).
Fetal movement spikes were coincided with the fetal motion detected by real-time ultrasound B-mode.
Commercial models
The author asked TOITU (Tokyo) to provide commercial model of actocardiogram, then MT-320, the first commercial model, was tested by perinatology expertsin the world, where all fetal movements were recorded by actocardiogram, namely movements of extremities and even fetal mouthing were conducted to fetal chest. Afterwards, many actocardiographs were provided and update model is MT-630 (Figure 3).
Since TOITU models recorded fetal heart rate (FHR), fetal movement spikes and uterine contraction simultaneously, it was an actocardiograph (ACG) when FHR and fetal movement were analyzed and it was cardiotocograph (CTG), when FHR and uterine contraction were analyzed. Thus, the machine could be called as acto-cardio-tocogram (ACTG), but usually called as actocardiogram from its main function.
Fetal behavior
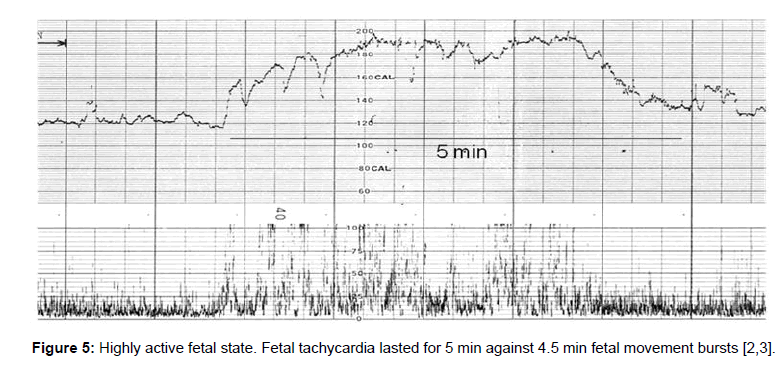
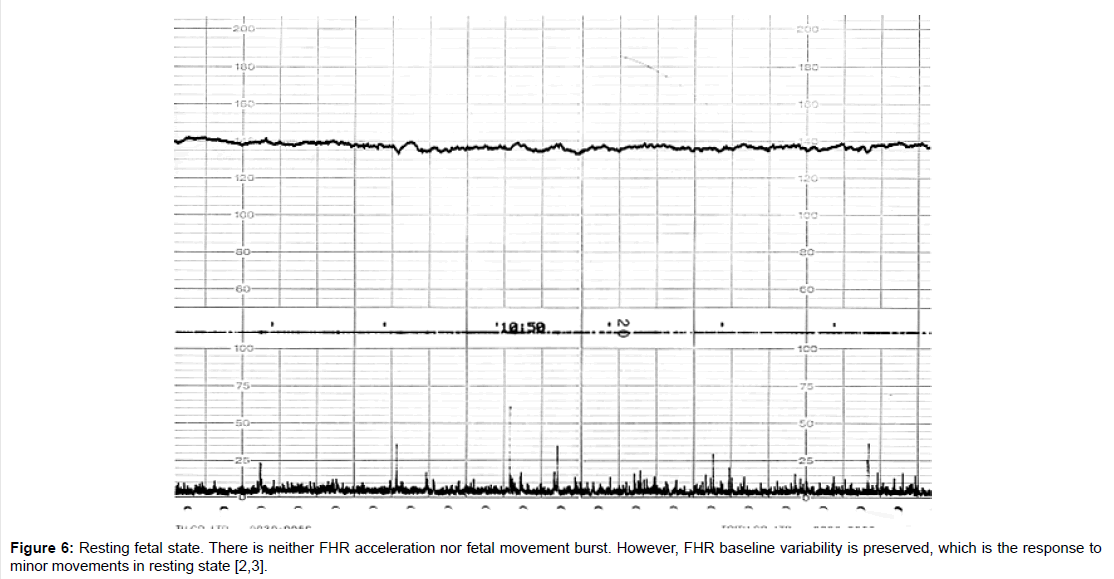
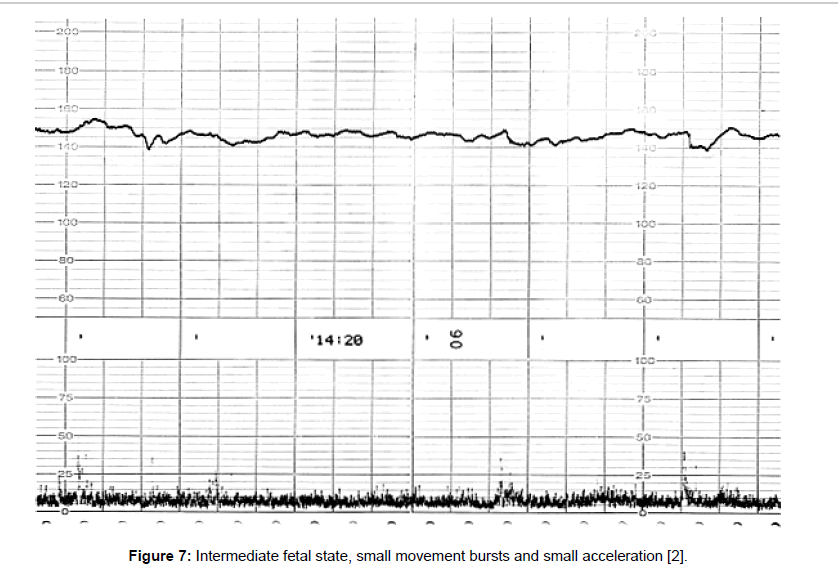
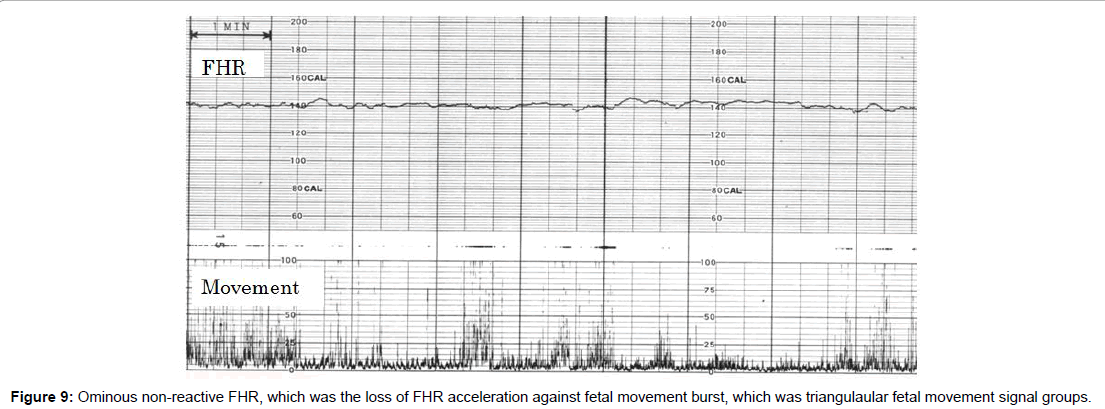
Fetal behavioral states were classified into active, highly active, resting and intermediate states classified by the active to resting states [2,3] (Figures 4-7).
Fetal behavioural states
They are divided into active, highly active, resting and intermediate states, of which details are shown in Tables 1-3 (Figures 4-7).
