Research Article Open Access
Fetal Heart Rate Changes are the Fetal Brain Response to Fetal Movement in Actoardiogram: The Loss of Fhr Variability is the Sign of Fetal Brain Damage
| Kazuo Maeda* | |
| Department of Obstetrics and Gynaecology, Tottori University Medical School, Yonago, Japan | |
| Corresponding Author : | Kazuo Maeda Department of Obstetrics and Gynaecology Tottori University Medical School Yonago, Japan Tel: 81-859-22-6856 E-mail: maedak@mocha.ocn.ne.jp |
| Received: February 10, 2016 Accepted: February 16, 2016 Published: February 23, 2016 | |
| Citation: Maeda K (2016) Fetal Heart Rate Changes are the Fetal Brain Response to Fetal Movement in Actoardiogram: The Loss of Fhr Variability is the Sign of Fetal Brain Damage. J Preg Child Health 3:219. doi:10.4172/2376-127X.1000219 | |
| Copyright: © 2016 Maeda K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Aims To study fetal brain response to fetal movements with fetal heart rate (FHR) changes. Methods FHR changing mechanism was investigated by simultaneously recorded FHR and fetal movements detected directly at fetal thorax with Doppler ultrasound in act cardiogram (ACG). FHR changing process was confirmed by electronic and physiologic simulations. Results and conclusion As recorded movement spike height was parallel to fetal movement amplitude in ACG, FHR increased when the fetus moved, and triangular FHR acceleration developed at fetal movement burst by the integral function of midbrain. Moderate fetal movements developed moderate FHR increase, and periodically changing fetal movements developed physiologic sinusoidal FHR separating pathologic sinusoidal one. FHR variability developed by minor fetal movements. FHR acceleration was lost in early stage of hypoxia, and then the loss of variability comparable to anencephaly appeared in severe hypoxic fetal brain damage, followed by cerebral palsy. Thus, early delivery is recommended before the loss of variability, instead of C-section after the loss of variability. Rabbit heart rate reduced along PaO2 drop, where parasympathetic centre was excited in medulla oblongata by low PaO2 developing fetal bradycardia, which showed environmental hypoxia, whereas the loss of variability was full brain damage due to severe hypoxia, where fetal brain could not respond fetal movements as anencephaly.
| Keywords |
| Fetal heart rate; Fetal movement; Acceleration; Sinusoidal heart rate; Baseline variability; Hypoxia; Bradycardia; Brain damage; PaO2 |
| Introduction |
| The cardiotocogram (CTG) studied FHR and uterine contraction [1], but it was unable to show developing mechanism of FHR acceleration, physiologic sinusoidal FHR and baseline FHR variability. Although FHR pattern classification was commonly used in obstetrics, the pattern diagnosis was frequently controversial, possibly due to empirical and subjective FHR patterns, though perinatal outcome was improved by the rapid delivery after introduction of CTG monitoring. As the actocardiogram (ACG) records FHR and fetal movement spikes [2], various problems in CTG were solved, e.g. the separation of physiologic sinusoidal FHR from pathologic one, and the mechanism to change FHR were clarified. |
| Methods |
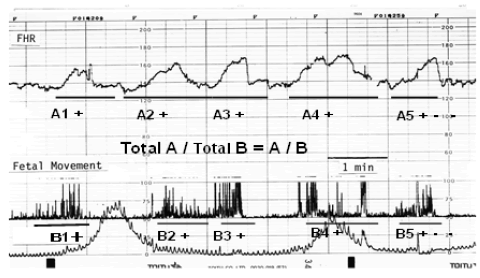
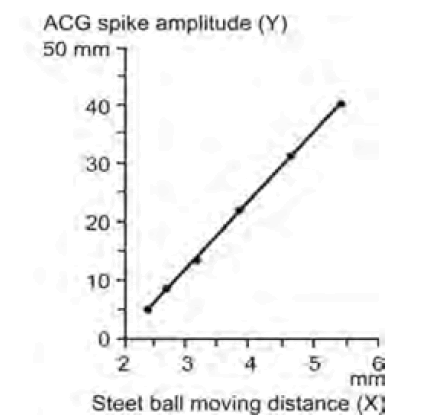
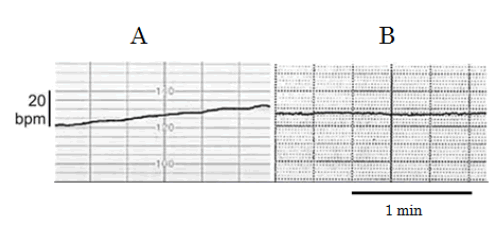
| Maeda [2] created the ACG, which was simultaneous records of FHR and fetal movement spikes detected directly at the surface of fetal thorax using ultrasonic Doppler method (Figure 1), because the transducer was set to detect fetal heartbeat. Fetal movements were fully detected by world researchers using the ACG [3-5], therefore, the relation between FHR change and fetal movement was objectively investigated in the off-line studies [6-13]. As the spike amplitude recorded on the chart was parallel to the moving width of the subject (Figure 2) [2], fetal moving size was correctly represented by the spike amplitude in ACG, i.e., the magnitude of fetal movement is able to be discussed measuring recorded movement spikes. |
