Research Article Open Access
Feeding Ecology and Prey Preference of Grey Mullet, Mugil cephalus (Linnaeus, 1758) in Extensive Brackish Water Farming System
Asish Mondal1,2*, Deepta Chakravortty2, Susmita Mandal1, Bhattacharyya SB1,2 and Abhijit Mitra2
1Kakdwip Research Centre of Central Institute of Brackish water Aquaculture Kakdwip, South 24 Parganas, West Bengal-743 347, India
2Department of Oceanography, Techno India University, Salt Lake campus, Kolkata, India
- *Corresponding Author:
- Asish Mondal
Kakdwip Research Centre of Central Institute of Brackish water Aquaculture Kakdwip
South 24 Parganas, West Bengal-743 347, India
Tel: +9007806599
E-mail: asish177@gmail.com
Received date: October 20, 2015; Accepted date: December 17, 2015; Published date: December 22, 2015
Citation: Mondal A, Chakravortty D, Mandal S, Bhattacharyya SB, Mitra A (2015) Feeding Ecology and Prey Preference of Grey Mullet, Mugil cephalus (Linnaeus, 1758) in Extensive Brackish Water Farming System. J Marine Sci Res Dev 6:178. doi:10.4172/2155-9910.1000178
Copyright: © 2015 Mondal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Feeding ecology and prey preference of Mugil cephalus reared in extensive brackish water farming system was studied based on monthly examination of stomach contents over a period of 10 months (February-November, 2014). Feeding intensity, planktonic constituents in water and food constituents in the stomach were estimated numerically. The percentage compositions of food items in the stomach falling under different groups were then compared with that of fish pond to evaluate prey preferences. Lower feeding intensity was observed during the initial months which gradually increased as the fish’s grew. The dominant phytoplankton groups in pond water according to the order of dominance were Chlorophyceae, Bacillariophyceae and Myxophyceae. Most abundant zooplankton group was Dinoflagellates followed by Copepods. The main phytoplankton groups found in the stomach according to the order of dominance were Bacillarophyceae, Chlorophyceae and Myxophyceae. Dominant zooplankton group in the stomachs were Dinoflagellets followed by copepods. Prey preference analysis revealed that M. Cephalus actively selected Bacillariophyceae as most preferred food material. Myxophyceae was also selected as second preference. Chlorophyceae was not at all selected and was probably swallowed mechanically during intake of other food stuffs. True positive selection of copepods during initial months of rearing indicates preference by M. cephalus juveniles.
Keywords
Mugil cephalus; Extensive farming; Feeding strategy; Prey preference; Sundarbans
Introduction
Feeding is the dominant activity in entire life cycle of fish; thus detailed knowledge on food and feeding habit of any fish is essential to get success in culture of that particular fish species. Food and feeding habits of a species of fish is intimately associated with the ecological niche that they occupy in the natural environment [1] and knowledge on this aspect is advantageous in their proper management and exploitation [2]. Quantitative and qualitative changes in fish food during the life span are useful tools to define the diet of a particular fish species [3,4]. Fish feed opportunistically yet are often selective in their diets [5-7]. The main factors that determine the type of prey are feeding preference [8-10], availability of prey, prey mobility and its distribution in the water column, catching efficiency of the predator, water temperature and turbidity [11]. Changes in feeding habits of a fish species are a function of the interactions among several environmental factors that influence the selection of food item [12]. Feeding behavior at the level of prey selection can have implications at the individual [13], population [14], and community levels [15].
Flathead grey mullet (Mugil cephalus L.) is an economically important euryhaline and eurythermal marine teleost contributing to sizable fisheries of estuarine and coastal regions in many countries [16] and has a cosmopolitan distribution between latitude 40°N and 40°S covering all the oceans [17-19]. The overall share of Aquaculture was 2.6% in the total production of Marine fishes and it was contributed substantially by flathead grey mullet (M. cephalus) as one of the species [20]. It is an excellent candidate species for both mono and polyculture as it feeds at the lower trophic level grazing on plant detritus and microflora [21,22]. This species is widely cultured in both brackish and freshwater semi-intensive fishponds [23] and can utilize both supplemental feed and/or natural food [24]. Studies on food and feeding habit of stripped grey mullet worldwide indicates that the food spectrum is mainly constituted with Diatoms, green and blue green algae, Dinoflagellates, Protozoa, macro plant detritus, Copepods, and Foraminifera according to the order of dominance [25-31].
