Feasibility and Acceptability of Over-The-Counter Distribution of Take-Home Naloxone: A Systematic Review
Received: 04-Apr-2022 / Manuscript No. jart-22-61053 / Editor assigned: 06-Apr-2022 / PreQC No. jart-22-61053 (PQ) / Reviewed: 20-Apr-2022 / QC No. jart-22-61053 / Revised: 22-Apr-2022 / Manuscript No. jart-22-61053 (R) / Accepted Date: 25-Apr-2022 / Published Date: 29-Apr-2022 DOI: 10.4172/2155-6105.100464
Abstract
Introduction: Opioid drugs or narcotic analgesics, are the most prescribed analgesics for effective pain management. Additional desirable effects of opioids lead to its high abuse risk. The most fatal outcome of overdose is death resulting from respiratory depression, hypoxia, brain damage and coma. Naloxone, a fast-acting opioid antagonist is proven to be effective for the reversal of opioid overdose effects, however with limited access. Over-thecounter distribution of take-home naloxone in community pharmacies is a medium of distribution that can increase accessibility to individuals at risk of opioid overdose deaths.
Methods: PubMed, Embase, Web of Science, PsycINFO and the Cochrane library were searched for peer reviewed literature using the main terms; opioids, opioid abuse, opioid overdose deaths, naloxone, take-home naloxone and over-the-counter distribution. Grey literature and other specialists’ websites were also searched. Seven papers met the inclusion criteria for this systematic review.
Results: Selected study results were synthesized qualitatively, and the results of this study indicates that overthe- counter distribution of take-home naloxone is acceptable and feasible in increasing the accessibility of takehome naloxone for the prevention of opioid overdose fatalities.
Conclusion: Over-the-counter distribution of take-home naloxone is an acceptable and feasible novel intervention that can increase the accessibility of this medicine to individuals at risk of opioid overdose deaths. Implementation challenges identified with over-the-counter distribution of take-home naloxone such as cost, lack of appropriately packaged naloxone products for over-the-counter distribution, elaborate licensing and certification procedures for pharmacist and legislations that affect the stocking and dispensing of naloxone, needs to be collaboratively tackled by all stakeholders to achieve successful outcomes of this intervention.
Keywords
Addiction; Addiction research; Addiction therapy; Opioids; Opioid dependence; Opioid overdose deaths; Naloxone; Takehome Naloxone; Over-the-counter distribution
Background
The term opioids refer to all compounds natural and synthetic functionally related to opium derived from poppies and endogenous opioid neuropeptides [1]. Opium is a naturally occurring mixture directly derived from the juice of opium poppy. Morphine is the main active alkaloid in opium and for the purpose of this paper, the term opioids will cover several exogenous opioids that are significant in the area of both medical and non-medical use of opioids [1]. They include but not limited to heroin, morphine, oxycodone, codeine, methadone, hydromorphine, pentazocine, and buprenorphine. Three major and most important opioid receptor subtypes exist, mainly μ-opioid receptor (mu), ẟ-opioid receptors (delta) and κ-opioid receptors (kappa). These receptor subtypes have unique distribution in the brain and spinal cords which suggest that they mediate a wide variety of effects [2] .
The mu opioid receptor has the highest affinity for morphine and related opioid drugs and have rich locations both in the brain and the spinal cord. These brain areas rich in mu receptors support their role in morphine-induced analgesia, for example, the medial thalamus, periaqueductal gray, median raphe, and clusters within the spinal cord [2,3]. Other high-density areas such as the nucleus accumbens suggest a role in positive reinforcement, brain stem controlling cardiovascular and respiratory depression, cough control, nausea and vomiting and the thalamus striatum responsible for sensorimotor integration.
The delta opioid receptors are also located in the brain and spinal cord like the mu receptors, but they are more restricted. They are predominantly found in the forebrain structures such as the neocortex, striatum, olfactory areas, substantia nigra and nucleus accumbens [2,3]. Many of these sites are consistent with a possible role for delta receptors modulating olfaction, motor integration, reinforcement, and cognitive function. Delta opioid receptors in areas overlapping mu opioid receptors suggest modulation of both spinal and supraspinal analgesia.
Compared to the mu opioid receptors and delta opioid receptors, the kappa opioid receptors have very distinct distribution. They are found in the striatum and amygdala but have unique distribution in the hypothalamus and pituitary. These locations maybe participated in the regulation of pain perception, gut motility, and dysphoria, as well as, modulate water balance, feeding, temperature control and neuroendocrine functions [1].
The multiple effects of morphine and other opioids on the central nervous system are dose-related and related to the rate of absorption. At low doses (5 to 10mg), pain is relieved, respiration is somewhat depressed, and pupils are constricted [1]. The principal subjective effects are drowsiness, decreased sensitivity to the environment and impaired ability to concentrate, followed by a dreamy sleep [1]. Due to the opiates’ action in the limbic system, some researchers suggest that the drug relieves ‘’psychological pain’’ including anxiety, feeling of inadequacy and hostility which may lead to increased drug use [1]. Opioids also supresses the cough reflex in a dose-dependent manner and has actions on the hypothalamus that lead to decreased appetite, drop in body temperature, reduced sex drive and a variety of hormonal changes [1].
With the use of slightly higher doses, particularly when the drug is administered intravenously or inhaled, the individual experiences an abnormal state of elation or euphoria, which is referred to as the ‘’kick’’, ’’bang’’, or ‘’rush’’ and is compared to a whole body orgasm, described by non addicts as a sudden flush of warmth located in the pit of the stomach [1]. To achieve the maximum euphoria, very rapid penetration into the brain is needed. Although it is experienced as intense pleasure, the ‘’rush’’ is not the principal basis for abuse but acts as a powerful reinforcer that encourages repeated drug use [1]. It is also important to know that the euphoric effects do not always accompany intravenous administration. For many individuals being medically treated, the drug may produce dysphoria, consisting of restlessness, anxiety as well as nausea and vomiting that may accompany low doses of morphine, this effect is increased with higher doses [1]. The nausea and vomiting are directly related to morphine’s effect on the chemoreceptor trigger zone (the area postrema) in the brain that elicits vomiting. Although clearly unpleasant for most individuals, for the individual dependent on opioids, the nausea may become a ‘’good sick’’ because it is closely associated with the drug induced euphoria by classical conditioning. At the highest doses, the opioids’ sedative effects become stronger and may lead to a typical acute intoxication state or overdose situation. This is the most fatal outcome of opioid use and misuse [1].
