Research Article Open Access
Female Gender and Hispanic Ethnicity are Associated with Increased Risk of Subacute Methotrexate Encephalopathy
Clara Y Lo1, Cynthia Campen1,2, Sandra Luna-Fineman1, Norman J Lacayo1, Paul G Fisher1,2 and Gary V Dahl1*
1Division of Pediatric Hematology/Oncology/Stem Cell Transplantation and Cancer Biology, California
2Department of Neurology, Stanford Cancer Institute, Stanford University School of Medicine, California
- *Corresponding Author:
- Dahl GV
Division of Pediatric Hematology/Oncology/Stem Cell Transplantation and Cancer Biology, California
Tel: 650-723-5535
Email: gary.dahl@stanford.edu
Received date: November 02, 2015 Accepted date: December 10, 2015 Published date: December 12, 2015
Citation: Lo CY, Campen C, Luna-Fineman S, Lacayo NJ, Fisher PG, Dahl GV (2015) Female Gender and Hispanic Ethnicity are Associated with Increased Risk of Subacute Methotrexate Encephalopathy. Toxicol open access 1: 104. doi:10.4172/2476-2067.1000104
Copyright: © 2015 Lo CY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
Subacute methotrexate (MTX) encephalopathy is poorly understood. We performed a historical cohort study of all cases of subacute MTX encephalopathy at Lucile Packard Children’s Hospital from January 1, 2005, to December 31, 2011. Of 272 patients receiving MTX, 21 patients were diagnosed with subacute MTX encephalopathy. Nine patients (42.9%) in this study were Hispanic females, whereas Hispanic females represented 56 of 272 (20.6%) total patients (p=0.0084). Further comparison with the 2010 California census confirms a Hispanic female predominance (p=0.032). We report a predominance of Hispanic females with subacute MTX encephalopathy, which suggests a genetic polymorphism that increases disease risk.
Keywords
Methotrexate; Female; Hispanic; Neurotoxicity of therapy
Introduction
Methotrexate (MTX) is a widely used chemotherapeutic agent for the treatment of pediatric malignancies, especially leukemia, lymphoma, and osteosarcoma. MTX use results in varied toxicities that include mucositis, hepatotoxicity, nephrotoxicity, and neurotoxicity. The mechanism(s) that lead to subacute MTX encephalopathy are not well understood. Furthermore, there are few known clinical characteristics, host genotypes, or preceding factors that identify patients at risk. We describe a cohort of patients with subacute MTX encephalopathy treated at Lucile Packard Children’s Hospital at Stanford University from 2005 to 2011.
Methods
Institutional review board approval was obtained for this historical cohort study. We defined subacute MTX encephalopathy as described previously in the literature [1-3]. We searched the leukemia (lymphoblastic and myeloblastic), lymphoma (non-Hodgkin’s, Hodgkin’s), and osteosarcoma databases of Lucile Packard Children’s Hospital to identify patients who had subacute MTX encephalopathy from January 1, 2005, to December 31, 2011. We reviewed all patients who developed neurologic symptoms within 2 weeks from MTX therapy. Ethnicities were defined by patient self-identification. Primary malignancy and central nervous system (CNS) tumor staging were defined as outlined by standard guidelines.
All patients were evaluated and diagnosed by a pediatric neurooncologist. We excluded patients who had other identifiable causes for their symptoms as determined by the neuro-oncologist, including posterior reversible encephalopathy syndrome (PRES) and ischemic or hemorrhagic infarct. All information was obtained from the patients’ medical records. Neuroimaging studies were retrospectively reviewed by a single neuro-radiologist. Gender and ethnicity demographics for California children were obtained from the California State Department of Finance open access data [4]. Most recent demographic data were available from the year 2010. Chi-squared analysis was performed for distribution statistical calculations.
Results
Patient demographics
Among the patients who received MTX from January 2005 to December 2011, 21 of 272 (7.7%) with leukaemia or lymphoma met the study criteria: 20 patients had leukaemia and 1 patient had lymphoma. There were no identified patients with osteosarcoma; therefore, further osteosarcoma data were excluded from this study. Patient clinical characteristics and demographics are outlined in Table I. None of the patients had a history of preceding neurologic disorders.