In India, M. cephalus occurs in the shallow coastal waters, lagoons, the coastal lakes and estuaries [32] and is mainly reared by polyculture method using seed collected from the wild and is restricted to traditional system [33] in extensive poly-farming impoundments. Studies on the food and feeding habits of M. cephalus in India were done earlier in Lake Pulicat [34], Thana creek [35], Gosthani estuary [36] and the East coast of Andhra Pradesh [37]. No such study has been reported from Hooghly-Matla estuarine complex, popularly known as ‘Sundarbans’ in spite of being a highly potential and ecologically important area where M. cephalus is an important component of extensive polyfarming. This study aimed to assess food and feeding ecology along with prey selectivity of grey mullet in extensive poly-culture system in Sundarbans as representative of natural environment.
Materials and Methods
The study was carried out at Gopalnagar Dakshin village (21.8029- 21.8073°N, 88.2962-88.2985°E) of Patharpratima block in South 24 Parganas district of West Bengal, India from February to November, 2014. Three tide fed extensive ponds (0.7-0.9 ha) locally known as ‘Bhery’ situated at the bank of a creek of Hatania-Doania river were selected. Ponds were dewatered and sun dried during December. During first week of January, unfiltered saline tidal water (18.2 ppt) was allowed to let in and the ponds were filled up to a depth of 110 cm and bamboo screen were fitted at the inlet. The traditional bamboo screen at the inlet allows entry of fry of different species during water exchanges but restricts exit of bigger individuals. No feed or fertilizer was applied following traditional practice. Entry of grey mullet fry along with other species was anticipated as seeds of M. cephalus remain available in north-east coast of India during January–March [16]. About 20-30% water was exchanged every lunar cycle following the common practice throughout the rearing period. Water and fish samples were collected from three ponds to eliminate any possible biasness. Both fish and water samples were frizzed in ice before those were carried to laboratory for subsequent analysis.
Water temperature, salinity, pH, dissolved oxygen (DO), nitritenitrogen (NO2-N), nitrate-nitrogen (NO3-N), ammonia-nitrogen (NH3-N)and phosphate-phosphorus (PO4-P) were analyzed following standard methods [38] from pond water samples collected between 09:00 and 10:00 hours at monthly interval. Salinity was recorded using a refractometer (ATAGO, Japan). Plankton samples were collected monthly by filtering 50 L of water through bolting silk plankton net (mesh size 64 μm). Plankton concentrates were immediately preserved in 5% buffered formalin for further qualitative and quantitative analysis.
From each three ponds, 10 fish were collected during middle of each month i.e., 30 fish in a month and total 300 fish were collected and analyzed throughout 10 months study period. Gravimetric data, namely, total length (TL, mm) was recorded with a slide caliper, while body weight (W, g) was measured using a digital electronic balance. The stomachs were removed intact by cutting above the cardiac and below the pyloric sphincters and preserved in a vial with 4% formalin. The stomach fullness degree was estimated and categorized as empty, ¼ full, ½ full, ¾ full, full and gorged [39]. Dissection entailed making an incision above the longitudinal axes of the stomach and intestine and stomach contents were transferred to a fixed volume of 4% formalin. Three 1ml sub samples of water and each stomach were then transferred to Sedgwick-rafter counting cell and planktonic constituents were counted and identified [40,41]. Food items of water and stomachs were grouped as Chlorophyceae (green algae), Bacillariophyceae (diatoms), Myxophyceae (blue-green algae), Dinoflagellates, Copepods and fish or shrimp parts and numeric percentages of each group were calculated. Additionally, organic matter and sand particles in stomach were also evaluated as major constituents. The percentage compositions of food items in the stomach falling under different groups were then compared with that of fish pond to evaluate prey preferences. Prey preferences were determined by the Ivlev Electivity Index [42] using the following formula:
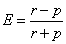
Where, r=percentage of dietary item in ingested food, p= percentage of prey in the environment.