| Umezawa [14] found that heart rate of adult rabbit closely correlated to rabbit’s PaO2, when it was lower than 50 mmHg, and Nakano [15] found that PaO2 of the umbilical cord arterial blood was 50 or less mmHg Therefore, fetal hypoxia was diagnosed by fetal bradycardia. |
| Fetal anencephalic ACG and heart rate of anencephalic neonates were studied in the comparison to normal fetus [16]. |
| Teshima [10] studied ACG of 20 fetal growth restriction (FGR) which lost FHR acceleration against fetal movement bursts preserving FHR variability (non-reactive FHR), comparing to the foetuses of 20 normal reactive FGR with positive acceleration against fetal movement burst. |
| Results |
| FHR acceleration developed against fetal movement bursts |
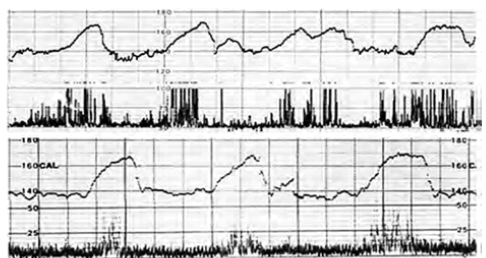
| Fetal movement bursts repeatedly associated triangular FHR accelerations in fetal active state (Figure 3), while neither FHR acceleration nor fetal movement was recorded in fetal resting state, namely, FHR acceleration was formed by the fetal movement burst. |
| Acceleration development was estimated by two simulations |
| FHR acceleration was reported to develop in the midbrain in the studies on anencephalic fetal heart rate [6]. Fetal movement burst was usually continuous in fetal active state, where all accelerations were triangular. Therefore, electronic simulation was studied by the input of 10 Hz wave groups into the integral circuit with 7 sec time constant, because the correlation coefficient was largest when the movement signal delayed for 7 sec in the correlation study of FHR and fetal movement [7]. The output of integral circuit was invariably triangular in the electronic simulation [8]. |
| Also in a physiologic simulation test, adult heart rate was recorded with a fetal heart rate meter in the repetition of leg motions for 1 min, where the heart rate repeatedly changed to triangular accelerations [8]. Since the experimental person did not recognize the heart rate change, the process of triangular heart rate increase would not be formed in the cerebrum, but in the midbrain. |
| Moderate fetal movements developed moderate FHR variations |
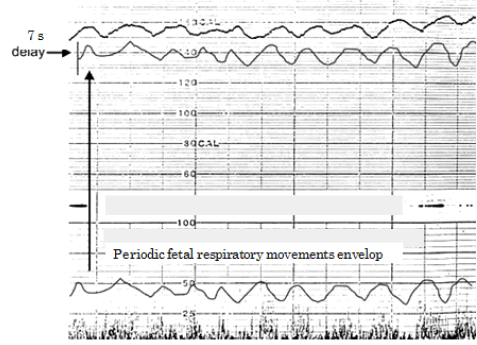
| Suitable example is fetal physiologic sinusoidal heart rate. The pathologic sinusoidal FHR developed in severe fatal anaemia, fetomaternal transfusion, Rh-incompatibility or Parbo-B 19 viral infection, while physiologic sinusoidal was healthy with favourable outcome, therefore, it should be differentiated from pathological one. The separation of physiologic sinusoidal from pathologic one was, however, unable with the CTG., while the differentiation was achieved in ACG, where moderate sine wave-like FHR variation was closely similar to the envelop of periodic fetal movements in the fetal cyclic respiration or mouthing movements (Figure 4) [9]. The FHR variation would develop also in the midbrain. |
| Minor fetal movement develops FHR baseline long term variability |
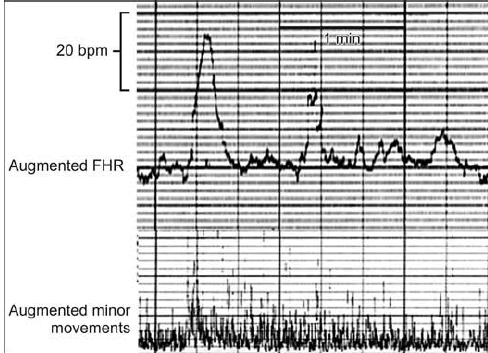
| Long term FHR variability amplitude is 5-24 beats per minutes (bpm) on the FHR baseline. The variability develops by minor fetal movements, which were recognized on enlarged ACG using computer processing (Figure 5). The variability would also develop in the midbrain. |
| Fetal brain damage is estimated by the loss of long term variability |
| FHR accelerations reduced in the early stage of fetal hypoxia, where the ratio of acceleration duration to fetal movement burst (A/B ratio) decreased, where short and long term outcomes closely correlated with the A/B ratio, and the outcomes were unfavourable when the A/B ratio was less than 1.0. Also the severity ranking of central nervous system lesions was determined by the A/B ratio [11-13]. |
| The loss of variability suggests the severe fetal brain damage |