Clinically, opioids are used for pain control and as maintenance medication for opioid addiction. For minor pain such as post dental procedures, opioids such as hydrocodone are used. For more chronic and severe pain, opioids such as morphine may be used. Chronic use of opioids subsequently leads to neuroadaptive changes in the nervous system, which are responsible for tolerance, sensitization, and dependence [1]. Tolerance refers to the diminishing effects of a drug with repeated use and it occurs for all opioids. Cross tolerance among opioids also exists, a process where tolerance developed to one opiate drug, leads to a reduced effectiveness of other chemically related drugs. For example, following chronic heroin use, treatment with codeine will elicit smaller-than-normal response even if the individual has never used it before [1].
The consequence of chronic use is the occurrence of physical dependence, which is a neuroadaptive state that occurs in response to the long-term occupation of opioid receptors [4. In the absence of the drug, cell function not only return to normal but overshoots basal levels leading to the effects of the drug withdrawal which are rebound in nature and are demonstrated by the occurrence of a pattern of physical disturbances called withdrawal or abstinence syndrome [4. Abstinence signs reflect a loss of inhibitory actions at the same receptor sites that opioids produce its effects, as blood levels of the drug decline. Withdrawal can also occur by administering an opioid antagonist that competes with the drug molecule and functionally mimics the termination of drug use [4. Notably the withdrawal following an antagonist administration is far more severe than that following drug caseation, due the rapid deprivation of the opioid receptors as compared to gradual caseation of drug use [4.
Acute opioid overdose is characterised by a triad of stupor or coma, respiratory depression, and pinpoint pupils [5]. Physical examination of the body may reveal needle marks especially on the arms. Individuals dosing and reliant on regular clinical doses are important as diminished respiration occurs with opioid until tolerance develops [5]. When any opioid is used beyond the degree of tolerance that is developed, reduced response to carbon dioxide centres in the pons and medulla can lead to carbon dioxide retention. Initially there is depressed cough which is modulated by the medulla, as well as nausea and vomiting, which is modulated by the postrema of the medulla. Constriction of the pupils is the result of parasympathetic nerve excitation. Convulsions may also be reported in some opioid overdose, probably because of the release of gamma amino butyric acid in the central nervous system [1]. Ultimately, without the appropriate emergency treatment, opioid overdose leads to death, resulting from respiratory failure [5].
Naloxone is a short–acting competitive opioid antagonist, which rapidly reverses the effect of opioid overdose within a given time when administered [6]. It is therefore the opioid antagonist medication of choice used to block or reverse the effects of opioid drugs, particularly within the setting of drug overdose. More specifically naloxone's binding affinity is highest for the μ-opioid receptor, then the δ-opioid receptor, and lowest for the κ-opioid receptor [7]. When naloxone binds to the opioid receptors it acts as an inverse agonist, causing the rapid removal of any other drug bound to these receptors [7]. Naloxone is an essentially pure opioid antagonist, that is, it does not possess the “agonistic” or morphine-like properties characteristic of other opioid antagonists [1]. Naloxone is indicated for the life-threatening symptoms of opioid overdose, particularly for the rapid reversal of central nervous system depression leading to respiratory depression, sedation, and hypotension [1].
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes; the onset of action is slightly less rapid when it is administered subcutaneously or intramuscularly [8]. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride. Intramuscular administration produces a more prolonged effect than intravenous administration [8]. Since the duration of action of naloxone may be shorter than that of some opiates, the effects of the opiate may return as the effects of the naloxone dissipates. The requirement for repeat doses of naloxone is required, however, will also be dependent upon the amount, type and route of administration of the opioid being antagonized [8].
Naloxone administered in usual doses and in the absence of opioids or agonistic effects of other opioid antagonists exhibits essentially no pharmacologic activity [7]. Naloxone has not been shown to produce tolerance or cause physical or psychological dependence. In the presence of physical dependence on opioids, naloxone will produce withdrawal symptoms. In the presence of opioid dependence, opiate withdrawal symptoms may appear within minutes of naloxone administration and will subside in about 2 hours later [7].
Burden of opioid misuse
According to the United Nations Office on Drugs and Crime (UNODC) report 2018, opioids are responsible for most of the negative health impact of drug use even though cannabis is the most used globally. In 2006, it was estimated that 34.3 million people globally reported opioid use in the past year, these included both people who use prescription opioids and those who used opioids for non-medical purposes [9]. This figure corresponds to 0.7 percent of the global population aged between 15 and 64 years [9].The prevalence of opioid use is high in North America at 4.2 percent, followed by Oceania at 2.2 per cent, Central Asia and Transcaucasia at 0.9 per cent, with eastern and Southern America at 0.7 percent .The misuse of pharmaceutical opioids such as tramadol is reported in many countries in Africa, particularly West and North Africa as well as Western and Central Europe [9].
In 2014, the United States of America recorded 18,893 overdose deaths related to prescription pain relievers, out of which 10,574 were deaths related to heroin overdose. This number of deaths corresponded to nearly 63% of all fatal drug overdose in USA with drug overdose deaths exceeding road traffic accidents as a cause of death in 2014 [10]. The highest increase in overdose deaths were recorded in 2016 with a record 63,632 number of deaths corresponding to 21.4 percent increase from 2015 [9]. The increase in overdose deaths was mainly associated with synthetic opioid use. Overdose deaths associated with heroin also increased by 19 per cent from 2015 to 2016 [9]. There was an indicated 150 percent increase in overdose deaths recorded over the period between 2002 and 2016 among heroin users [9].