Results
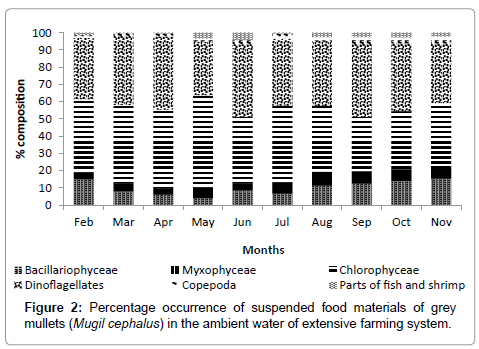
Water quality parameters of the three studied ponds are presented in Table 1. Salinity showed wide variations in all three ponds throughout the culture duration and was highest during May and lowest during September. Temperature ranged between 18.7 and 33.1°C. Highest temperature was recorded during April and lowest during November. Dissolved oxygen (DO) and pH remained almost steady throughout the rearing period and ranged between 5.80 to 9.10 ppm and 7.92 to 8.72, respectively. Those were similar in pond 1 and 2, however, significantly (p<0.05) lower pH and DO were observed in pond 3. Concentrations of toxic nitrogenous metabolites like nitrite-nitrogen (NO2-N) and total ammonia nitrogen (NH3-N) varied between 9.33-24.47 and 21.83-44.08 μg/l, respectively considering all the ponds. Significantly (p<0.05) higher NH3-N concentration was observed in pond 3 while it was similar in pond 1 and 2. Concentrations of nutrients like nitratenitrogen (NO3-N) and phosphate-phosphorous (PO4-P) ranged between 69.62-111.04 and 21.58-43.27 μg/l, respectively showing no significant difference among ponds. Both phytoplankton and zooplankton concentration showed marked difference (p<0.05) among ponds with highest value in pond 1 and lowest in pond 3 (Figure 1). The dominant phytoplankton groups in pond water according to the order of dominance were Chlorophyceae, Bacillariophyceae and Myxophyceae in all ponds. Most abundant zooplankton group was Dinoflagellates followed by Copepods, fish and shrimp parts were also present in the water column (Figure 2). Among Chlorophyceae, Pediastrum, Chlorellaand Tetraedron were found to be the most abundant genera. In addition, Ankistrodesmus, Coilastrum, Crucigenia, Scenedesmus and Pandorina were recorded. Percentage composition of Chlorophyceae varied between 31.49-53.52% with highest and lowest value during May and September, respectively. The most abundant genera of Bacillariophyceae were Navicula, Nitzschia, Cyclotella and Melosira. Other genera of Bacillariophyceae were Gyrosigma, Cymbella, and Synedra. Bacillarophyceae constituted 4.06-15.36% of planktonic forms. Anabaena and Oscillatoriawere the dominant genera among Myxiphyceae. Other genera such as Chroococcus, Gleocapsa, and Merismopedia were also present in the pond water. Composition of Myxophyceae was 3.88-8.09% throughout the study period. Common zooplankton groups were Dinoflagellates and Copepods. Rotifers and Cladocera represented other less abundant zooplankton groups. Maximum abundance of Dinoflagellates were observed during April (42.56%) and minimum during November (34.62%) whereas Copepoda constituted 0.22-4.35% of planktonic constituents. Common Dinoflagellate genera were Ceratium and Peridinium whereas; Calanus was the most common Copepod genera.
