Research Article Open Access
Feasibility of a Randomized Controlled Trial of Light Therapy in Cancer Patients with Insomnia
Dev R1*, Delgado-Guay MO1, De La Cruz M1, Rhondali W2, Hui D1 and Bruera E11Department of Palliative Care and Rehabilitation Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
2Centre Hospitalier de Lyon Sud, Hospices Civils de Lyon, Lyon, France
- *Corresponding Author:
- Rony Dev DO
Department of Palliative Care and Rehabilitation Medicine
Unit 1414, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
Tel: 713-792-6072
E-mail: rdev@mdanderson.org
Received date: June 19, 2014; Accepted date: August 26, 2014; Published date: September 06, 2014
Citation: Dev R, Delgado-Guay MO, De La Cruz M, Rhondali W, Hui D, et al. (2014) Feasibility of a Randomized Controlled Trial of Light Therapy in Cancer Patients with Insomnia. J Palliat Care Med 4:183. doi: 10.4172/2165-7386.1000183
Copyright: © 2014 Dev R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Purpose: The primary objective of our study was to compare bright light therapy versus dim red light for global sleep quality in palliative care patients with cancer. Methods: The study was designed as a randomized, double blind, placebo controlled trial. Patients initiated blinded phase, either daily bright light versus red light placebo, from day 1 to day 14, then proceeded to an open label phase between day 15 to day 28. Results: Of the 319 outpatients assessed for eligibility, 97 patients (30%) fulfilled criteria for the study. Of the 97 patients, only 12 patients (12%) enrolled in the study with the majority unwilling to participate or reported a lack of interest in light therapy. Only 4 patients (33%) completed the trial to the primary endpoint at 2 weeks. Conclusion: At our institution, a randomized controlled trial examining bright light therapy, a potentially safe and effective non-pharmacological approach to treat sleep disturbances, was not feasible for palliative care patients with cancer. Future studies should be tailored to advanced cancer patients who are often frail and have a high symptom burden, incorporate alternative trial designs such as randomization without a placebo arm, and consider integration of home visits or assessment by phone calls to lessen the burden of participation in a clinical trial.
Keywords
Light therapy; Insomnia; Sleep disturbances; Cancer
Introduction
For cancer patients, sleep provides respite from physical and psychological distress, [1] restores a sense well-being [2,3] and maintains cognitive function [4]. Insomnia has a negative impact on quality of life [5], ability to engage in work or recreational activities, and is associated with neuro-endocrine abnormalities [6,7].
The prevalence of sleep disturbance in cancer patients ranges from 24% to 95% [8-14]. Insomnia affects about 70% of hospice patients, with frequent waking being the most common problem reported [15]. Hypersomnolence, excessive daytime sleepiness, is also common in cancer patients and frequently associated with opioids.
Patients with insomnia frequently develop tolerance to hypnotics and their use may result in fragmented sleep and dependence [16]. Undesirable side effects of hypnotics include day-time sedation, delirium, fatigue, and respiratory depression. A recent study reported that hypnotic use was associated with a greater than threefold increased risk for death even when patients were prescribed less than 18 pills per year [17]. In cancer patients, 23% of the populations were utilizing hypnotics and their use was associated with older age, increased stress and anxiety, greater use of opioids, and history or current chemotherapy treatment [18].
One promising non-pharmacologic treatment for insomnia is bright light therapy (BLT). BLT has been studied in institutionalized elderly patients [19-23] and adolescents with delayed sleep phase disorder. [24] In breast cancer patients receiving chemotherapy, stage I-III, BLT was shown to prevent an increase in total fatigue scores [25].
Advanced cancer patients often have altered sleep patterns resulting in disruptions in their circadian rhythm [26,27] which may be restored with BLT. The primary objective of our study was to compare BLT with dim red light on global sleep quality in advanced cancer patients. We hypothesized that BLT was more effective than dim red light in improving sleep disturbances.
Methods
Participants
We planned to recruit a total of 152 patients for the two arms (i.e. 76 per arm) at a single site, MD Anderson Cancer Center (MDACC) – Supportive Care Clinic. Patients were eligible if they had advanced cancer with an average sleep disturbance rating of ≥ 4 out of 10, as measured by the Edmonton Symptoms Assessment Scale (ESAS), [28] with a rating of 0 having no problems sleeping and 10 being severe difficulties sleeping for at least one week; age 18 or greater; Karnofsky performance status score of ≥40; agreeable for follow-up visits at MDACC; and English speaking. Patients were excluded if they had congenital blindness or acquired blindness; history of retinal disease; current diagnosis of major depression or generalized anxiety disorder; received light therapy in the past; currently on amiodarone, thiazide diuretics or EGFR inhibitors (erlotinib, gefitinib, cetuximab, panitumumab); receiving UVA/UVB therapy; diagnosis of obstructive sleep apnea or narcolepsy; and patients with >2 hours of direct exposure to outdoor natural light per day. Patients who were using pain medications and/or had a history of taking any hypnotics for sleep disturbance on a regular or “as needed” (PRN) basis, but still rate insomnia ≥ 4 on scale ranging from 0 to 10, were eligible.