| Nijhuis's States | Fetal Movements | Fetal Heart Rate | Actocardiogram States | ||
|---|---|---|---|---|---|
| Body | Eye | Acceleration | Bandwidth | ||
| 1F | ‒ (or brief) | ‒ | ‒ (or isolated) | narrow | resting |
| 2F | ++ | + | ++ | >1F | active |
| 3F | ‒ | + | ‒ | >1F The regularity and frequency of variability are more than 1F | intermediate |
| 4F | +++ | + | +++ | unstable or tachycardia | highly active |
Table 1: Actocardiographic fetal behavioral states were compared to that reported by Nijhuis [4].
| Parameters | |
|---|---|
| Burst duration (sec) | Mean duration of fetal movement bursts |
| Occupancy (%) | Summarized fetal movement durations divided by the recording duration |
| Frequency (cycles per minute, cpm) | The frequency of movement bursts in a minute. |
Table 2: Three quantitative parameters of fetal movement.
| Normal active state after 22 W | Intermediate States | p | |
|---|---|---|---|
| N=14 | N=5 | (st-t test) | |
| Burst duration (Sec) | 29.69 ± 10.27 (18.9-57.8) | 17.78 ± 3.68 (12.1-21.1) | 0.023 |
| Occupancy (%) | 32.7 ± 14.76 (12.9-58.8) | 6.44 ± 1.75 (3.5-7.9) | 0.001 |
| Frequency (cpm) | 0.7 ± 0.22 (0.23-0.99) | 0.17 ± 0.03 (0.13-0.2) | <0.001 |
| state duration (min) | <50 | 28.4 ± 7.09 (20-35) |
Table 3: Parameters of active and intermediate states [2,3].