Overdose deaths in Europe rose consecutively to reach highest peak levels in 2015 [9]. Opioid overdose deaths accounted for 79 percent of deaths, mainly from heroin use [9]. Among the European countries, United Kingdom recorded the highest number of deaths, with a record of 31 percent, accounting for approximately one third of the total recorded for Europe[11]. The number of drug misuse deaths in England and Wales began to rise from 1993 mostly due to the use of heroin and morphine. By the year 2016, a total of 2,593 deaths were recorded and represented the highest records over the years. Similarly, Australia recorded its highest number of drug overdose deaths in 2016, with a substantial increase detected from the year 2011[9]. Unintentional overdose accounted for 71 per cent of these deaths recorded and majority were caused by opioid overdose [9].
Take-home naloxone as an intervention
Naloxone as an opioid antagonist was synthesized and patented in the early 1960’s and approved by the Food and drugs Authority in the United States of America, for intramuscular, subcutaneous and intravenous use in 1971, for the reversal of opioid overdose partially or completely [12]-[14]. Even though naloxone was not the first opioid antagonist that existed at the time it was the first to be identified to be largely free of agonist effects. Naloxone was included in the World Health Organization’s list of essential medicines in 1982 and it became a standard rescue medication in hospitals and ambulance service [15].
Naloxone is proven to be a safe medication with opioid withdrawal as the primary adverse effect [16]. Naloxone has value for all individuals who have developed dependence to opioids either through illegal use for non-medical purposes, or those receiving high doses of prescription opioids for legitimate pain relief [16]. The 2016 Centre for Disease Control and Prevention Guidelines for Prescribing Opioids for chronic pain recommend that as a harm reduction strategy, a receipt of naloxone should accompany any opioid doses greater than 50mg of morphine or its equivalent.
Take-home naloxone is a harm reduction intervention that aims at reducing opioid overdose related mortality by distributing naloxone to people at risk of experiencing and or witnessing an overdose situation [17]. During the 3rd international harm reduction conference in Melbourne in 1992, the notion of take–home naloxone provision to at risk populations was propounded based on the idea that, opioid users and or family and friends can take home naloxone and use it in the event of an emergency overdose [18]. Early calls for the implementation of take-home naloxone programmes highlighted the need to make this intervention readily accessible to at risk population namely, (a) opioid users already enrolled in treatment programmes as they remain at risk even though treatment was a protective factor; (b) active users; (c) individuals at risk of overdose due to lose of tolerance, resulting from incarceration, detoxification or abstinence based treatment, as well as those leaving emergency care following an overdose[19].
Early implementation of take-home naloxone was started by user advocates in collaboration with physicians who despite the medicolegal barriers were willing to prescribe naloxone [20]. By the 1990’s the first take-home naloxone was being provided in the United Kingdom (Jersey), United States of America (Chicago and San Francisco), Germany (Berlin), and Italy (Turin, Bologna and Padua) [20]. The inception of take–home naloxone over the past two decades has moved from its initial concepts of harm reduction measure for the prevention of opioid overdose deaths to an effective evidence based public health strategy [21, 22].
There is considerable evidence that take-home naloxone is effective in reducing the risk of opioid overdose deaths [23,24].The take-home naloxone programme has been found to increase opioid overdose knowledge and reduce opioid related deaths amongst opioid abusers [25-27]. Naloxone distribution programmes have been proven to be widely successful in that people who use drugs can be trained to respond to overdose effectively [6, 28, 29].
In the United States of America between 1996 and 2014, organisations distributed over 152,000 naloxone kits to laypersons and received reports of over 26,000 overdose reversals [30]. Naloxone is also associated with reduction in heroin use among its recipients and a population-level reduction in overdose mortality [8, 24, 31-34].
However, despite the proven benefits of take-home naloxone as a harm reduction strategy there are barriers to the accessibility of this medication. These barriers have been identified to exist within the medical professionals who provide care for opioid users, the community of opioid users themselves and from administrators and legislators in their approach to policy formulations that places restrictions on the accessibility of naloxone.
Barriers from the medical professionals who provide care to opioid users may be attributed to lack of knowledge on the process of addiction and dependence on drugs .This results in some level of judgemental attitude and active discrimination that leads to lack of best practices of care given to opioid abusers [35]. The medical community sometimes hold the view that, an intervention such as take-home naloxone will only encourage riskier drug use behaviour [35]. Additionally, the existence of shortages in staff resourcing in terms of professionals who are appropriately trained to deliver naloxone programmes results in disparities in care for opioid abusers in the healthcare delivery system [36].
Barrier from within the community of drug users may also exist. Users may fail to seek for medical help during an overdose for fear of police and legal actions. Study shows that drug users are fearful of facing legal prosecution if they disclose their drug use status and might not call for professional help should they witness an overdose of peer user [25]. The third form of barrier relates to legislations that regulate or restrict the use of naloxone. Two relevant laws exist, on the possession and use of naloxone. The ‘good Samaritan laws’ which extend immunity from procession beyond physicians to any responders witness who extend care in emergency situations, and laws that enable naloxone access through standing orders [27].
Additionally, the requirement of prescription for the purchase of naloxone remains a barrier to its easy accessibility and with the international expansion of naloxone supply a range of supply models have been established including standing orders, collaborative practice agreements and pharmacist initiated supply [28]. An additional model to facilitate supply is over-the-counter (OTC) pharmacy supply [37]. In general, medicines are scheduled according to their therapeutic potential, side effects, abuse liability, the likelihood of a consumer understanding the safe use of a product and benefits of self-medication [38].
In the United Kingdom naloxone is under the ‘’schedule 7’’ class of drugs in the ‘’United Kingdom medicine Act’’ which allows any member of the general public to administer naloxone with the aim of saving lives, likened to glucagon and adrenaline [39]. The United Kingdom department of health ‘’orange guidelines’’ states that naloxone must be prescribed to named patients or supplied to individual by means of a patient group direction [39]. Naloxone therefore remains a prescription only medication in the United Kingdom. In furtherance, in the United States of America naloxone is a prescription only medicine at the federal level however variations exist at the state legislation and lower court’s rulings [20]. In 2007 New Mexico passed the good Samaritan law that grant legal immunity to bystanders who use naloxone for the purposes of saving lives [40]. New York and Connecticut also have laws that grant immunity from liability to health care providers with prescribing authority [41]. In 2006, Massachusetts take-home naloxone pilots programme used standing order to enable public health care workers to provide take home naloxone without a prescription [42]. Standing order model allows a physician within given jurisdiction to issue a written order that naloxone can be distributed by designated pharmacies or other qualified professionals [43]. Most opioid overdoses occur in private homes and as such, most of these are witnessed [39, 44]. Close friends, a partner or family member are most likely to witness an opioid overdose [45,46]. The other key group of individuals likely to witness overdoses are people working with those who use drugs. They include trained health professionals and first responders, such as ambulance, police fire, community pharmacist and drug-treatment workers as well as outreach workers.