Hispanic ethnicity was the largest racial group in the cohort, representing 13 patients (61.9%). This was followed, in descending order, by Caucasian, Asian, and African-American ethnicity (Table 1).
| Age of presentation | Median age: 11 years | Number of patients |
|---|---|---|
| Age range: 1 year - 20 years | ||
| Gender | Female | 14 |
| Male | 7 | |
| Ethnicity | Hispanic | 13 |
| Asian | 3 | |
| African-American | 1 | |
| Caucasian | 4 | |
| Malignancy | VHR preB ALL | 3 |
| HR preB ALL | 12 | |
| Infant ALL | 1 | |
| T-cell ALL | 1 | |
| CNS relapse preB ALL | 2 | |
| SR ALL | 1 | |
| T-cell Lymphoblastic Lymphoma | 1 | |
| CNS status at malignancy diagnosis | CNS 1 | 15 |
| CNS 2 | 5 | |
| CNS 3 | 1 | |
| CNS status at encephalopathy symptom onset | CNS 1 | 21 |
| MTX exposures | HD MTX | 4 |
| IT MTX | 8 | |
| HD+IT MTX | 4 | |
| IV MTX+IT MTX | 2 | |
| TIT | 1 | |
| HD MTX+TIT | 2 | |
| Time from exposure to symptom onset | Average time: 7.7 days | |
| Time range: 5-14 days | ||
| Signs and Symptoms# | Hemiparesis: 15 | |
| Headache: 1 | ||
| Speech disturbance: 15 | ||
| Seizure: 3 | ||
| Confusion: 5 | ||
| MRI imaging | White matter changes consistent with leukoencephalopathy. No bleed or infarct | |
| Acute Treatment | Leucovorin | 2 |
| None | 19 | |
| Time to resolution | <48 hours | 5 |
| 2 days- 14 days (none treated) | 10 | |
| 14 days – 3 months (1 received leucovorin treatment) | 3 | |
| Continued symptoms (1 received leucovorin treatment) | 3 | |
| Subsequent | Aminophylline prophylaxis alone | 3 |
| Prophylactic | Aminophylline prophylaxis+MTX dose reduction | 2 |
| Management | Aminophylline prophylaxis+TIT substitution | 1 |
| MTX dose reduction alone | 14 | |
| Cytarabine substitution alone | 1 | |
| Encephalopathy relapse | --All female | 5 |
| -- 2 Hispanic, 2 Caucasian (both with Trisomy 21), 1 African-American | ||
| --All after IT MTX reintroduction | ||
| --Prophylactic Management History: 1 previously given aminophylline, 4 previously with MTX dose-reduction |
Table 1: Patient Demographics and clinical characteristics (total n=21).
Abbreviations: MRI, magnetic resonance imaging; MTX, methotrexate; HD, high dose (5 grams/square meter); TIT, triple intrathecal (methotrexate 15 mg, hydrocortisone 15 mg, cytarabine 30 mg); IV, intravenous (range 50-250 mg/square meter); IT, intrathecal (12 mg or 15 mg); CNS, central nervous system; preB, precursor B cell; ALL, acute lymphoblastic leukemia; VHR, very high risk; HR, high risk; SR, standard risk.
#: total incidence of signs and symptoms among 21 patients
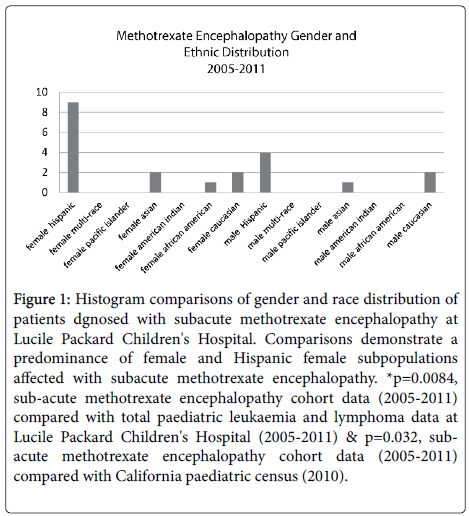
However, there was not a significantly increased proportion of affected Hispanics when compared to the total patients with leukaemia or lymphoma (p=0.1741) or compared to the 2010 California census data (p=0.17). Female gender predominated in this cohort, representing 14 of the 21 (66.7%) patients. In comparison, females represented 116 of 272 (42.6%) total patients diagnosed with leukaemia or lymphoma from 2005-2011 (p=0.0275). However, when compared to the 2010 California census data, there was not a significantly increased number of affected females (p=0.052). Nine patients (42.9%) in this study were both Hispanic and female (median age 10 years, range 1 year – 18 years), whereas Hispanic female patients represented 56 of 272 (20.6%) total patients (p=0.0084). Furthermore, there remained a significantly increased proportion of affected Hispanic females when compared to the 2010 California census data (p=0.032) (Figure 1).
