Research Article Open Access
Feasibility of a Bioremediation Process Using Biostimulation with Inorganic Nutrient NPK for Hydrocarbon Contaminated Soil in Tunisia
| Abdeldjalil Abid1*, Kaouther Zaafouri1, Abdelwaheb Aydi2, Iman Manai1, Aida Ben Hassen Trabesli3, Chokri Messaoud4 and Moktar Hamdi1 | |
| 1Laboratory of Microbial Ecology and Technology, Department of Biological and Chemical Engineering, National Institute of Applied Science and Technology (INSAT), Tunis, University of Carthage, Tunisia | |
| 2National School of Engineers of Sfax, BP: 1173, 3038 Sfax. University of Sfax, Tunisia | |
| 3Centre de recherche et de technologie de l'énergie, Technopôle de Borj Cédria-, BP N ° 95 2050 - Hammam Lif, Tunisie | |
| 4Laboratory of Plant Biotechnology, National Institute of Applied Science and Technology (INSAT) Tunis, University of Carthage, Tunisia | |
| Corresponding Author : | Abdeldjalil Abid Laboratory of Microbial Ecology and Technology Department of Biological and Chemical Engineering National Institute of Applied Science and Technology (INSAT) Tunis, University of Carthage, Tunisia Tel: 216-71-70-38-29 Fax: 216-71-70-43-29 E-mail: abid213jalil@gmail.com |
| Received April 12, 2014; Accepted April 28, 2014; Published May 02, 2014 | |
| Citation: Abid A, Zaafouri K, Aydi A, Manai I, Trabesli ABH, et al. (2014) Feasibility of a Bioremediation Process Using Biostimulation with Inorganic Nutrient NPK for Hydrocarbon Contaminated Soil in Tunisia. J Bioremed Biodeg 5:224. doi:10.4172/2155-6199.1000224 | |
| Copyright: © 2014 Abid A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
This study focused on the processing time reduction of soils microcosm process, during the biological restore of diesel-contaminated soil under natural conditions. Biodegradation activity of hydrocabonoclastic microflora and biostimulation with inorganic nutrient (NPK) were studied through the determination of optimal conditions which improve bioremediation process. Indeed, after 56 days, about 98% removal rate of total petroleum hydrocarbon (TPH) in soil treated by microcosm’s technique were observed relative to approximately 2.22×107CFU/g soil of bacterial number. This performance was achieved with microbial (bacteria) metabolism which accompanies biodegradation of hydrocarbons. Moreover, the hydrocarbon fractions (alkanes and aromatics) were rapidly degraded then the other complex fractions according to GC/MS and FT-IR analysis
Microbial community structures in hydrocarbon-contaminated soil are influenced by a number of factors, such as soil type, concentration and bioavailability of the contaminants, nutrient contents, temperature, oxygen content and pH [4,5].
Bioremediation, which is defined as a process that uses microorganisms, plants or enzymes in order to transform contaminants in a less toxic species, is a very attractive method due to its costs, and benefits that result from the pollutant mineralization [6].
Various forms of treatment technologies, employed in the bioremediation of diesel-contaminated soil, are reported in the literature [7-14].
Cunningham and Philp investigated several factors bioaugmentation, biostimulation via inorganic fertilizer and bulking agents that influenced the removal rate of diesel- contaminated soil [8]. Frankenberger et al. [7] reduced the petroleum constituents’ concentration at a diesel-contaminated site by injected nutrients (nitrogen and phosphorus) and hydrogen peroxide was added to provide molecular oxygen to the subsurface microflora in degrading the petroleum. More so, Chemlal et al. [10] were restored diesel-contaminated soil by using biological process on biopile system, stimulated with nutrients.
The aim of this study is the evaluation of the processing time reduction of soils microcosms’ process, by enriching soil diesel-contaminated with inorganic nutrient during the bioremediation process. Then, we studied the microbial metabolism followed by biodegradation of hydrocarbons through the determination of optimal conditions during the bioremediation process.
The climate of Ariana is arid, characterized by dry and warm summers (from June to August), and cool, wet winters (from December to February). The annual precipitation in the region is 220 mm. The annual predominant wind direction in Ariana is northward. The annual average wind speed is 2.5 m/s, while the maximum monthly average wind speed occurs in April with a magnitude of 2.9 m/s. The annual average temperature of Ariana is around 16-19°C.