The question therefore is, is there a means by which opioid users, their families, other professionals who work closely with opioid abusers and the general public who are most likely to witness opioid overdose, will be able to access naloxone and use it effectively as required?
3.3. Scope of distribution of take-home naloxone
Naloxone has been available as a non-prescription drug in Italy since 1996, as the Italian ministry of health classification of naloxone as over-the-counter medication allowed pharmacist to issue it without prescription [47, 48]. However, it must be requested by the customer directly from the pharmacist, as it is not openly displayed on shelves. This has been associated with a steady decline in opioid overdose mortality rates in Italy, with 470 deaths in 1990, 280 in 2005 and 101 as at 2015 [47].
The second country to introduce over-the-counter distribution of naloxone was Australia even though their take-home naloxone programmes began later than many other countries, only in 2011. A decision of the therapeutic goods administration placed naloxone on schedule 3, thereby approving the over-the-counter status [49]. Since 2016, Australian community pharmacists have been able to supply naloxone without prescription. Take-home naloxone programmes widely exist in Canada and in 2016 an interim order by Health Canada approved the previously FDA licensed nasal naloxone product to be available without prescription [50].
In the United States of America there are selected pharmacies in at least 15 of its states where special agreements allow pharmacist to sell naloxone without prescription [51]. However not all pharmacies stock or dispense naloxone. Depending on the pharmacy, a pharmacist may have to write a prescription or may not be able to give naloxone to comply with rules regarding prescription medication as naloxone is still considered a prescription only medication under FDA rules with formal reclassification yet to be considered [52].
The United Kingdom Public Health England guidelines published in 2015, allow people who work or are involved with the National Health Service drug treatment services to make take-home naloxone available to opioid users, family members and hostel staff without prescription once accurate documentation of the naloxone supply is ensured [53]. This guideline makes naloxone distribution easier, as staff without prescribing authority can distribute take-home naloxone, however naloxone remains a prescription only drug [20,43].
Existing literature on take-home naloxone
Literature search for systematic reviews conducted on takehome naloxone revealed a range of aspects of conclusions and recommendations to improve naloxone accessibility. A systematic review of community opioid overdose prevention and naloxone programmes showed that bystanders (mostly opioid users) can and will use naloxone to reverse opioid overdose when properly trained and that this training can be done successfully through opioid overdose prevention programs [25].
A scoping review by Nielson and Hout Van in 2016 also confirmed that provision of naloxone for bystander administration to prevent opioid overdose deaths appears increasingly feasible. However, barriers such as cost and remuneration for community pharmacist time and how pharmacist may effectively identify and train naloxone recipients still exist [54].
Strang and McDonald [55] report the impact of take-home naloxone distribution on overdose-related mortality and to assess the safety of take-home naloxone in terms of adverse effects using the Bradford Hill Criteria. They concluded that take-home naloxone provision reduced outcomes of overdose among programme participants themselves, among fellow opioid users and the wider community, as evidenced by public vital statistics records. Additionally, the risk associated with take-home naloxone programmes is relatively low especially when considering the life-threatening nature of overdose [55]. They also found no empirical evidence to support the concern that take-home naloxone programmes might encourage heroin use and therefore recommend take-home naloxone distribution to at risk users to be introduced as standard care for community-based prevention of heroin overdose deaths [55].
A systematic review aimed at summarizing the known benefits of naloxone access and detailing the knowledge gap of unanswered questions about overdose education and naloxone rescue kits was conducted in 2017 [56]. There was the need for federal government to realise the need to prioritise increasing prescriber education, improving access to treatment for opioid use disorder and naloxone [56].
In 2017, Lewis and colleagues in their literature review identified that strategies to make naloxone available for laypersons or take-home use should include development of naloxone formulations that are easier to administer for non-medical users, such as intranasal and autoinjector intramuscular delivery systems. There should be increased efforts to distribute naloxone to potential users, high impact categories of non-medical users as well as efforts to reduce regulatory barriers to more widespread distribution and use [57].
A systematic review on twenty years of take-home naloxone for prevention of overdose deaths from heroin and other opioids from its conception to maturation was conducted in 2017 [20]. The outcome was that framed as a public health tool for harm reduction, take-home naloxone has overcome social, clinical and legal barriers in many jurisdictions [20]. Nonetheless the rising death toll of opioid overdose illustrates that current take-home naloxone coverage is insufficient and greater public investment in overdose prevention will be required if take-home naloxone is to achieve its full potential impact [20].
In exploring the willingness of providers to prescribe naloxone to patients, there appears to be an increase from the early 2000s to date, in parallel to increasing attention and concern regarding the opioid crisis [6].
An ecological study of the geographical distribution of drug overdose education and naloxone distribution programmes which trains laypersons (people who use drugs), family members, peers as prospective responders in overdose events by providing access to naloxone and directions for delivery was carried out in 2018 [58]. The review concluded that some counties that experienced the highest rate of drug overdose mortality did not have the highest percentage of overdose education and naloxone distribution programmes as these programmes were operating in only 13% of the high burden counties [58]. The relatively low volume of overdose education and naloxone distribution programme coverage throughout the United States highlights a critical implementation gap in the delivery of these programs even in areas with highest rates of overdose deaths. The study identified areas such as impact of naloxone laws, provider level-stigma, cost of naloxone and staff time as barriers [58].