Figure 1: Histogram comparisons of gender and race distribution of patients dgnosed with subacute methotrexate encephalopathy at Lucile Packard Children's Hospital. Comparisons demonstrate a predominance of female and Hispanic female subpopulations affected with subacute methotrexate encephalopathy. *p=0.0084, sub-acute methotrexate encephalopathy cohort data (2005-2011) compared with total paediatric leukaemia and lymphoma data at Lucile Packard Children's Hospital (2005-2011) & p=0.032, subacute methotrexate encephalopathy cohort data (2005-2011) compared with California paediatric census (2010).
Outcome
There was no correlation between length or severity of symptomatology with patient demographics, cumulative MTX dose, or type of preceding MTX exposure. Five patients (23.8%) experienced recurrent symptoms when rechallenged with MTX. All 5 were female, and all recurrences occurred after intrathecal MTX reintroduction. Of these 5 females, 2 were Hispanic, 2 were Caucasian, and 1 was African- American. There were no correlations between cumulative MTX dose, timing of MTX reintroduction, and relapse incidence.
Discussion
Subacute MTX encephalopathy is an uncommon treatment complication. It can be disturbing to patients and their families due to the unique neurologic disturbances (e.g., seizures, motor deficits, speech disturbances, and emotional lability). We performed an historical cohort study of patients with this diagnosis to attempt to identify risk factors. There are epidemiologic findings revealed in this patient cohort that have not previously been reported. Compared to the hospital data, there is a predominance of females affected with subacute MTX encephalopathy. This finding is not replicated when compared to the California census data, although it approaches statistical significance. Furthermore, though small numbers preclude power for statistical significance, it is important to note that all 5 recurrences in this cohort occurred in female patients. Secondly, while Hispanic ethnicity alone was not found to be statistically significant, we found there was also a significantly increased number of affected Hispanic females, which also was not reflective of a predominance of Hispanic females treated at this institution or a predominance of Hispanic females in California
To the authors’ understanding, this is the first cohort in which there is a predominance of Hispanic female patients. The underlying pathophysiology of this interaction is not well understood. One speculation is a possible ethnic link to methylenetetrahydrolate reductase (MTHFR) polymorphisms. MTHFR polymorphisms have been linked with acute MTX toxicities, including hepatoxicity, neurotoxicity, nephrotoxicity, and mucositis [5-8]. MTHFR plays a key role in folate metabolism, catalyzing the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. Antimetabolites such as MTX block this enzymatic pathway, resulting in organ toxicities. The most common MTHFR polymorphism, 677C>T, has been particularly implicated in MTX toxicity [5-8] and 1 case report of subacute MTX encephalopathy [9]. This polymorphism has been reported to be of especially high incidence in Hispanics [10-12]. This phenomenon may help explain the predominance of affected Hispanics on this cohort, but does not explain the female predominance; this second finding could interact with the distribution of the polymorphism and warrants future investigation.
One potential confounding reason for the predominance of females reported in this cohort is the relatively high incidence of affected female patients with HR B-ALL treated at this institution. However, from 2005 to 2011, there were 43 total males who had HR B-ALL treated, but only 2 developed subacute MTX-encephalopathy. Alternatively, there were 45 total females who had HR B-ALL treated during this time frame, and 8 developed subacute MTXencephalopathy. Again this highlights a possible inherent difference between females and males in MTX metabolism and MTX encephalopathy risk.
There are potential confounding variables that may contribute to the symptomatology described in this patient cohort. Six patients were exposed to low-dose cytarabine (75mg/square meter) preceding encephalopathy onset; however, cytarabine neurotoxicity presents more commonly with cerebellar symptoms (specifically ataxia and nystagmus), in patients given high-dose cytarabine (3g/square meter) [13-15]. Furthermore, MRI findings in patients with cytarabine neurotoxicity are predominantly normal [16]. Several patients also received vincristine prior to symptom onset; nevertheless, vincristine neuropathy typically affects peripheral nerves and presents with such symptoms as jaw pain, extremity weakness or paresthesia, and constipation. Five patients also had evidence of CNS disease at malignancy diagnosis, which has been associated with neurologic symptoms [17-19]. However, these patients did not have any evidence of leukemic cells in the cerebrospinal fluid at the time of encephalopathy symptoms. Finally, cranial irradiation can exacerbate encephalopathy symptoms . This may explain why 2 patients in this cohort with CNS relapse did not have neurologic sequelae during preceding treatment courses but developed encephalopathy symptoms with CNS relapse treatment.