Sampling soils were divided into three tow-liters sterile flasks each containing 1 kg of soil. The height of the flask was 13 cm with 20 cm internal diameter; every flask has many openings at the base to prevent water logging. Every soil sample occupied approximately half the volume of the flask leaving enough head space.
In the first flasks, the soil sample was thoroughly mixed with a hand trowel sanitized with 80% ethanol. The inorganic nutrient was dissolved in 200 mL sterile distilled water and mixed with the contaminated soil to distribute the nutrient through the soil particles and also to enhance good aeration. In the second flask, the soil was taken without nutrient as control system.
Microcosms were kept at room temperature in a laboratory incubator; nutrient-treated soils were regularly watered weekly with 200 mL sterile distilled water to substitute for evaporated water and also mixed every other day for aeration. The total hydrocarbon compounds were controlled every 7 days, as various physical-chemical and biological analyses.
GC–MS analysis was performed with a HP model 5975B inert MSD, equipped with a capillary DB-5MS column (30m length; 0.25mm i.d; 0.25m film thickness (Agilent Technologies, J&W Scientific Products, U.S.A.). The carrier gas was the helium, and chromatogram peaks were identified by comparing their mass spectra with Wiley and NIST library database and standards of the main components and quantified using the retention time and response factors of these compounds, correlating chromatographic areas to molar concentrations.
The pH of the medium was adjusted to 7; all media and solution were prepared with 1 Liter distilled water and autoclaved at 121°C for 20 min. After isolation, and for the working cell banks, the microbial suspension was resuspended in fresh Minimal Medium (MM) containing 15% of glycerol and stored in cryo-vials -80°C.
The guide les of the [30]; suggest that bioremediation is feasible when there is about 106 CFU/kg soil of the microbial population. According to the results of the physic-chemical and microbiological analysis were reported in the Table 1. The number of total germs at 30°C (bacteria) is 1.43×107 (CFU/g soil), which confirmed that this soil could be treated by biological process using microorganisms.
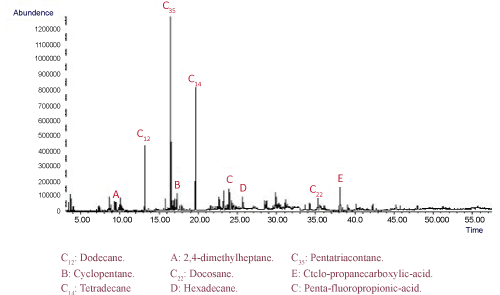
The GC/MS analysis was performed to identify the presence of hydrocarbon petroleum in the soil sample. The results confirmed that the soil samples consisted mainly of n-alkanes C10 through C40 approximately lower than 80%, with intermediate branched chain hydrocarbon, along with cycloalkanes, aromatic compounds and other petroleum-based compounds (Figure 3).
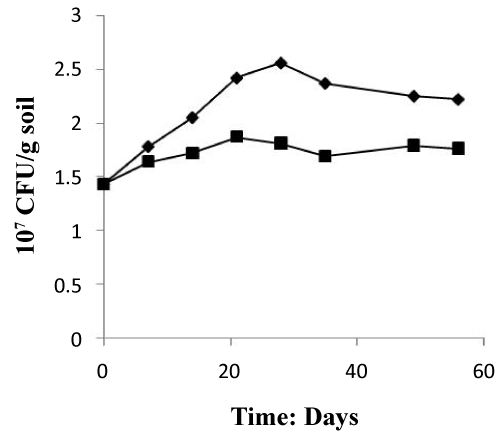
Figure 4 depicted that the nutrient induced a vigorous microbial propagation which increases after 30 days of treatment from 1.43×107 (CFU/g soil) to 2.56×107 (CFU/g soil) and from 1.43×107 (CFU/g soil) to 1.81×107 (CFU/g soil) in the two systems treated and control respectively. The treated system showed decreases progressively during last study, while the control system showed a slowly increase in microbial numbers. This value is considered normal if compared with the results of Wibbe and Blanke [31], who mentioned much higher values.
The Microflora responsible for biodegradation was predominantly bacterial population and this biological microcosms study was done through co-metabolism phenomenon. During the bioremediation process the number of dominant microorganisms increased slightly as the biodegradation ratio.