A systematic review identified the emergency department as a potential setting for naloxone distribution for overdose reversal in the community. This is due to an increase over the years in the rate of opioid-related emergency department visits that has nearly doubled from 89.1 per 1000,000 persons in 2005 to 177.7 per 100,000 persons in 2014 [59].
Study rationale
Increasing access to naloxone medication is an effective and costeffective method of reducing opioid overdose deaths [60], however local legislation is a key factor in the evolution of naloxone access initiatives. The United Nations Commission on Narcotic Drugs passed the resolution 55/7 in 2012, which identified the need for more effective prevention of drug overdose [61]. In this resolution, member states are encouraged to include effective elements for the prevention and treatment of drug overdose particularly opioid overdose in national drug policies, including the use of opioid receptor antagonist such as naloxone [61].
The World Health Organisation’s guidelines on ‘’community management of opioid overdose’’ recommends that there should be increased access to naloxone and instructions to use to people who are likely to witness an opioid overdose [48].
Despite the resolution by the UNODC in 2012 and recommendations by the WHO in 2014, which has led to some impact on efforts by member states to increase access to take home naloxone the burden of opioid overdose deaths continues to increase as indicated by statistics [11,61]. Most countries experienced peak numbers of overdose deaths between the year 2014 and 2016 [11,61].
Knowledge on other facets of existing take home naloxone distribution programmes as a harm reduction strategy has been explored in various reviews. These include the willingness of bystander to administer naloxone and barriers preventing the provision of naloxone for bystander administration [25]. Others explored the safety of take-home naloxone and found no empirical basis for concerns on it encouraging illicit opioid use, [55]. There are studies that have examined the role of federal Governments enacting policies that can enhance the accessibility to naloxone and possible increase in the current take-home naloxone coverage by making it a greater public investment [56]. Other studies examine the role of new formulations of naloxone that will enhance its administration by bystanders and highlights the increase concern and attention by healthcare providers in prescribing naloxone over time [6,57].
There is evidence that, pharmacy-based interventions implemented are generally effective. Community pharmacy based interventions have been successfully implemented for annual influenza immunization [62], smoking cessation interventions, screening for diabetes and risk factors of cardiovascular disease [63], early cancer detection initiatives [64], and assessing worsening heart failure [65]. Other successfully implemented pharmacy-based interventions include, improving pneumococcal vaccination coverage for at risk patients [66], emergency hormonal contraception [67]. A systematic review about the feasibility and acceptability of community pharmacy-based screening for major diseases found high patient satisfaction rates with such services [68].
Additionally, the role of pharmacist in opioid harm reduction programmes have evolved beyond their traditional roles as pharmacist engage in medication-assisted treatment, to obtaining licence for prescription and dispensing of naloxone, developing and monitoring first responder naloxone initiatives and, instituting safe avenues for disposal of medications [69]. As demonstrated by Italy, Australia, Canada and some selected states in the United States of America, takehome naloxone distributed through pharmacies due to its over-thecounter status increased its accessibility and resultantly decreases in opioid overdose mortalities recorded [47,50,51].
Systematic reviews are relevant to healthcare decision and practice. Healthcare decisions for individual patients and for public health policies should be informed by the best available research evidence. The practice of evidence-based medicine is the integration of individual clinical expertise with the best available external clinical evidence from systematic research and patient's values and expectations [70]. Primary care physicians as well as other health professionals need evidence for both clinical practice and for public health decision making. This evidence comes from reviews which is a state-of-the-art synthesis of current evidence on a given research question [70]. Given the explosion of medical literature, systematic reviews aim to identify, evaluate, and summarize the findings of all relevant individual studies over a healthrelated issue, thereby making the available evidence more accessible to decision makers [70].
Public health and medical practice are moving towards the goal of implementing evidence-based interventions and therefore the need for comprehensive evaluation of multiple interventions through feasibility studies. The two important aspects of feasibility studies are to determine its acceptability and practicality of implementing such an intervention. In studying the feasibility of an identified intervention, the under listed core areas are addressed;
• The acceptability of the intervention gathered through data on how both the target population and health professionals who react to it.
• Estimating the demand for the intervention by gathering data on its estimated use in the defined target population.
• Gathering concerns from target group and health professionals on their concerns regarding anticipated problems with implementation of the intervention.
• Exploring the practicality of the implementation and delivery of the intervention within limited resources, time and commitment.
• Assessment of the actual modifications at system levels that is necessary to accommodate and integrate the intervention successfully.
Generally, feasibility studies seek to answer the following questions in relation to an intervention, ‘’can it work’’, ‘’does it work’’ and finally ‘’will it work’’.
The search for available literature on take-home naloxone identified that there is a gap in policies and the implementation of naloxone programmes. Despite the proven efficacy of take-home naloxone, it appears to be an intervention that is widely under-utilized or nonexistent in many countries. It is evident through existing literature that, limited accessibility is linked with the corresponding restrictions that accompany existing distribution methods for take-home naloxone. The few countries that have implemented the distribution of take-home naloxone over-the-counter in community pharmacies have recorded decreases in opioid overdose deaths, as this mode of distribution increases its accessibility.
A systematic review to consolidate and evaluate the available literature to highlight the feasibility and acceptability of over-thecounter distribution of take-home naloxone is therefore warranted. This will add to the already existing knowledge on the other facets of take-home naloxone intervention. This systematic review will specifically highlight the willingness of people at risk of overdose deaths and pharmacist to partake in this intervention. The laws and policies that affect this mode of distribution as well as system level changes and modification that are necessary for the implementation of this intervention will be analysed. Both anticipated and practically experienced implementation challenges and how this intervention will fit into the existing scarce financial and human resource for addiction treatment, specifically opioid dependence treatment programmes will be assessed.
Findings from this systematic review in addition to existing literature is aimed at providing a go to reference point for health care professionals who are involved with take-home naloxone programmes in the framework of evidence-based practice. It will help with the implementation and expansion of take-home naloxone programmes through this mode of distribution, with the resultant aim of increasing its accessibility and reducing the rising statistics of opioid overdose deaths.
Methodology
Research design
This is a systematic literature review of available literature on overthe overthe- counter distribution of take-home naloxone as a harm reduction programme for opioid overdose deaths, following the PRISMA statement guidelines for reporting systematic reviews.