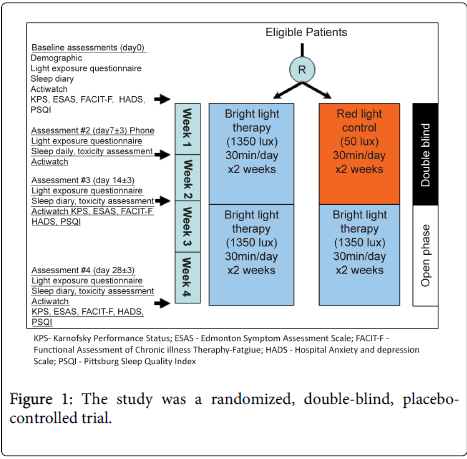
Patients initiated the blinded phase of study intervention (i.e. daily bright light or red light) from day 1 to day 14 ± 3, then proceeded to an open label phase and received daily bright light between day 15 ± 3 and day 28 ± 3.
Study intervention
The active treatment arm received BLT via a Litebook device (The Litebook Company Ltd., Alberta, Canada). The Litebook device, a small and lightweight box, consists of 60 LEDs with spectral emission with a peak at approximately 464 nm and fluorescent phosphors which provide a broader, secondary spectral peak near 564 nm.
The control, previously used in randomized trials of BLT, is a red light device was also produced by Litebook and was identical in appearance and dimensions, with the exception that it emits light at a wavelength of 680nm (i.e. red light) and at an intensity of 50 lux [29-31]. Subjects were instructed on the use of the Litebook devices with regard to proper positioning, duration, and start of therapy - 30 minutes each morning, within 2 hours of awakening on a daily basis.
A randomization list was prepared by our study biostatistician in advance of trial initiation. Once enrolled, study participants were issued an active or control treatment device by a research staff (different from the blinded staff who performed study assessments).
Outcomes
Patient demographics and performance status, as well as Karnofsky Perfomance Scale, were recorded. A light exposure diary, the participants were asked to transcribe their daily indoor and outdoor activities with duration at baseline, day 7, 14, and 28 (Figure 1). In addition, a sleep diary, a subjective tool utilized in a number of studies on sleep disorders and in general practices [32], was also recorded.
The following assessments were conducted at baseline, day 14, and day 28 (Figure 1).
The Edmonton Symptom Assessment Scale (ESAS) measures the patient’s response to 9 common symptoms in the past 24 hours (pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, sleep problems) and the feeling of well-being [28].
The Pittsburgh Sleep Questionnaire Inventory (PSQI), a validated tool for insomnia, is an effective instrument for measuring the quality and patterns of sleep [33].
The Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) subscale has been used primarily in cancer patients to measure fatigue; [34,35]. It consists of 27 quality-of-life questions which cover the following domains: physical, social, emotional, and functional. The Hospital Anxiety and Depression Scale (HADS) is a brief, self-administered, and widely used screening tool to measure psychological distress in patients. It is sensitive to change both during the course of disease and in response to medical and psychological interventions [36,37].
Continuous assessment of activity was measured by actigrapy (Actiwatch 64 IPX7 portable recorder; Mini Mitter Company, Inc. A Respironics, Inc., OR, USA) which is a small wrist-worn device optimized for highly effective sleep-wake inference from wrist activity which has been previously validated [38,39]. Patients were instructed to wear the actigraphs throughout the study period and remove them during bathing.
Results
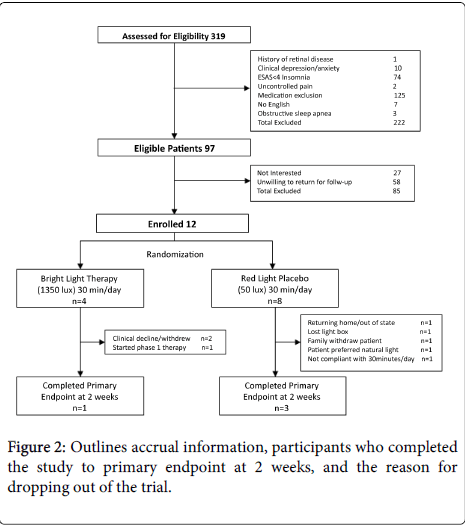
Of the three hundred and nineteen patients assessed for eligibility, only 97 patients (30.4%) fulfilled inclusion and exclusion criteria. The majority of patients excluded from the study were due to treatment with a medication with which light exposure was contraindicated; mainly phase 1 therapy with EGFR inhibitors (erlotinib, gefitinib, cetuximab, panitumumab). Seventy four patients with difficulty sleeping did not rate insomnia greater than 4 on the ESAS. Ten patients were excluded secondary to depression.