| Water parameters | P1 | P2 | P3 |
|---|---|---|---|
| Water temperature (°C) | 29.9 ± 1.7 | 29.9 ± 1.7 | 29.7 ± 1.9 |
| pH | 8.04 ± 0.23a | 7.96 ± 0.25a | 7.78 ± 0.31b |
| DO (mg L-1) | 6.06 ± 0.42a | 5.99 ± 0.52a | 5.69 ± 0.52b |
| Salinity (ppt) | 12.87 ± 5.34 | 12.74 ± 5.32 | 12.89 ± 5.19 |
| NO2-N (µg L-l) | 16.35 ± 5.83 | 15.91 ± 5.62 | 16.11 ± 6.63 |
| NO3-N (µg L-l) | 93.12 ± 15.41 | 92.66 ± 11.14 | 92.97 ± 8.94 |
| NH4-N (µg L-l) | 30.96 ± 5.61b | 31.19 ± 7.91b | 34.89 ± 6.27a |
| PO4-P (µg L-l) | 32.07 ± 13.43 | 31.91 ± 11.98 | 31.89 ± 12.74 |
| Phytoplankton (numbers/L-1×103) | 15.38 ± 1.62a | 15.12 ± 1.94b | 14.95 ± 1.73c |
| Zooplankton (numbers/L-1×103) | 3.05 ± 0.25a | 2.91 ± 0.23b | 2.83 ± 0.17c |
Table 1: Water quality parameters of three extensive brackish water farming impoundments used for grey mullet feeding ecology study.
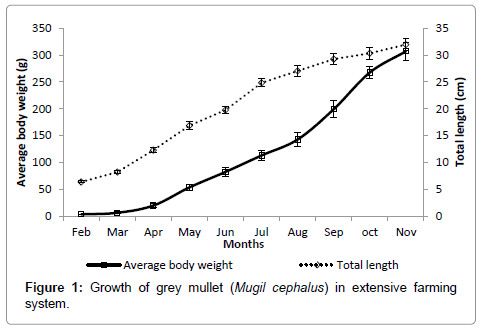
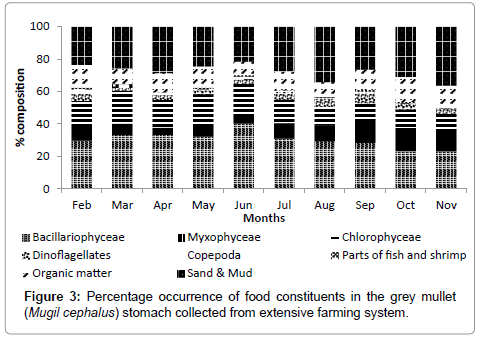
Growth of grey mullet in terms of weight and length over time is presented in Figure 1. Fishes were grown from 3.36 ± 0.15 g (6.37 ± 0.18 cm) as measured during 30 DOC to 307.20 ± 16.55 g (31.97 ± 1.14 cm) in 300 days. Feeding intensity of grey mullets in terms of stomach fullness is presented in Table 2. Lower feeding intensity with highest number of empty stomachs was observed during the initial months of rearing. Feeding intensity gradually increased as the fishes grew, indicated by more number of gorged and full stomachs in the later phase of rearing. Constituents of stomach contents throughout the study period are presented in Figure 3. The main phytoplankton groups found in the stomach according to the order of dominance were Bacillarophyceae (23.04-40.10%), Chlorophyceae (9.59-20.39%) and Myxophyceae (4.45-14.36%). Dominant zooplankton group in the stomachs were Dinoflagellets (1.60-6.58%) followed by copepods (0.20- 2.97%). Among non-planktonic stomach constituents, parts of fish and shrimp constituted 0.12-0.98%, organic matter composed mainly of scraps of macrophytes and organic particles constituted 8.37-15.36%; sand and mud constituted 23.52-36.38% of stomach content. Organic matter and sand particles were not considered for prey preference analysis.
| Months | Gorged | Full | 3/4 Full | 1/2 Full | 1/4 Full | Little | Empty |
|---|---|---|---|---|---|---|---|
| Feb | 0 | 6.66 | 9.99 | 16.65 | 20 | 33.3 | 13.32 |
| Mar | 0 | 3.33 | 9.99 | 9.99 | 26.6 | 40 | 9.99 |
| Apr | 3.33 | 6.66 | 6.66 | 16.65 | 23.3 | 30 | 13.32 |
| May | 3.33 | 9.99 | 9.99 | 19.98 | 23.3 | 26.6 | 6.66 |
| Jun | 6.66 | 9.99 | 13.32 | 16.65 | 26.6 | 20 | 6.66 |
| Jul | 9.99 | 13.3 | 13.32 | 19.98 | 26.6 | 13.3 | 3.33 |
| Aug | 6.66 | 13.3 | 19.98 | 26.64 | 23.3 | 9.99 | 0 |
| Sep | 13.32 | 20 | 23.31 | 13.32 | 16.7 | 13.3 | 0 |
| Oct | 16.65 | 9.99 | 23.31 | 19.98 | 20 | 6.66 | 3.33 |
| Nov | 13.32 | 13.3 | 16.65 | 23.31 | 26.6 | 6.66 | 0 |
Table 2: Feeding intensity of grey mullet in extensive brackish water farming impoundments used for grey mullet feeding ecology study.