Aims
To critically examine and summarise existing literature on over-the-counter distribution of take-home naloxone to access the feasibility and acceptability of this medium of distribution as a means of increasing accessibility to naloxone.
Study objectives
• To determine the perceptions both pharmacist and atrisk population hold on over-the-counter distribution of take-home naloxone.
• To examine the willingness and acceptance of over-thecounter distribution of take-home naloxone in community pharmacies
• To examine available policies towards over-the-counter distribution of take-home naloxone in community pharmacies.
• To examine practical challenges experienced with the implementation of over-the-counter distribution of take-homenaloxone as an intervention.
Eligibility criteria
Inclusion Criteria: Inclusion Criteria can be shown in table 1 (Table 1).
| Population | Individuals at risk of or likely to witness an overdose |
|---|---|
| Interventions | Interventions should indicate use of over the counter distribution of take-home naloxone |
| Outcomes |
|
|
|
|
|
|
|
|
|
|
|
| Study Design | Research data from cross-sectional surveys, cohort studies, systematic reviews and meta-analysis |
| Intervention studies: pre and post intervention studies and meta-analysis | |
| Data from observational studies, commentaries, case reports, qualitative and quantitative studies. |
Table 1: Inclusion Criteria of Population.
Exclusion Criteria: Studies were excluded if interventions did not specify use if over-the-counter distribution of take-home naloxone.
Search methods
Electronic searches: The search of electronic sources was conducted using Medical Subject Heading (MESH) as well as freetext terms. The under listed are the summary of the main terms used for the search; opiates, Heroin, fentanyl, morphine, oxycontin, diamorphine,prescription opioids, addiction, opioid dependence, opioid use, opioid misuse, opioid overdose, opioid overdose deaths, substance use, Pharmacy, Pharmacist, community pharmacies, Access, Distribution, Naloxone, Narcan, opioid antagonist, distribution of THN, OTC distribution of THN, Non-prescription dispensing of THN, harm reduction.
A detailed list of free text and mesh terms for electronic literature search is represented in appendix 1 (Appendix 1).
Peer reviewed literature: The following data bases were consulted for peer reviewed literature: PUBMED, EMBASE, WEB OF SCIENCE, PsycINFO and the COCHRANE library.
Grey literature: Grey literature included in the studies included search from, HMIC (Health management information consortium), Open Grey database, OCLC, for dissertations and thesis with relevant information related to this topic.
Consultation with experts and user groups: In addition to the above sources of literature, the website of governmental and nongovernmental agencies who play leading role in the field of addiction, including but not limited to, National Institute on Drugs Abuse (NIDA), United Nations Office on Drugs and Crime (UNODC), International Narcotics Control Board (INCB), Substance Abuse and Mental Health Services Administration (SAMHSA),European Monitoring Centre for Drugs and Drug Addiction,(EMCDDA)and International Policy Drug Consortium, were also searched. Both published and unpublished literature from the community of drug users, as well as, community Pharmacist was also consulted in order to elicit some useful information. Experts in the field were also contacted to identify articles not found in initial searches.
Data extraction and analysis
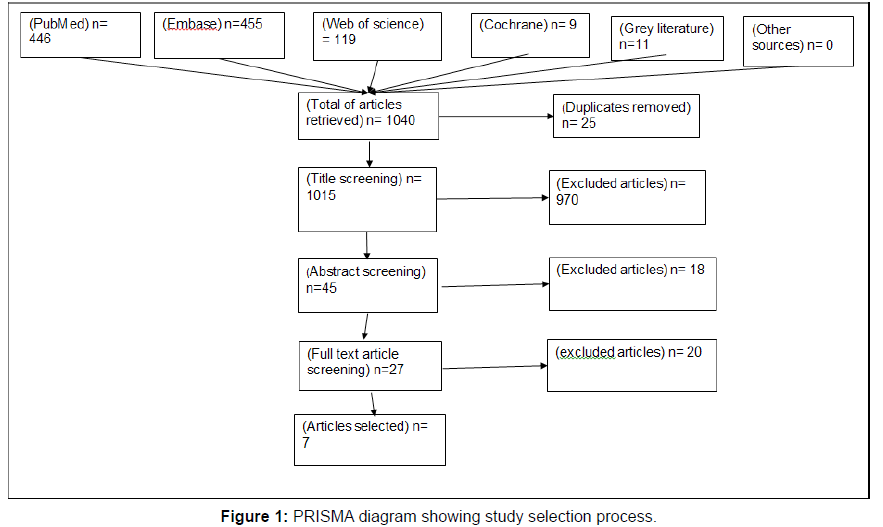
Study selection: The study selection process detailed in figure I appendix A, was initiated with a search of multiple scientific online database for peer reviewed literature and grey literature for articles of relevance to this topic under study. This was done using both free terms and mesh terms, as mentioned above. Search was first done on 29th May 2019 and the final searches on 15th June 2019. Initial evaluation was focused on titles retrieved for further inclusion. There was removal of duplicate search results generated across the various database searches. After this process, database search was done using non duplicate titles to retrieve abstract of articles for the next step of study selection process. There were several studies eliminated at this abstract screening as they did not broadly meet the inclusion criteria.
The search for articles from grey literature and other sources was done following the same procedure as for peer reviewed literature. The final number of full text articles that were included in this review was determined after the thorough process of article selection from scientific database and grey literature. The search of other sources yielded no relevant articles deemed fit for this study as per the inclusion criteria.
The final stage of the study selection process was search of the databases for full length articles of abstract that were selected when the inclusion criteria was applied. The full-length article review screening yielded narrowed down number of relevant articles. Reasons for eliminating full length articles included, mixed methods of distribution of take home naloxone, a combination of naloxone and buprenorphine as the drug for intervention, sample not representative primarily of those at risk of opioid overdose or poly substance users and the full length of some articles were not available for retrieval. The study selection process was carried out independently by the primary investigator of this systematic review.