Of the 97 patients who were eligible, very few patients, 12 (12.4%), enrolled in the study with the majority of patients, 58 (59.8%), unwilling to participate in the trial due to the need to return for follow-up visits. Another 27 patients (27.8%) were not interested in BLT.
Of the 12 patients enrolled, only 4 (33.3%) were able to complete the primary endpoint at 2 weeks. Various reasons for patients dropping out of the trial were provided and included patient’s clinical health declining, initiation of phase1 treatment with a contraindication to light exposure, preference for natural light exposure, and patient or family members lack of interest in enrollment (Figure 2).
Discussion
Sleep disturbances are common in patients with cancer. Despite the ease of use and limited toxicity [40], a randomized controlled trial evaluating BLT in advanced cancer patients at our institution was not feasible secondary to poor accrual and a high attrition rate. Even if the study were to continue, the high percentage of non-eligible patients and low completion rate would limit generalizability.
Using a similar research design, Ancoli-Israel and colleagues completed a study of BLT which included 39 patients with stage I-III newly diagnosed breast cancer undergoing chemotherapy; authors reported that light therapy prevented deterioration in total fatigue scores [25]. However, studies of patients with early stage disease may not be applicable in patients with advanced disease receiving palliative care secondary to their frail condition with increased symptom burden, intensity of other treatment modalities (i.e. chemotherapy, radiation treatment), and increased susceptibility to side effects. In our study, patients, despite being functional, were in the late stages of their disease trajectory. In the palliative care setting, it is not uncommon for eligible patients decline to enroll in studies, which has been reported to exceed 50 percent [41-43].
Barriers to enroll in BLT study included the large number of patients receiving phase 1 therapy with epidermal growth factor receptor (EGFR) inhibitors. Treatment with EGFR inhibitors may lead to a skin reaction which can be exacerbated by sun exposure [44] and was an exclusion criteria. In addition, the study was randomized, Figure 1, with a 2 week placebo controlled phase prior to open label treatment which may have discouraged patient participation. As more patients have knowledge and access to BLT, the ability to distinguish BLT from placebo makes blinded randomization problematic. Also, the need for return visits to complete assessments for the trial discouraged potential participants, and in future studies, follow-up assessments by phone call should be considered.
Other factors contributing to the failure of the study include the possible lack of enthusiasm by physicians and also, arguably, a preference for patients for pharmaceutical intervention for their sleep disturbances. A limitation of the current study is the lack of specific detailed information for why patients were not interested in participation which may due to fear of side effects, inability to comply with daily use, or cancer patients may have felt that BLT was inadequate to alleviate their symptoms. Enlight of a recent study which reported an increased mortality associated with the use of hypnotics in cancer and non-cancer patients [17], future studies may have to highlight the risks of hypnotics for the treatment of insomnia in order to persuade patients to use a safer non-pharmacological intervention.
Conclusion
Patients with advanced cancer in a tertiary cancer center lacked interest in receiving BLT for symptoms of insomnia. Of those that choose to enroll in a randomized control trial, therapy was not tolerated resulting in a high attrition rate. Future studies should be tailored to advanced cancer patients who are often frail and have a high symptom burden, incorporate alternative trial designs such as randomization without a placebo arm, and consider integration of home visits or assessment by phone calls to lessen the burden of participation in a clinical trial.
Acknowledgement
Eduardo Bruera is supported in part by the National Institutes of Health grant numbers: RO1NR010162-01A1, RO1CA122292-01, RO1CA124481-01, and in part by the MD Anderson Cancer Center support grant # CA016672.
References
- Kvale EA, Shuster JL (2006) Sleep disturbance in supportive care of cancer: a review. J Palliat Med 9: 437-450.
- Zisapel N (2007) Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci 64: 1174-1186.
- Maquet P (1995) Sleep function(s) and cerebral metabolism. Behav Brain Res 69: 75-83.
- Maquet P (2001) The role of sleep in learning and memory. Science 294: 1048-1052.
- Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA (1999) Quality of life in people with insomnia. Sleep 22 Suppl 2: S379-385.
- Savard J, Laroche L, Simard S, Ivers H, Morin CM (2003) Chronic insomnia and immune functioning. Psychosom Med 65: 211-221.
- Lamberg L (2000) Sleep disorders, often unrecognized, complicate many physical illnesses. JAMA 284: 2173-2175.