| The acceleration of fetal growth retardation (FGR) cases disappeared in early stage of hypoxic suppression in FGR, where the variability was preserved, while severe NRFS asphyxia including the loss of variability appeared after the loss of acceleration within 2 weeks, and its outcome was ominous in spite of all C-section comparing to normally reactive FHR acceleration of FGR [10]. |
| The variability was preserved when the acceleration was lost, then it was lost finally at the most severe hypoxic fetal brain damage, which was experienced in a case of severe late decelerations, where the baseline was as smooth as an anencephalic fetus (Figure 6). Also the variability was lost in a case of severe FHR variable decelerations, later accompanied cerebral palsy (CP) after birth. |
| Comparison of FGR who lost FHR acceleration and reactive FGR |
| Sixteen cases developed severe asphyxia showing the loss of variability, bradycardia, and severe variable and late decelerations among 20 non-reactive FGR cases within 2 weeks after the loss of FHR acceleration. Although all non-reactive FGR received C-section, 2 neonates died and 4 severely asphyxiated, while reactive FGR cases that normally developed acceleration against fetal movement had normal vaginal deliveries developing neither neonatal death nor asphyxia. Significant outcome differences were noted between reactive and non-reactive groups in outcomes [10]. Thus, it is recommended to perform C-section when the acceleration is lost in high-risk cases before the loss of variability. |
| Discussion |
| Fetal heart rate variations are caused by fetal movements |
| Fetal heart rate had been discussed with the uterine contraction in the CTG of fetal monitoring, where various problems remain unsolved in the use of uterine contraction, and they were clearly solved studying simultaneously recorded fetal movements in ACG as seen in this article, particularly in the developmental mechanism of FHR acceleration, physiologic sinusoidal heart rate and baseline variability. The outcome of fetal disorder is predicted, ranking of fetal CNS lesions is determined, paradox in fetal monitoring was solved, namely, almost all of controversy problems in CTG were solved by the introduction of simultaneous fetal movement studies using ACG, e.g., healthy neonates were obtained in fetal bradycardia associated with FHR acceleration [17,18]. Fetal brain reaction to fetal movement is indispensable in the discussion of fetal pathophysiology. |
| Fetal bradycardia as the sign of low PaO2 |
| Adult rabbit heart rate did not respond the PaO2 change when it is higher than 50 mmHg, while the heart rate highly correlated PaO2 when the PaO2 was lower than 50 mmHg, i.e. heart rate dropped showing bradycardia when PaO2 decreased by the nitrogen gas inhalation [14]. Since human umbilical arterial blood PaO2 was usually lower than 50 mmHg [15], the fetus shows bradycardia in low fetal PaO2, i.e. fetal deceleration can be reversible from transiently low PaO2, but bradycardia itself would not indicate brain damage in transiently mild to moderate decelerations. The parasympathetic centre located in the medulla oblongata is stimulated and excited by the hypoxia, producing bradycardia, actually, experimental hypoxia produced bradycardia, but it did not appear under general rabbit anaesthesia [14]. Also, remained medulla oblongata was excited by the hypoxia after the delivery of anencephalic apnoeic neonate, developing severe bradycardia which returned to normal heart rate after injection of oxygenated blood [19], namely, bradycardia is an nervous reaction to low PaO2, but not fetal brain damage in short duration of hypoxia, as confirmed in moderate variable deceleration, showing fetal environmental hypoxia tolerating for some duration. Urgent C-section in prolonged irreversible bradycardia is valuable to prevent hypoxic damage due to pro longed hypoxia. |
| Severely asphyxic fetus should deliver before the loss of variability |