Synthesizing the information: Information was reviewed in a standardised form to include; date, location, study design, study population, sample size, method of data collection, intervention, key findings and outcomes. Articles also included patient or pharmacist perceptions on this topic, an evaluation or feasibility studies, programme evaluations, qualitative or quantitative analytic methods or mixed methods. Search results were exported to a reference manger, Mendeley. A PRISMA diagram was used to illustrate the article selection process.
To access the acceptability of over-the-counter distribution of naloxone, articles that focus on pharmacist awareness and willingness, attitudes and anticipated barriers or concerns with this mode of distribution were evaluated. To access the feasibility of over-thecounter distribution of naloxone, articles that focus on programmatic implementation, education and experienced challenges with this mode of distribution were evaluated.
Data analysis: The data retrieved was analysed using thematic analysis. Thematic synthesis as developed by Thomas and Harden [71] involves a combination and adaptation of approaches from both meta-ethnography and grounded theory. This was used to analyse and organise the results into themes that have been discussed fully in the results section of this paper. Organizing results into themes help conduct reviews that addresses questions relating to interventions in terms of its need, appropriateness, facilitators, barriers to acceptability and issues relating to effectiveness.
Critical appraisal: Critical appraisal of literature of qualitative research forms an essential part of systematic analysis of this nature. In using critical appraisal, a variety of key questions were addressed to whether the study addresses a clearly focused question /issue, is the research design used appropriate for answering the research question, was sampling, collection and data analysis done appropriately. Additionally, was there a description of the fieldwork taken, could the evidence be inspected independently by others, are procedures theoretically justified, and were analysis repeated by more than one researcher to ensure reliability. Also, are the conclusions justified by the results and are credible to clinical practice. Attention was also given to how the research conforms to ethical conducts and how generalizable the results are.
The 10-item critical appraisal skills programme (CASP), quality appraisal tool for qualitative studies was used for the appraisal of peer review literature. The Authority accuracy coverage objectivity date and significance (AACODS) checklist was used to appraise grey literature retrieved.
Ethical consideration: The ethical considerations in each study reviewed was analysed, to ascertain if this conforms to the ethical principles for conducting research, particularly, appropriate ethical approval and consent from study participants. Conflicts of interest that could arise from funding from pharmaceutical companies and other entities were also considered.
Results
Study selection
A total of 1,040 articles relevant to this study were retrieved during the initial title search, however this number was narrowed down substantially to only 7 articles. The 1,040 articles retrieved from the multi-database search consisted of 446 from PubMed, 455 from Embase,119 from web of science, 9 from Cochrane, 11 collectively from grey literature sources and none from the other sources sought for information. After removal of 25 duplicates, 970 articles were excluded at the stage of title screening. The remaining 45 titles were used for retrieval of abstract and then an abstract screening was performed, leading to removal of 18 articles.
Resultantly, 27 articles were selected to be included in the full text or article screening. At this stage, all the articles had opioid dependants as sample population and naloxone as the opioid antagonist of choice for reversal and prevention of opioid overdose deaths. Of the 27, 5 full length articles were unavailable so only 22 studies were assessed in full. Of these, 7 papers had mixed methods of distribution of take home naloxone including over-the-counter in community pharmacies, 3 articles had a combination of naloxone and buprenorphine formulation as the drug of choice for interventions, 5 papers had samples that were poly-substance users, including opioid use and thus at risk of overdose deaths from drug use. A total of 15 articles were excluded, leaving 7 studies meeting the inclusion criteria set for papers to be selected for this systematic review. The PRISMA diagram below represents the study selection process (Figure 1 and Table 2).
| Article | N | Study sample | Study design | Primary research question | Summary of key findings |
|---|---|---|---|---|---|
| Abouk et al. [73] | 7344 | Patients with an opioid addiction | Programme Evaluation | Are state laws regarding Naloxone access associated with reductions in fatal overdose involving opioids? | Naloxone access laws policies that granted direct authority to pharmacists to dispense were estimated to reduce opioid-related fatal overdoses by 0.387(95% CI,0.119-0.656; p =0.007) per 100,000 people in 3 or more years after adoption. There was little evidence of an association for indirect authority to dispense (increased by 0.121;95%CI, -0.014 T0 0.257; P=0.09) and other NALS,( increased by 0.094;95% CI.-0.040 to 0.0227;p=0.17). Naloxone access laws granting direct authority to pharmacist were associated with significant reductions in fatal overdoses, however there were increases in non-fatal overdoses seen in emergency department visits. Other types of NALS appear not to be associated with decrease or increase in mortality. |
| Donovan et al., [72] | 52 | 1.individuals who obtained naloxone from the pharmacy in the past year 2.individuals who did not obtained naloxone in the past year but reported a risk for opioid overdose because; a. obtained an over the counter syringe from a pharmacy in the past month and had used an opioid in the past month b. had used a prescribed opioid pain medication in the past month |
Semi – structured interview | What are the factors that impact the likelihood pf getting naloxone from the pharmacy | Key factors specific to individual, interpersonal, pharmacy, community and society–level were identified to play a role in the likelihood of obtaining naloxone from a pharmacy. Individual factors; helplessness and fear, naloxone as empowerment to help and past experiences at the pharmacy Interpersonal factors; concern for family and friends, sources of harm reduction information. Pharmacy; perceived stigma from pharmacist, confusion at the counter and receptiveness to offer naloxone from pharmacist. Community; community caretaking and the need for education and training Society; generational crisis and frustration at lack of response to opioid crisis. There were differences in the beliefs of PWID and the people who are prescribed opioid pain relief on the need to make naloxone readily available in community pharmacies |
| Stopka et al, 2017 | 809 | Retail pharmacies (pharmacist, pharmacy technicians, pharmacy managers) |