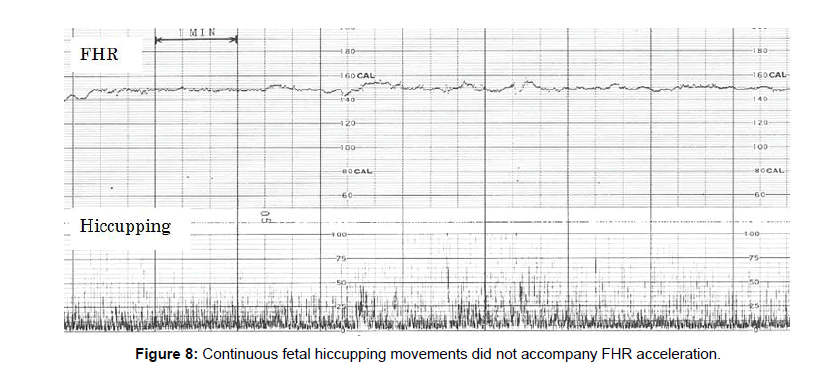
Fetal hiccupping movements
Fetal hiccupping was clearly shown by sudden and large stretcking motion in the real-time B-mode ultrasound image, which is continuous regular repetition of sharp spikes appearing suddenly and repeating 10 or more min. Twice appearances were experienced in day time in late pregnancy.
Fetal hiccupping is a problem in fetal heart rate diagnosis, because the continuous fetal hiccuppings did not accompany fetal heart rate acceleration and the loss of accelration is the sign of fetal hypoxic brain suppression in actocardiogram, however the outcome is favorale and there was no hypoxic asphyia in fetal hiccupping (Figures 8 and 9).
As the hiccupping does not accompany FHR acceleration, hiccupping should be separated from non-reactive FHR by the loss of movement burst formation.
How to separate fetal hiccupping from the non-reactive loss of acceleration?
It is possible because fetal hiccupping does not form grouped movement burst, but it is single and very sharp single repeating continuous spikes, which suddenly starts and finishes, while it makes no triangular grouped feal movement bursts, which is found in active fetal state (Figure 4).
Real-time ultrasound B-mode more clearly shows that hicupping, which is strong convulsive stretching, whch is regular solid actions, forming solid and very sharp actographic spikes.
In electronic simulation, 10 Hz grouped pulses for 30 sec formed triangular wave after passing through an integral circuit, while continuous signals for some minutes formed no acceleration after passing through the integral circuit, namely, fetal hicupping, which is continuous sharp spikes form no acceleration after passing through integral circuit. These facts explains the no acceleration in the fetal hiccupping, namely, short duration of spikes make acceleration, however, 10 min continuously repeated regular spikes form no acceleration [4].
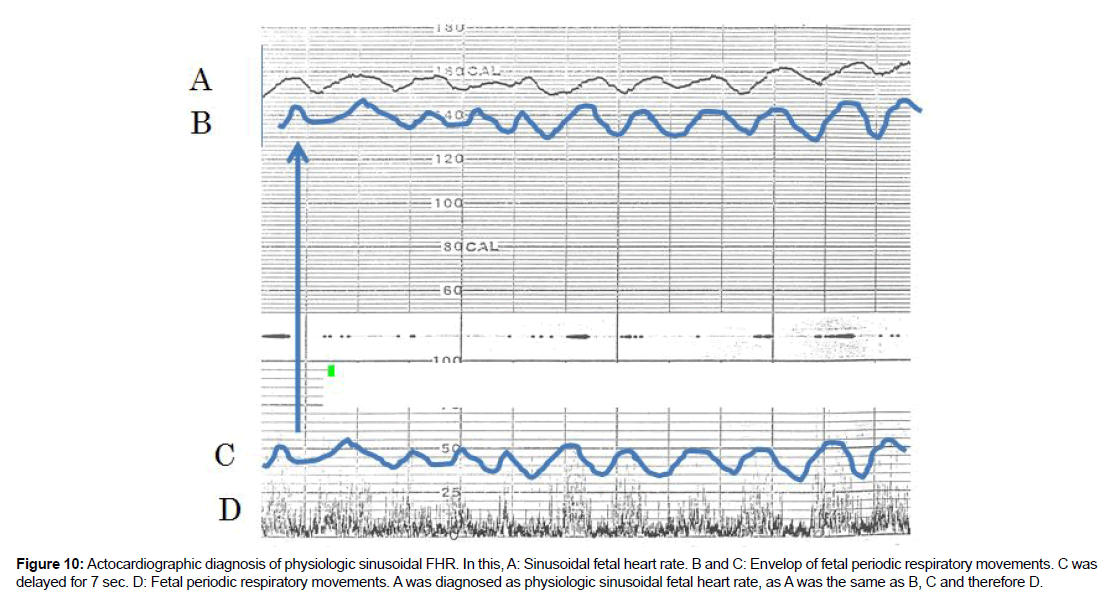
How to separate physiologic sinusoidal FHR from the pathologic sinusoidal?