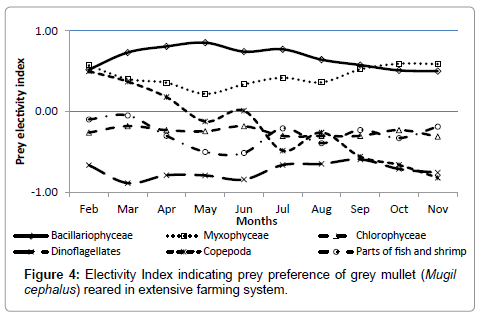
Electivity Index (E) for Bacillariophyceae varied between 0.50- 0.85 with highest value during the month of May whereas E for Myxophycese ranged between 0.22-0.59 showing highest value during October and November (Figure 4). Chlorophyceae showed negative value throughout the study period and ranged between -0.18 to -0.31. Among zooplanktonic stomach constituents, Copepods showed higher values during early months of rearing which gradually decreased as the fishes were grown. Dinoflagellets and fish or shrimp parts showed negative values almost throughout the rearing period.
Discussion
Recorded water quality parameters were within optimum ranges for brackish water aquaculture [43] and differed significantly (P<0.05) with time. Concentrations of toxic gases like nitrite-nitrogen (NO2-N) and ammonia-nitrogen (NH4-N) remained lower than the critical level and concentrations of nutrients like nitrate-nitrogen (NO3-N) and phosphate-phosphorous (PO4-P) was much lower than fertilized ponds reported from Sundarbans [16,44]. Lower nutrient concentrations in the studied extensive system may be attributed to complete dependence on natural productivity without any additional input. Order of dominance of the planktonic groups in the ambient water in the present study was 0 corroborated with that reported from the Hooghly estuary [45].
M. cephalus is a diurnal and opportunistic feeder and feeds almost continuously throughout the day [22]. The higher feeding frequency in bigger fishes than smaller ones may be attributed to the fear of potential predators by the smaller fishes while feeding as they are more vulnerable and would rather feed more cautiously than their bigger counterpart [46]. Larger fish may require more food to obtain the necessary energy for reproductive activity than smaller ones require for growth. Moreover, a wider mouth opening in larger fish helps to ingest relatively larger quantity food items at a time [47].