In summary, subacute MTX-induced encephalopathy is a poorly understood treatment complication. In this historical cohort review we report a predominance of female and Hispanic female patients. All recurrences occurred in female patients after repeat intrathecal MTX exposure, despite dose-reduction and/or aminophylline prophylaxis. These findings highlight possible gender and ethnic risk factors of disease, which may indicate a genetic polymorphism that increases risk. Future prospective and genome-wide association studies will provide better understanding of the incidence of this entity and may validate female gender and Hispanic ethnicity as risk factors.
Conflict of Interest
The authors do not have any conflicts of interest to disclose
References
- Walker RW, Allen JC, Rosen G, Caparros B (1986) Transient cerebral dysfunction secondary to high-dose methotrexate. J Clin Oncol 4: 1845-1850.
- Inaba H, Khan RB, Laningham FH, et al. (2008) Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol.19:178-184
- Borgna-Pignatti C, Battisti L, Marradi P, Balter R, Caudana R (1992) Transient neurologic disturbances in a child treated with moderate-dose methotrexate. Br J Haematol 81: 448.
- Müller J, Kralovánszky J, Adleff V, et al. (2008) Toxic encephalopathy and delayed MTX clearance after high-dose methotrexate therapy in a child homozygous for the MTHFR C677T polymorphism. Anticancer Res.28:3051-3054
- Spyridopoulou KP, Dimou NL, Hamodrakas SJ, et al. (2012) Methylene tetrahydrofolate reductase gene polymorphisms and their association with methotrexate toxicity: a meta-analysis. Pharmacogenet Genomics.22:17-33
- Cáliz R, Del Amo J, Balsa A, et al. (2012) The C677T polymorphism in the MTHFR gene is associated with the toxicity of methotrexate in a Spanish rheumatoid arthritis population. Scand J Rheumatol.41:10-14
- Tasbas O, Borman P, Gürhan Karabulut H, et al. (2011) The Frequency of A1298C and C677T Polymorphisms of the Methylentetrahydrofolate Gene in Turkish Patients with Rheumatoid Arthritis: Relationship with Methotrexate Toxicity. Open Rheumatol J.5:30-35
- Vagace JM, Caceres-Marzal C, Jimenez M, et al. (2011) Methotrexate-induced subacute neurotoxicity in a child with acute lymphoblastic leukemia carrying genetic polymorphisms related to folate homeostasis. Am J Hematol.86:98-101
- Chowdary D, Streck D, Schwalb MN, Dermody JJ (2003) High incidence of two methylenetetrahydrofolate reductase mutations (C677T and A1298C) in Hispanics. Genet Test 7: 255-257.
- Abratte CM, Wang W, Li R, Moriarty DJ, Caudill MA (2008) Folate intake and the MTHFR C677T genotype influence choline status in young Mexican American women. J Nutr Biochem 19: 158-165.
- Schneider JA, Rees DC, Liu YT, Clegg JB (1998) Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet 62: 1258-1260.
- Lazarus HM, Herzig RH, Herzig GP, Phillips GL, Roessmann U, et al. (1981) Central nervous system toxicity of high-dose systemic cytosine arabinoside. Cancer 48: 2577-2582.
- Winkelman MD, Hines JD (1983) Cerebellar degeneration caused by high-dose cytosine arabinoside: a clinicopathological study. Ann Neurol 14: 520-527.
- Herzig RH, Herzig GP, Wolff SN, et al. (1987) Central nervous system effects of high-dose cytosine arabinoside. Semin Oncol.14:21-24
- Véra P, Rohrlich P, Stiévenart JL, et al. (1999) Contribution of single-photon emission computed tomography in the diagnosis and follow-up of CNS toxicity of a cytarabine-containing regimen in pediatric leukemia. J Clin Oncol.17:2804-2810
- Bleyer WA (1988) Central nervous system leukemia. Pediatr Clin North Am 35: 789-814.
- Ingram LC, Fairclough DL, Furman WL, Sandlund JT, Kun LE, et al. (1991) Cranial nerve palsy in childhood acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Cancer 67: 2262-2268.
- Paryani SB, Donaldson SS, Amylon MD, Link MP (1983) Cranial nerve involvement in children with leukemia and lymphoma. J Clin Oncol 1: 542-545.
- Pochedly C (1977) Neurotoxicity due to CNS therapy for leukemia. Med Pediatr Oncol 3: 101-115.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 10928
- [From(publication date):
December-2015 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 10033
- PDF downloads : 895