Answer is to use actocardiogram which records fetal movement simultaneously with fetal heart rate, which changes according to fetal movement amplitudes, due to fetal brain function. Thus, the CTG did not separate physiologic sinusoidal from pathologic one because it did not record fetal movement. There are periodic fetal movements, e.g. periodic fetal respiration and fetal mouthing movements, of which motion was detected by fetal brain to change fetal heart rate periodically with 7 sec delay, that is the time constant of integral function of fetal brain, of which presence was confirmed in adult brain too, namely, regular leg motion of an adult changed its heart rate and formed triangular heart rate in a physiologic experiment.The envelope of fetal respiratory movements formed physiologic sinusoidal FHR [5] (Figure 10).
Figure 10:Actocardiographic diagnosis of physiologic sinusoidal FHR. In this, A: Sinusoidal fetal heart rate. B and C: Envelop of fetal periodic respiratory movements. C was delayed for 7 sec. D: Fetal periodic respiratory movements. A was diagnosed as physiologic sinusoidal fetal heart rate, as A was the same as B, C and therefore D.
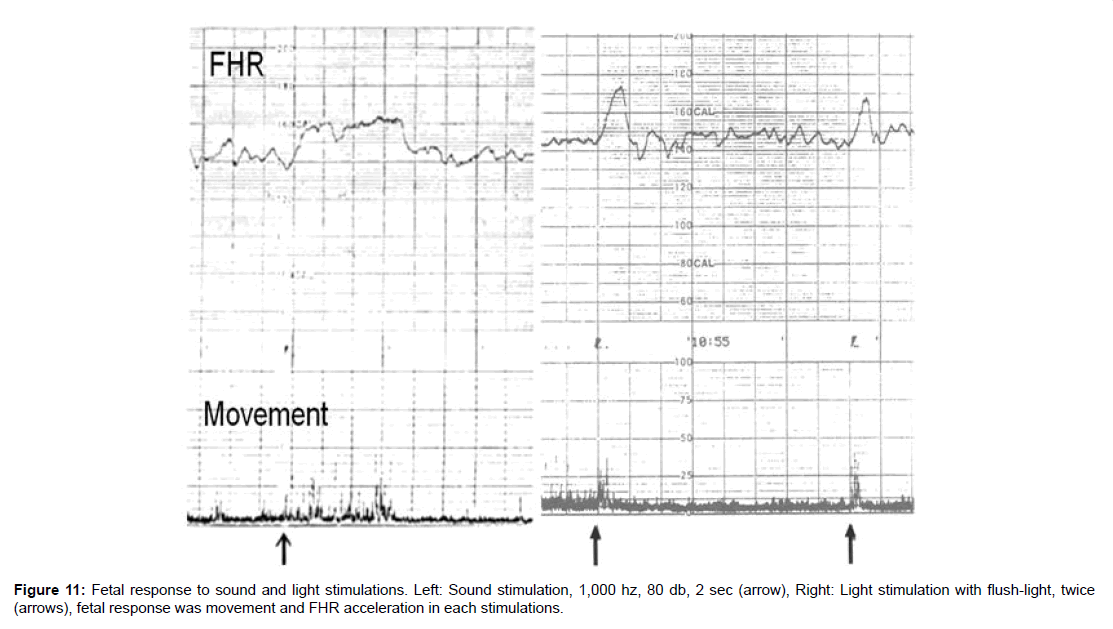
Fetal response to sound and light
Intrauterine fetus responded external sound and light.
1. Fetal response to sound
200, 500 and 1,000 Hz sine waves were generated by HP signal generator and amplified with power amplifier and the sound was radiated by 10 inch dynamic loud speaker on maternal abdomen for 2 sec, 2-3 times repeatedly, Fetal behavioral state was resting state, because false fetal reaction in active state was avoided. Loud speaker output was measured with an audiometer at 1 m apart frrom speaker. Fetal heart rate and movement were recorded by a TOITU actocardiogram (Tokyo).