| Since the loss of variability appears in the most severe fetal brain damage similar to the loss of brain in anencephalic fetus [16], the highly asphyxiated fetus is recommended to deliver before the loss of variability. Since total CP is 0.2% of birth and the brain damage of mature fetus develops in 10% of CP, the CP developed in term fetus will be 0.02% of births that is 1 in 5,000 births. It is rare; however, it will be 200 cases in yearly one million deliveries in Japan. Therefore, the CP in the loss of variability would be prevented by the delivery before the loss of variability. The delivery timing would be severe bradycardia, severe variable or late decelerations, loss of acceleration, decreased variability, high FHR score, low A/B ratio, high hypoxia index etc. CP was reduced in old time when C-section was performed by above indications, where the fetus might deliver unexpectedly before the loss of variability [20-23]. |
| Hypoxia index |
| Hypoxia index was studied to indicate the timing of C-section in severe FHR changes. The index is the duration of bradycardia (min) X100/the lowest heart rate (bpm) of bradycardia. The author studied the index of the cases that showed the loss of variability, which was 25 to 26, while the FHR changes who did not accompany the loss of variability was 20 to 24. Therefore the timing of C-section would be indicated when the hypoxia index is more than 20 but lower than 24. |
| Is it possible to treat fetal hypoxia to avoid maternal morbidity due to C-section? |
| Although some one announced coming treatment of hypoxic damaged fetus by anti-glutamate and free radical scavenger, they had not succeeded. Maternal oxygen inhalation is always applied but the effect was insufficient. However, two strategies will be proposed, one of which was already successful to increase placental function, which was; transfer oxygen from maternal to fetal blood. |
| Solution of fibrin deposit of intrervillous fibrin deposit |
| The treatment was successful in a case of placental fibrin deposit solute by heparin treatment [24]. Fibrin deposit was diagnosed to detect placental B-mode echogenicity by ultrasound tissue characterization, GLHW, which can be determined using common Bmode histogram, of which higher value than normal placenta showed the fibrin deposit. Dr. Utsu treated the mother with 5000 U heparin in a case of FGR in the 2nd trimester, where fetal weight increased, showing normal fetal growth, and then normal neonate was obtained, instead of IUFD in previous pregnancy. |
| Anti-sympathiotonic therapy in preeclampsia |
| Sympatico-tony is characteristic in preeclampsia [25], where uterine arterial constriction would be characteristic, showing the abnormal uterine arterial Doppler flowIt will be effective to administer antisympaticotonic medicine to the preeclampsia patient to treat the mother and fetus at the same time. |
| CP prevention in preterm labor |
| Since CP is definitely more in preterm babies than those in term delivery [22], strategy to prevent CP in Obstetrics will be preventing preterm birth, that is term delivery by various tocolyses, including pharmaceutical or uterus-brain nerve sedation [23], etc., and the treatment of periventricular echo density (PVE) in preterm neonatal brain [19], possibly by administration of growth factor, etc.. These strategies are problems to be solved in the future. |
References
- Hon EH (1968) An Atlas of Fetal Heart Rate Patterns. HartyPresss, New Haven, Connecticat.
- Maeda K (1984) [New ultrasonic Doppler fetalactograph and continuous recording of fetal movement]. Nihon SankaFujinkaGakkaiZasshi 36: 280-288.
- DiPietro JA, Costigan KA, Pressman EK (1999) Fetal movement detection: comparison of the Toituactograph with ultrasound from 20 weeks gestation.J MaternFetal Med 8: 237-242.
- Schwöbel E, Fallenstein F, Huch R, Huch A, Rooth G (1987) Combined electronic fetal heart rate and fetal movement monitor-a preliminary report.J Perinat Med 15: 179-184.