Bacillariophyceae followed by chlorophyceae and myxophyceae as most dominant food constituents of M. cephalus in brackish water environments has been reported from various parts of the world like East coast of Andhra Pradesh, India [37], coastal waters of Bangladesh [48], Northern Pacific coast of Ecuador [49], along Bulgarian Black Sea coast [31], Senegal River estuary [50] and earthen fishponds in Egypt [51]. Similar order of dominance of prey groups in different environments with varied planktonic constituents indicates that M. cephalus does not feed on whatever available, but have some preference. Chlorophyceae, in spite of being dominant group in water column ranked second in order of dominance in the stomach content in the present study further supports this view. Considering the complex nature of grey mullet feeding ecology, electivity index (E) analysis is necessary to throw some light on food preferences. As per Ivlev’s equation, E ranges from -1 to +1, where -1 to 0 stands for negative selection, while values 0 to +1 can be interpreted as positive selection of that prey item. Subsequent investigation [52] suggested that a true positive or negative prey selection can be interpreted only at values >0.3 or <-0.3 respectively. Grey mullets in the present study actively selected bacillariophyceae as most preferred food material. In spite of ranking third in the order of dominance in the stomach content, myxophyceae was selected as second preference except during the month of May characterized with high temperature. Although being second dominant planktonic constituent in the stomach content, chlorophyceae was not at all selected and was probably swallowed mechanically during intake of other food stuffs. True positive selection of Copepods during initial months of rearing indicates preference by M. cephalus juveniles. Studies on the prey preference of M. cephalus is rare, however, it has been suggested that the organization of the alimentary system of a particular species, as for example in the relative concentrations of its digestive enzymes, may be such as to obtain maximum advantage for only a limited part of the range of material which the animal is actually capable of ingesting [53]. Feeding ecology study along Bulgarian Black Sea coast revealed that M. cephalus is able to utilize either the direct grazing or plant detritus food chains as an energy source depending upon which is the easiest to exploit. When both food sources are present in abundance, the mullet exhibits a preference for living microalgae. Such an algal diet has a higher caloric content than a macro plant detritus diet [31]. These might be the guiding force behind prey selection. Based on the present observations, it may be inferred that grey mullet (M. cephalus) does not feed at random but prefers diatoms (bacillariophycae) followed by blue green algae (myxophyceae) and higher production can be expected in extensive brackish water farming systems which are rich in those phytoplankton groups.
Acknowledgement
The authors are indebted to the authorities of Techno India University, Salt Lake, Kolkata and the Officer-in Charge, KRC of CIBA, Kakdwip for providing infrastructural support. Authors are thankful to the faculty members and scientists of both the organizations for their valuable guidance. Help from the supporting staffs and continuous encouragement by colleagues are thankfully remembered.
References
- Oren OH (1981) Aquaculture of Grey mullets (International Biological program No. 26). Cambridge University press, Cambridge, England. 507.
- Khan AA, Fatima M (1994) Feeding ecology of the Grey Mullet, Rhinomugilcorsula (Hamilton) from the River Yamuna, North India. Asian Fish Sc 7: 256-266.
- Shamsi M1K, Niamat R, Jafri AK (1995) Planktonic biota and food selection of freshwater fish puntiussophore in a perennial and sewage-fed tropical fish pond from Northern India. Egypt J ApplSc 10: 217-224.
- A1-Ake1 AS, A1-Kahem HF,Shamsi M1K, Ahmed Z (1996) Selection of food in various size groups of Nile Tilapia, Oreochromisniloticus (L.) in WadiHaneefah stream, Saudi Arabia. Pak J Zool 27: 271-275.
- Ehlinger TJ, Wilson DS (1988) Complex foraging polymorphism in bluegill sunfish. Proc Nat Ac Sc 85: 1878-1882.
- Gerking SD (1994) Feeding Ecology of Fish, San Diego: Academic Press Inc 416.
- Fry B, Mumford PL, Tam F, Fox DD, Warren GL, et al. (1999) Trophic position and individual feeding histories of fish from Lake Okeechobee Florida. Canadian J Fish Aquatic Sc 56: 590-600.
- Shaw GW, Pankhurst PM, Purser GJ (2003) Prey selection by greenback flounder Rhombosolea (Günther) larvae. Aquaculture 228: 249-265.
- Hagiwara A, Suga K, kazawa A, Kotani T, Sakakura Y (2007) Development of rotifer strains with useful traits for rearing fish larvae. Aquaculture 268: 44-52.
- Nunn AD, Harvey JP, Cowx IG (2007) The food and feeding relationships of larval and 0+ year juvenile fishes in lowland rivers and connected water bodies II Prey selection and the influence of gape. J Fish Biol 70: 743-757.
- Moore JW, Moore IA (1976) The basis of food selection in flounders, Platichthysflesus in the Sever Estuary. J Fish Biol 9: 139-156.
- Ribeiro DFO, Nuner APO (2008) Feed preferences of Salminusbrasiliensis (Pisces, Characidae) larvae in fish ponds. Aquaculture 274: 65 -71.
- Fraser D, Huntingford FA, Adams CE (2007) Foraging specialisms, prey size and life-history patterns a test of predictions using sympatric polymorphic Arctic charr (Salvelinusalpinus). Ecol Freshwater Fish published online June 7.