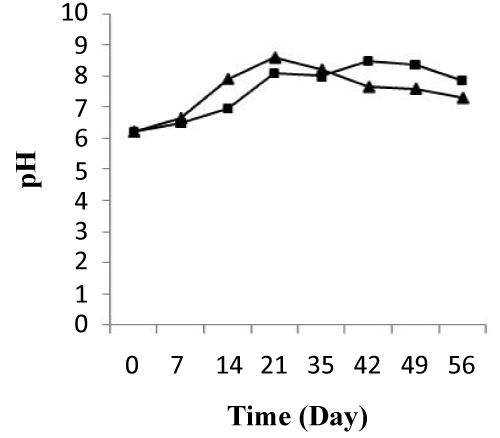
The pH values were similar within the start of treatment and had an average of 6.2 in two microcosms, treated and control. During the first 10 days of treatment, pH showed an increase from 6.2 to 7.91 and from 6.2 to 6.95 in two systems, respectively. At the end of this period (10 days), the pH ranged from 7.91 to 8.21 and depicted rapid increases in the treated system. At the same, in the control system pH increase from 6.95 to 8.11. After 37 days of treatment, the pH value decreased progressively for the two system and the abatement values reached 91.717% and 97.758%, respectively (Figure 5). The average of pH changing observed was confirmed by same result of Greer et al. [32], that microbial community structures in hydrocarbon-contaminated soil are influenced by a pH value during the biodegradation.
In other hand, soil temperature and soil moisture affect the kinetics of hydrocarbon soil reactions during the bioremediation process. Microbial activities in soil involve enzymatic and biochemical processes related to temperature sensitive.
The optimal temperature depends on the volatility and the hydrocarbons solubility pollutants that be treated. In this case, Microcosms were kept at room temperature around 27 ± 4°C in a laboratory incubator in order to facilitate metabolic activity, diffusion, and mass transfer.
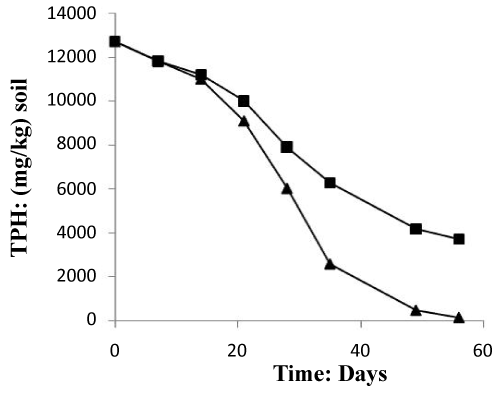
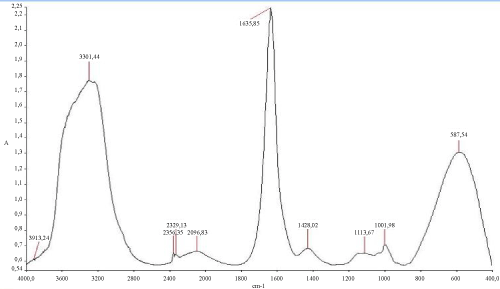
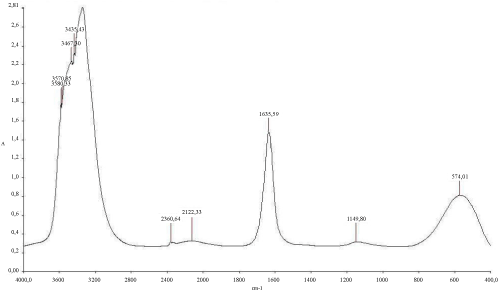
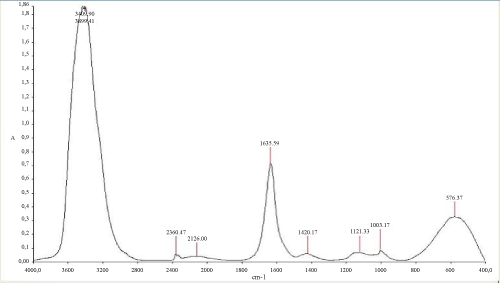
The (Figure 6) mentioned that the TPH concentration decreased slightly from 12700 mg/kg to 11000 mg/kg in the soil treated with inorganic NPK during the first 13 days of treatment. In the control system, the same profile changing was observed where the TPH concentration value ranged from 12700 mg/kg to 11200 mg/kg. In the first system, the TPH concentration decreased rapidly by about 98% at the end of treatment, it is relatively high when compared to the control system which have an average of 67% removal. The removal simulation process is significantly higher if compared to the work of [31] and [10]. Unexpectedly, the differences in hydrocarbon biodegradation between the treated and control system were due to the large differences between bacterial numbers. Moreover, in the other hand The FTIR spectrum (Figure 7) of shows peaks at the frequency level of 3913–3250 cm-1 representing –OH stretching of carboxylic groups and also representing stretching of –NH groups. Peak OH groups were observed at 3301.44 cm-1. The peak observed at 2356.35 was indicative of the C–H group. Comparing the final spectrum of the two systems (Figures 8 and 9), it found that aliphatic hydrocarbon C-H bonds are mainly degraded, in contrary the C-H aromatic hydrocarbons bonds (due to bonded OH) are largely degraded during the treatment. This could be explained by the fact that sample aromatic fraction are degraded in the treated system followed by complex fraction. The same result of [33] and [10].