Fetal response to the sound was fetal movement followed by FHR acceleration in positive reaction.
All of 200, 500 and 1,000 Hz sound stimulation in 2 sec achieved positive feral reaction, but significantly positive reaction was achieved only by 1,000 Hz sound stimulation [6-10]. Positive reaction was obtained by 80 dB intensity sound in a case of 28 weeks of pregnancy, while the same fetus respnded to 60 dB ultrasound in 40 weeks of pregnancy, namely, fetal hearing function icreased about 10 times in 2 weeks in late pregnancy (Figure 11).
Figure 11:Fetal response to sound and light stimulations. Left: Sound stimulation, 1,000 hz, 80 db, 2 sec (arrow), Right: Light stimulation with flush-light, twice (arrows), fetal response was movement and FHR acceleration in each stimulations.
Is fetal education is possible by maternal voice? Answer is negative because maternal voice is lower than 1,000 Hz, to which the fetus responded [11-13].
2. Fetal response to light
Photographic strobolight of 20 guide-number was flushed on maternal abdomen around at fetal head. Positive reactions were fetal movement then FHR acceleration. The first positive reaction was achieved at 23 weeks of pregnancy, when fetal retina was formed. Positive reaction rate to the light was increased to 77% in 40 weeks of pregnancy [13] (Figure 11).
Discussion
Intrapartum fetal monitoring
As stated above, continuous recording of fetal heart rate and movement with actocardiogram was convenient to assess the cause of fetal heart rate change, fetal behavior, detection of fetal hypoxia by the loss of heart rate acceleraton and the loss of baseline variability, where it is able to diagnose more fetal states than cardiotocogram, which is the recording of fetal heart rate and uterine contraction, while actocardiogram records also fetal movements, namely, actocargiogram also prepares the function of cardiotocogram too, if the user hopes to monitor heart rate and uterine contraction simultaneously. As fetal heart rate is recorded with ultrasonic Dopplor autocorrelation heart rate meter, its heart rate tracing is external through pregnancy and labor and it is as clear as fetal scalp lead ECG with needle electrode, of which use is limited only after the rupture of membrane and in case of no maternal viral disease [6]. As ultrasound intensity of autocorrelation is only 1 mW/cm2, which is very low and safe.
Novel hypoxia index to numerically prevent cerebral palsy
Since 3 connective late decelerations (LD) achieved normal neonate with Apgar score 9, though ominous outcome was expected, when particular pattern of LD was recorded, namely, fetal abnormality should be expected in all of LD in FHR record, while, 50 min continuation of LD patterns resulted Apgar 3, severe neonatal asphyxia with severe brain damage, characterized by the loss of FHR variability, where repeated decelerations, which is characterized by overlapping transient hypoxia will be the target, namely, novel hypoxia index was the sum of deceleration duraton (min) divided by the lowest FHR (bpm) and multiplied by 100. Heart rate was used instead of PaO2, because heart rate was completely linarly correlated to PaO2 if it is lower than 50 mmHG and cord blood PaO2 is lower than 50 mmHg. The hypoxia index is recommended to apply to all decelerations, including socalled early, late and variable decelerations and also fetal continuous bradycardia because the equation does not include the lag time. Also the author recommend to try to change maternal posture to lateral one, because various fetal bradycardia is caused by the compression of large blood vessels by contracted pregnant uterus to limit the blood flow through the placenta at supine postuture. Typical disappearance of late deceerations after laeral posture was reported by Poseiro 1969. You do not need to calculate the index, if the deceleration disappears after taking lateral posture. In a retrospective study, the author collected 6 cerebral palsy cases and 16 normal cases without cerebral palsy with intraprtum FHR chart and calculated the Hypoxia Index by the author, where Hypoxia Index was 25 or more in cases of cerebral palsy and 24 or less in caes of no cerebral palsy. Thus, the numeric threshold exists between positive and negative cerebral palsy, it is located between 24 and 25 of hypoxia index (Table 4).
| Hypoxia Index | Cerebral Palsy | |
|---|---|---|
| Yes | No | |
| 25 or more | 6 | 0 |
| 24 or less | 0 | 16 |
* Chi square test: p=0.000008<0.05, significant difference.
Table 4: The relation of cerebral palsy and Hypoxia Index.