- Herwig BR, Zimmer KD (2007) Population ecology and prey consumption by fathead minnows in prairie wetlands importance of detritus and larval fish. Ecol Freshwater Fish 16: 282-294.
- Schleuter D, Eckmann R (2007) Generalist versus specialist the performances of perch and ruffe in a lake of low productivity. Ecol Freshwater Fish published online August 16.
- Biswas G, De D, Thirunavukkarasu AR, Natarajan M, Sundaray JK, Kailasam M, et al. (2012) Effects of stocking density, feeding, fertilization and combined fertilization-feeding on the performances of striped grey mullet (Mugilcephalus L.) fingerlings in brackishwater pond rearing systems. Aquaculture 338-341: 284–292
- De Silva SS (1980) The biology of juvenile grey mullet a short review. Aquaculture 19: 21-36.
- Thomson JM (1966) The grey mullet. Barnes H (Ed.) Ocean and Mar Biol-an Annual Review. Allen and Unwin, London. 4: 301-355.
- Well RDS (1984) The food of the grey mullet (Mugilcephalus L.) in Lake Waahi and the Waikato River at Huntly. New Zealand J Mar and Freshwater Res18: 13-19.
- FAO (2010) The State of the World Fisheries and Aquaculture Food and Agricultural Organization of the United Nations Rome Italy.
- Moriarty DJW (1976) Quantitative studies on bacteria and algae in the food of the mullet (Mugilcephalus L) and the prawn Metapenaeusbennettae (Recek&Dall). J Exp Mar Biol 22: 131-143.
- Odum W (1970) Utilization of the direct grazing and plant detritus food chains by the striped mullet (MugilcephalusL.): Steele, JJ (Eds) Marine Food Chains. Oliver and Boyd, Edinburgh 222-240.
- Lupatsch I, Katz T, Angel DL (2003) Assessment of the removal efficiency of fish farm effluents by grey mullets a nutritional approach. Aquacult Res 34: 1367-1377.
- Abdel-Tawwab M, Abdel-Hamid ME, Ali EA, El-Marakby HI (2005) The Assessment of Water Quality and Primary Productivity in Earthen Fishponds Stocked with Stripped Mullet (Mugilcephalus) and Subjected to Different Feeding Regimes. Turk J Fish and Aquatic Sc 5: 1-10.
- Thompson J (1954) The organs of feeding and the food of some Australian mullet. Aust J mar and Freshwater Res 5:469-485.
- Wood E (1964) Study on the microbial ecology of Australian region Nova Hedwigia 8:461-568.
- Odum W (1968) Mullet grazing on a dinoflagellate bloom. Chesapeake Center for Sc 9: 202-204.
- Brusle J (1981) Food and feeding in grey mullet: OH Oren (Ed.) Aquaculture of Grey Mullet. Cambridge University Press Cambridge UK: 185-217.
- Porter CB, Krost P, Gordin, H, Angel DL (1996) Preliminary assessment of grey mullet (Mugilcephalus) as a forager of organically enriched sediments below marine fish farms. Israel J. Aquaculture 48: 47-55.
- Jana SN, Garg SK, Patra BC (2004) Effect of periphyton on growth performance of grey mullet Mugilcephalus (Linn.) in inland saline ground water ponds. J. Appl. Ichthyol 20: 110-117.
- Bekova R, Raikova-Petrova G, Gerdzhikov D, Petrova E, Vachkova V, et al. (2013) Food spectrum of grey mullet (Mugilcephalus L.) along the Bulgarian Black Sea coast. AgrilSc and Tech 5: 173-178.
- Rheman S, Islam ML, Shah MMR, Mondal S, Alan MJ (2002) Observation on the fecundity and Gonadosomatic Index (GSI) of Grey mullet Liza parsia (Ham.). Online J. Biol. Sci. 2: 690-693.
- Abraham M, Kailasam M, Kishore Chandra P, Sihranee P, Rajendran KV et al. (2000) Development of captive broodstock of the grey mullet, Mugilcephalus (L). Ind J Fish 47: 91-96.