In the most case the removal rate of TPH value obtained depend with macronutrients soil for microorganisms and pollution natural. Soil organic matter is an important source of nutrients for microorganisms, hence decreases in organic matter content with depth are often linked with decreases in microbial population density and decreased ability to degrade hydrocarbon pollution, Moreover, a decrease in organic matter with depth can also reduce the soil’s sorption capacity, hence increasing the ability of the pollutant.
Moreover, the aerobic biodegradation of hydrocarbon compound is the modification and decomposition of the compound by soil bacterial to produce ultimately cells, carbon-dioxide CO2 and water H2O, this modification is carried out entirely by enzymes located within the microbial cells. This hydrocarbon biodegradation is a biologically catalyzed reduction in complexity of organic hydrocarbon composite through mineralization process. The transformation of composite after its collision with enzymes of the cells depends upon the compound binding to the enzyme and conformational changes at enzyme’s active site.
References
- Plohl K, Leskovsek H, Bricelj M (2002) Biological degradation of motor oil in water. ActaChimicaSlovenica 49: 279-290.
- Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54: 305-315.
- Wolicka D (2008) Biodegradation - The Natural Method for Liquidation Environmental Polluted by Petroleum Products. Oil and Gas Institute,Prace 675-680.
- Margesin R, Schinner F (2001) Biodegradation and bioremediation of hydrocarbons in extreme environments. ApplMicrobiolBiotechnol 56: 650-663.
- Greer CW, Whyte LG, Niederberger TD (2010) Microbial Communities in Hydrocarbon- Contaminated Temperate, Tropical, Alpine, and Polar Soils: Handbook of Hydrocarbon and Lipid Microbiology. (Timmis K edn), Springer Berlin Heidelberg, 2313-2328.
- Okoh A, Trejo-Hernandez M (2006) Remediation of petroleum hydrocarbon polluted systems: Exploiting the bioremediation strategies. AfrJ Biotechnol 5.
- Frankenberger WT, Emerson KD, Turner DW (1989) In situ bioremediation of an underground diesel fuel spill: A case history. Environmental Management 13: 325-332.
- Cunningham C, Philp J (2000) Comparison of bioaugmentation and biostimulation in ex situ treatment of diesel contaminated soil. Land Contamination and Reclamation 8: 261-269.
- Chemlal R, Tassist A, Drouiche M, Lounici H, Drouiche N, et al. (2012) Microbiological aspects study of bioremediation of diesel-contaminated soils by biopile technique. International Biodeterioration& Biodegradation 75: 201-206.
- Chemlal R, Abdi N, Lounici H, Drouiche N, Pauss A, et al. (2013) Modeling and qualitative study of diesel biodegradation using biopile process in sandy soil. International Biodeterioration& Biodegradation 78: 43-48.
- Qin G, Gong D, Fan MY (2013) Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. International Biodeterioration& Biodegradation 85: 150-155.
- Dias RL, Ruberto L, Hernández E, Vázquez SC, Lo Balbo A, et al. (2012) Bioremediation of an aged diesel oil-contaminated Antarctic soil: Evaluation of the “on site” biostimulation strategy using different nutrient sources. International Biodeterioration& Biodegradation 75: 96-103.
- Grace Liu PW, Chang TC, Chen CH, Wang MZ, Hsu HW (2013) Effects of soil organic matter and bacterial community shift on bioremediation of dieselcontaminated soil. International Biodeterioration& Biodegradation 85: 661-670.