- Wheeler T, Roberts K, Peters J, Murrills A (1987) Detection of fetal movement using Doppler ultrasound.ObstetGynecol 70: 251-254.
- Terao T, Kawashima Y, Noto H, Inamoto Y, Lin TY, et al. (1984) Neurological control of fetal heart rate in 20 cases of anencephalic fetuses.Am J ObstetGynecol 149: 201-208.
- Takahashi H (1990) Studies on cross correlation coefficient of fetal heart rate and fetal movement signals detected by ultrasonic Doppler fetalactocardiogram. ActaObstetGynecolJpn 42: 443-449.
- Maeda K (2013) Actocardiographic analysis of fetal hypoxia detected by the bradycardia, loss of fetal heart rate acceleration and long term variability. J Health Med Inform 4: 118-122.
- Ito T, Maeda K, Takahashi H, Nagata N, Nakajima K, et al. (1994) Differentiation between physiologic and pathologic sinusoidal FHR pattern by fetalactocardiogram.J Perinat Med 22: 39-43.
- Teshima N (1993) [Non-reactive pattern diagnosed by ultrasonic Doppler fetalactocardiogram and outcome of the fetuses with non-reactive pattern].Nihon SankaFujinkaGakkaiZasshi 45: 423-430.
- Maeda K, Morokuma S, Yoshida S, Ito T, Pooh RK, et al. (2006) Fetalbehavioranalyzed by ultrasonic actocardiogram in cases with central nervous system lesions.J Perinat Med 34: 398-403.
- Morokuma S, Fukushima K, Yumoto Y, Uchimura M, Fujiwara A, et al. (2007) Simplified ultrasound screening for fetal brain function based on behavioral pattern.Early Hum Dev 83: 177-181.
- Maeda K, Iwabe T, Yoshida S, Ito T, Minagawa Y, et al. (2009) Detailed multigrade evaluation of fetal disorders with the quantified actocardiogram.J Perinat Med 37: 392-396.
- Umezawa J (1976) Studies on the relation between heart rate and PaO2 in hypoxic rabbit: a comparative study for fetal heart rate change during labor. ActaObstetGynecolJpn 28: 1203-1212.
- Maeda K, Kimura S, Nakano H (1969) Pathophysiology of Fetus. Annual Conv Jap SocObstetGynecol 1: 150-155.
- Takahashi H, Ito T, Maeda K (1989) Actocardiogram in anencephalic fetus. ActaObstetGynecoljpn Chugoku-Shikoku 37: 295-298.
- Maeda K, Kimura S, Nakano H (1969) Studies on fetalpathophysilogy. ActaObstetGynecol 8: 877-886.
- Minagawa Y, Akaiwa A, Hidaka T, Tsuzaki T, Tatsumura M, et al. (1987) Severe fetal supraventricular bradyarrhythmia without fetal hypoxia.ObstetGynecol 70: 454-456.
- Yamamoto N, Utsu M, Serizawa M, Ohki S, Murakoshi T, et al. (2000) Neonatal periventricular leukomalacia preceded by fetal periventricular echodensity.FetalDiagnTher 15: 198-208.
- Takeshita K, Ando Y, Ohtani K, Takashima S (1989) Cerebral palsy in Tottori, Japan. Benefits and risks of progress in perinatal medicine.Neuroepidemiology 8: 184-192.
- Tsuzaki T, Sekijima A, Morishita K, Takeuchi Y, Mizuta M, et al. (1990) [The survey on the perinatal variables and the incidence of cerebral palsy for 12 years before and after the application of the fetal monitoring systems].Nihon SankaFujinkaGakkaiZasshi 42: 99-105.
- Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M; Neonatal Research Network, et al. (2013) Outcomes of infants born at 22 and 23 weeks' gestation.Pediatrics 132: 62-71.
- Maeda K (1999) A proposal to reduce congenital cerebral palsy. J Health Med Info 201: 111-199.
- Maeda K, Utsu M, Kihaile PE (1998) Quantification of sonographic echogenicity with grey-level histogram width: a clinical tissue characterization.Ultrasound Med Biol 24: 225-234.
- Maeda K (2014) Preeclampsia is caused by continuous sympathetic center excitation due to an enlarged pregnant uterus.J Perinat Med 42: 233-237.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12964
- [From(publication date):
February-2016 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 12033
- PDF downloads : 931