The author would like to recommend to try lateral posture when there is fetal bradycardia. Hypoia Index is calculated, when the bradycardia does not disappear after taking lateral posure, then the Index should be kept to be less than 24 until delivery. It will be the way to prevent cerebral palsy. The author is planning a prospective study to reduce cerebral palsy by numeric hypoxia index.
Computerized fetal monitoring
The computerized intrapartum fetal monitoring is convenient and useful by introduction of FHR score with its regression equqtions to predict Apgar score and UApH [8]. Novel hypoxia index prevents cerebral palsy if the index is less than 24, Normal Apgar score is predicted by the duration ratio of FHR acceleration to movement burst (A/B ratio) and artificial neural network computer can predict the probabilities to be normal or pathologic outcome [9,11,14-17]. The author is planning a new fetal computer with FHR score, hypoxia index and frequency spectrum to detect pathologic sinusoidal heart rate [7]. Clinical results were already improved by a centralized computer system to significantly reduce periata mortality and no cerebral palsy [10].
Conclusion
Fetal movement was studied to objectivly and numerically diagnose intrapartum fetus and to reduce cerebral palsy due to intrapartum damage.
References
- Maeda K, Kimura S, Nakano H (1969) Pathophysiology of fetus. Fukuoka Printing, Fukuoka, Japan.
- Maeda K (2016) Actocardiogram: Analysis of fetal motion and heart rate.
- Maeda K (2016) Invention of ultrasonic Doppler fetal actocardiograph and continuous recording of fetal movements. J Obstet Gynaecol Res 42: 5-10.
- Nijhuis JG (1993) Fetal behavioral states. In: Chervenak, Isacson, Campbel (Eds). Ultrasound Obstet Gynecol. Pp: 447-455.
- Ito T, Maeda K, Takahashi H, Nagata N, Nakajima K, et al. (1994) Differentiation between physiologic and pathologic sinusoidal FHR pattern by fetal actocardiogram. J Perinat Med 22: 39-43.
- Cosumi K, Gardosi J, Maeda K (1995) FIGO news: Intrapartum surveillance: Recommendations on current practice and overview of new developments. Int J Gynecol Obstet 49: 213-221.
- Maeda K, Nagasawa T (2005) Automatic computerized diagnosis of fetal sinusoidal heart rate. Fetal Diagn Ther 20: 328-334.
- Maeda K (2006) Quantitative FHR evaluation without pattern classification. Kurjak (Edtr), textbook of Perinatal Medicine, 2nd edition, Informa. Pp: 1496.
- Maeda K, Iwabe T, Yoshida S, Ito T, Minagawa Y, et al. (2009) Detailed multigrade evaluation of fetal disorders with the quantified actocardiogram. J Perinat Med 37: 392-396.
- Maeda K, Utsu M, Noguchi Y, Matsumoto F, Nagasawa T, et al. (2012) Central computerized automatic fetal heart rate diagnosis with a rapid and direct alarm system. The Open Medical Devices J 4: 28-33.
- Maeda K, Noguchi Y, Matsumoto F, Nagasawa T (2012) Artificial neural network system in the automated diagnosis of fetal heart rate. J Health Med Informat S5.
- Maeda K (2013) Origin of the long-term variability and acceleration of FHR studied for the prevention of cerebral palsy in fetal hypoxia and general insults. J Perinat Med 42(3):401-403.
- Maeda K, Tatsumura M (2017) Fetal response to sound and light: Possible fetal education? J Neonatal Biol 6: 247.
- Maeda K (2017) Hypoxia index precisely covers the roles of FHR deceleration and bradycardia in fetal monitoring. J Pregnancy Reprod 1(4): 1-2.
- Maeda K (2018) Novel hypoxia index in fetal heart rate monitoring. J Gynecol Reprod Med 2: 1-2.
- Maeda K (2018) Hypoxic fetal brain damage followed by cerebral palsy will be prevented by computerized fetal monitoring. J Applied Bioinforma Comput Biol 7:1.
- Maeda K (2018) Novel hypoxia index in fetal heart rate monitoring. J Gynecol Reprod Med 2: 1-2.
Citation: Maeda K (2018) Fetal Movement in Actocardiogram and Prevention of Cerebral Palsy with Hypoxia Index. J Preg Child Health 5: 380. DOI: 10.4172/2376-127X.1000380
Copyright: © 2018 Maeda K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7143
- [From(publication date): 0-2018 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 6197
- PDF downloads: 946