- Abed RMM, Al-Sabahi J, Al-Maqrashi F, Al-Habsi A, Al-Hinai M (2014) Characterization of hydrocarbon-degrading bacteria isolated from oil-contaminated sediments in the Sultanate of Oman and evaluation of bioaugmentation and biostimulation approaches in microcosm experiments. International Biodeterioration& Biodegradation 89: 58-66.
- De la Torre-Sánchez R, Baruch I, Barrera-Cortés J (2006) Neural prediction of hydrocarbon degradation profiles developed in a biopile. Expert Systems with Applications 31: 383-389.
- Kriipsalu M, Marques M, Nammari DR, Hogland W (2007) Bio-treatment of oily sludge: the contribution of amendment material to the content of target contaminants, and the biodegradation dynamics. J Hazard Mater 148: 616-622.
- Hunt ME, Floyd GL, Stout BB (1979) Soil algae in field and forest environments. Ecology 60: 362-375.
- MODECOM (1993) Methodology for municipal solid waste characterization. (2ndedn), ADEME Report, 1601-2766.
- Watanabe F, Olsen S (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society of America Journal 29: 677-678.
- Bremner J, Mulvaney C (1982) Nitrogen-total: Methods of soil analysis Part 2 Chemical and microbiological properties. 595-624.
- Nelson D, Sommers L (1982) Total carbon, organic carbon, and organic matter, Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties (AL Page, RH Miller, DR Keeneyedn), Am Soc of Agron, Soil SciSoc of Am, Madison, Wis, 539-580.
- Agency UEP (1986) EPA Method 7610. Potassium (Atomic Adsorption, Direct Aspiration): Test Method for Evaluating Solid Wastes. 3rd Ed Update, SW-846.
- Agency UEP (1986) EPA Method 7450. Magnesium (Atomic Adsorption, Direct Aspiration):. Test Method for Evaluating Solid Wastes. 3rd Ed Update, SW-846.
- Agency UEP (1986) EPA Method 7140. Calcium (Atomic Adsorption, Direct Aspiration): Test Method for Evaluating Solid Wastes. 3rd Ed Update, SW-846.
- Agency UEP (1986) EPA Method 7380. Iron (Atomic Adsorption, Direct Aspiration): Test Method for Evaluating Solid Wastes. 3rd Ed Update, SW-846.
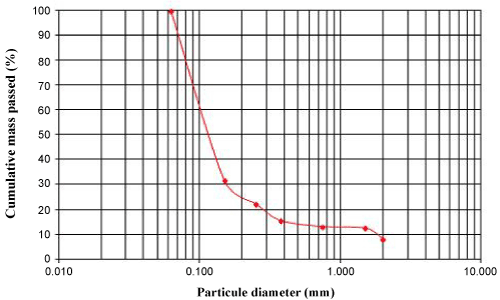
- ASTM (2002) Standard test method for particle-size analysis of soil: 2004 Annual book of ASTM Standards. (D422-63), ASTM, Philadelphia, Section 8, 4: 10-17.
- Method UE (9071B) n-Hexane Extractable Material (HEM) for Sludge, Sediment and Solid Samples.
- Standard F (NFT 90-114, AFNOR (French Association of Standardization) (1987) Collection of the French Standards on the Quality of the Grounds, Methods of Analysis. (1stedn), TCE & DOC, Paris 131.
- Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacterpostgatei gen. nov., sp. nov. Arch Microbiol 129: 395-400.
- Agency. USEP (2006) A Citizen’s Guide to Bioremediation. April 1996, 24 Nov 2006 Verified 12/15/2006.
- Wibbe ML, Blanke MM (1999) Aliphatic hydrocarbons in an oil-contaminated soil: Carbon economy during microbiological decontamination. Environ SciPollut Res Int 6: 2-6.
- Greer C, Whyte L, Niederberger T (2010) Microbial communities in hydrocarboncontaminated temperate, tropical, alpine, and polar soils: Handbook of Hydrocarbon and Lipid Microbiology. Springer, 2313-2328.
- Coulon F, Al Awadi M, Cowie W, Mardlin D, Pollard S, et al. (2010) When is a soil remediated? Comparison of biopiled and windrowed soils contaminated with bunker-fuel in a full-scale trial. Environ Pollut 158: 3032-3040.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 17431
- [From(publication date):
July-2014 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 12661
- PDF downloads : 4770