- Rangaswamy CP (1973) Studies on the age and growth and food habits of the grey mullet Mugilcephalus Linnaeus of the Lake Pulicat. J Inland Fish Soc India 5: 9-22.
- Tandel SS, Athalye RP, Gokhale KS (1986) On the seasonal changes in food habit of Mugilcephalus of the Thana Creek. Ind J Fish 33: 270-276.
- Rao LM, Sivani G (1996) The food preferences of five commercially important fishes of Gosthani estuary. Ind J Fish 43: 199-202.
- Rao RK, Babu KR (2013) Studies on food and feeding habits of Mugilcephalus (linnaeus, 1758) east coast off Andhra Pradesh, India. Can J Basic and ApplSc 7: 2499-2504.
- APHA (1998) Standard Methods for the Examination of Water and Wastewater, 20th ed, American Public Health Association Washington DC USA.
- Abdelghany AE (1993) Food and feeding habits of Nile tilapia from the Nile River at Cairo, Egypt: L Reintertsen, A Dhale, C Jorgensen, R Twinnereim (Eds) Fish Farm Technology AA Balkema, Rotterdam, The Neatherland447-453.
- Jhingran VG, Natarajan AV, Banerjee SM, David A (1969) Methodology on reservoir fisheries investigation in India. Central Inland Fisheries Research Institute, Barrackpore, India Bulletin No. 12. 109.
- Prescott GW (1961) Algae of the Western Great Lakes Area. WMC Brown Company Publishers Dubuque IA USA 990.
- Ivlev VS (1961) Experimental ecology of the feeding of fisheries. Yale University Press, New Haven CT, USA 322.
- Chakraborti RK, Sundaray JK, Ghoshal TK (2002) Production of Penaeusmonodon in the tide fed ponds of Sundarban. Ind J Fish 49: 419-426.
- Saha SB, Bhattacharyya SB, Mitra A and Choudhury A (2001) Quality of shrimp culture farm effluents and its impact on the receiving environment. Bangladesh J Zool 29: 139-149.
- Dutta S, Maity S, Bhattacharyya SB, Sundaray JK, Hazra S (2013) Diet composition and intensity of feeding of Tenualosailisha (Hamilton, 1822) occurring in the Northern Bay of Bengal, India. ProcZoolSoc Ind. 67: 33-37.
- Akpan AW, Isangedighi IA (2004) Aspects of the Feeding Ecology of three Species of Pseudotolithus (Sciaenidae) in the Inshore waters of Southeastern Nigeria, East of the Niger Delta, Nigeria. J AquatSc 19: 51-58.
- Isangedighi I, Uudo PJ, Ekpo IE (2009) Diet composition of Mugilcephalus (pisces: mugilidae) in the Cross River estuary, Niger delta, Nigeria. Nigerian J of Agril Food and Env 5:10-15.
- Islam R, Hossain MB, Das NG, Rafi RUN (2009) Food and feeding behaviour of grey mullet, Mugilcephalus of Bangladesh coastal water. Bangladesh J Pro Sc& Tech 7: 56-61.
- Rramirez LV, Navia AF, Rubio EA (2008) Food habits and feeding ecology of an estuarine fish assemblage of northern Pacific Coast of Ecuador. Pan-Am J Aquatic Sc 3: 361-372.
- Modou SS, Mouhameth C, Tinkoudgou KJA (2014) Seasonal feeding variation of the yellow mule (Mugilcephalus, Linnaeus 1758, Mugilidae) in Senegal River estuary fishery. Int J Agril Pol and Res 2: 125-131.
- El-Marakby HI, Eid AM, Abdelghany AE, Abdel-Tawwab M (2006) The impact of stripped mullet Mugilcephalus on natural food and phytoplankton selectivity at different feeding regimes in earthen ponds. J Fish Aquatic Sci 1:87-96.
- Lazzaro X (1987) A review of planktivorous fishes: Their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146: 97-167.
- Hartley P (1947) The natural history of some British freshwater fish. Proceedings of the Zoological Society of London 117: 129-206.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 13805
- [From(publication date):
specialissue-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12769
- PDF downloads : 1036