- Savard J, Simard S, Blanchet J, Ivers H, Morin CM (2001) Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep 24: 583-590.
- Savard J, Villa J, Ivers H, Simard S, Morin CM (2009) Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J ClinOncol 27: 5233-5239.
- Fiorentino L, Ancoli-Israel S (2007) Sleep dysfunction in patients with cancer. Curr Treat Options Neurol 9: 337-346.
- Fiorentino L, Ancoli-Israel S (2006) Insomnia and its treatment in women with breast cancer. Sleep Med Rev 10: 419-429.
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH (2002) Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 24: 471-480.
- Graci G (2005) Pathogenesis and management of cancer-related insomnia. J Support Oncol 3: 349-359.
- Zammit GK (2007) The prevalence, morbidities, and treatments of insomnia. CNS NeurolDisord Drug Targets 6: 3-16.
- Hugel H, Ellershaw JE, Cook L, Skinner J, Irvine C (2004) The prevalence, key causes and management of insomnia in palliative care patients. J Pain Symptom Manage 27: 316-321.
- Poyares D, Guilleminault C, Ohayon MM, Tufik S (2004) Chronic benzodiazepine usage and withdrawal in insomnia patients. J Psychiatr Res 38: 327-334.
- Kripke DF, Langer RD, Kline LE (2012) Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open 2: e000850.
- Casault L, Savard J, Ivers H, Savard MH, Simard S (2012) Utilization of hypnotic medication in the context of cancer: predictors and frequency of use. Support Care Cancer 20: 1203-1210.
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, et al. (2003) Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's disease patients. Behav Sleep Med 1: 22-36.
- Satlin A, Volicer L, Ross V, Herz L, Campbell S (1992) Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's disease. Am J Psychiatry 149: 1028-1032.
- Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, et al. (1994) Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. ActaPsychiatrScand 89: 1-7.
- Van Someren EJ, Kessler A, Mirmiran M, Swaab DF (1997) Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry 41: 955-963.
- Mishima K, Hishikawa Y, Okawa M (1998) Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer's type. ChronobiolInt 15: 647-654.
- Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, et al. (2011) A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep 34: 1671-1680.
- Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, et al. (2012) Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer 20: 1211-1219.
- Lack LC, Wright HR (2007) Chronobiology of sleep in humans. Cell Mol Life Sci 64: 1205-1215.
- Lack LC, Wright HR (2007) Treating chronobiological components of chronic insomnia. Sleep Med 8: 637-644.
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7: 6-9.
- Martiny K, Lunde M, Undén M, Dam H, Bech P (2006) The lack of sustained effect of bright light, after discontinuation, in non-seasonal major depression. Psychol Med 36: 1247-1252.
- Loving RT, Kripke DF, Elliott JA, Knickerbocker NC, Grandner MA. Bright light treatment of depression for older adults [ISRCTN55452501]. BMC Psychiatry. 2005;5:41.
- Lieverse R, Nielen MM, Veltman DJ, Uitdehaag BM, van Someren EJ, et al. (2008) Bright light in elderly subjects with nonseasonal major depressive disorder: a double blind randomised clinical trial using early morning bright blue light comparing dim red light treatment. Trials 9: 48.
- Currie SR, Wilson KG, Curran D (2002) Clinical significance and predictors of treatment response to cognitive-behavior therapy for insomnia secondary to chronic pain. J Behav Med 25: 135-153.
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28: 193-213.
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, et al. (2005) Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 32: 811-819.
- Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC (2007) Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med 10: 1068-1075.
- Snaith RP (2003) The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes 1: 29.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. ActaPsychiatrScand 67: 361-370.
- Gibbins J, McCoubrie R, Kendrick AH, Senior-Smith G, Davies AN, et al. (2009) Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage 38: 860-870.
- Tonetti L, Pasquini F, Fabbri M, Belluzzi M, Natale V (2008) Comparison of two different actigraphs with polysomnography in healthy young subjects. ChronobiolInt 25: 145-153.
- Terman M, Terman JS (1999) Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry 60: 799-808.
- Rinck GC, van den Bos GA, Kleijnen J, de Haes HJ, Schadé E, et al. (1997) Methodologic issues in effectiveness research on palliative cancer care: a systematic review. J ClinOncol 15: 1697-1707.
- Ross C, Cornbleet M (2003) Attitudes of patients and staff to research in a specialist palliative care unit. Palliat Med 17: 491-497.
- Jordhøy MS, Kaasa S, Fayers P, Ovreness T, Underland G, et al. (1999) Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 13: 299-310.
- Eaby B, Culkin A, Lacouture ME (2008) An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J OncolNurs 12: 283-290.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 15270
- [From(publication date):
August-2014 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10678
- PDF downloads : 4592