Brief telephone surveys | What are the current non-prescription sales practices related to sale of naloxone and sterile syringes, and how it determines the public health role of local pharmacies in reducing risk related to opioid overdose and infectious disease transmission | Findings indicated that,97.5% of pharmacies reported selling syringes non-prescription. Nearly all chain pharmacies reported selling non-prescription syringes (99%) verses 83.5% of independent pharmacies. Out of the total number of pharmacies, only 365 (45.1%) reported stocking or selling naloxone, a higher proportion of chain pharmacies,( 49.4% 343/694)reported stocking or selling as compared to independent pharmacies,( 19.1%,22/115).Whereas non-prescription sales pf syringes are available in nearly all retail pharmacies in Massachusetts with excellent geographical access, naloxne non-prescription sales re available in less than half of all retail pharmacies across the state. Factors identified for the above variation include, PWID reporting still feeling demeaned or stigmatized at pharmacies. Some pharmacists also report not wanting ‘’to condone drug use’’ and or do not feel ‘’adequately trained’’ |
| Nielson et al., [75] | 595 | Community Pharmacist | Online survey | What are pharmacist knowledge and attitudes towards OTC supply of naloxone to expand its accessibility? | Pharmacist were willing to be involved in the supply of naloxone and many had positive attitudes towards harm reduction interventions, however it was clear that, there is needed support for the implementation of this among pharmacists in general. Barriers identified included, training and knowledge on laws around naloxone, time for naloxone education and pharmacist being less comfortable to supply naloxone to customers already on OST programmes as opposed to supply to those on chronic pain patients on high doses of opioids. |
| Bachyrycz et al. [74] | 133 | Pharmacist who have received Naloxone Authority verification and can prescribe and supply naloxone rescue kits | Programme evaluation | What are the emerging trends in Naloxone rescue kits prescription patterns by pharmacist in New Mexico as an example of a unique healthcare delivery system | Most Naloxone rescue kits (89.5%) were first time prescriptions. The most common reason for an NRK prescription was patient request (56.4%), followed by a pharmacist’s prescription due to high dose of prescription opioids (28.6%). The results indicate that, patients at risk of opioid overdose might feel comfortable solicit for an NRK from a pharmacist. Participation of pharmacist in naloxone prescription authority highlights the opportunity for this novel healthcare delivery model. Potential barriers identified included, normative attitudes toward opioid dependent patients and variability of coverage for NRK’s by health insurance, from the perspective of the pharmacist. |
| Pricolo and Nielson, et al. [38] | The article aims to describe the process of the rescheduling of Naloxone in Australia to an OTC status and highlight the challenges that encountered through this process | Following the initial submission to the therapeutic goods administration to down-schedule naloxone in Australia put forward by a qualified pharmacist, the development of a case for review of the available literature, which revealed few jurisdictions where naloxone was available without a prescription. In October 2015, the TGA announced their interim decision to reschedule naloxone to ‘’schedule 3’’, this is a pharmacist only medicine’’ that can be supplied without a prescription. The following challenges arose as a result of the implementation of the down scheduling:
|
|||
| Cressman et al., [37] | 429 | Community pharmacies in Canada | Telephone based cross-sectional survey | Is naloxone available in community pharmacies in Canada after down scheduling to OTC status in Canada | Of the 429 pharmacies studied, only 103 (24.0%) had naloxone on date of contact and generally naloxone was not available in pharmacies despite its non-prescription status. Availability prices varied considerably On sites without naloxone,50% cited a perceived lack of demand as the reason for not stocking. Other reasons included: pharmacies did not simply prioritise stocking the product, perceived lack of availability from suppliers and pharmacist not yet receiving straining to provide the drug. |
Table 2: Detailed Characteristics of articles selected.
Thematic analysis
The thematic analysis resulted in a classification of the papers in two categories: 1.Studies discussing the acceptability of over-thecounter distribution of take-home naloxone (N= 3 or 2) [54,72]. Studies discussing the feasibility of over-the-counter distribution of take home naloxone (N=4) [38,73,74].
Articles categorised as acceptability papers were those that discussed both pharmacist and patients’ concerns raised about over-the-counter distribution of take-home naloxone as an intervention. They discussed the willingness of patients and pharmacist to get involved with this intervention. They were also articles that discussed the attitudes and anticipated barriers to be encountered. Articles categorised as feasibility studies are those that described programme implementation: training of pharmacist, types of naloxone formulations suitable for over-thecounter distribution, stocking and dispensing of take-home naloxone kits and challenges experienced. Feasibility papers also discussed patients and family members’ attitudes and challenge experienced with over-the-counter distribution of take-home naloxone.
Studies assessing the acceptability of over-the-counter dispensing of take-home naloxone obtained data through semi-structured interviews, brief telephone survey and online survey. Analytic methodology included, qualitative and quantitative analysis, with period of studies ranging between 2015 and 2017.
Except for one feasibility study which collected data through a telephone based cross-sectional survey, the rest of the studies largely focused on policy formation and implementation as well as programme evaluation. Period of studies were between 2013 and 2018.The feasibility study articles used varied analytic methodology including quantitative analysis, descriptive statistics and descriptive analysis.
Acceptability of over-the-counter distribution of take-home naloxone
Willingness to distribute take-home naloxone over-the-counter, (N=2): Two articles assessed and discussed the willingness of pharmacist to dispense naloxone over-the-counter. The Nielson et al. [54] paper which was a study conducted to estimate the knowledge, attitudes and confidence of Australian pharmacist regarding naloxone for overdose reversal reports that in general, participants did not express high level of confidence about naloxone supply, as one in three pharmacist reported confidence in identifying appropriate patients [54]. Despite the low level of confidence participants indicated more willingness to engage in actions related to naloxone supply [54]. 49% of verbalized being able to identify individuals proactively for naloxone supply, whereas forty one percent were willing to dispense naloxone as an over-the-counter medication [54]. More positive attitudes towards naloxone supply were reported by pharmacist that provide opioid substitution therapies and or are involved in a needle exchange programme [54].
Stopka and colleagues compared the sale of non-prescription naloxone to that of non-prescription syringes in Massachusetts. The results indicated that a greater percentage of pharmacies sold nonprescription syringes at 97%, whiles 45% sales of non-prescription naloxone in pharmacies were recorded. None the less the authors concluded that, this represents a window of opportunity for growth of opioid overdose prevention through pharmacies under novel models such as over-the-counter distribution. The paper report that the 45% of pharmacies reporting stocking and sale of non -prescription naloxone highlights there has been some progress made in non-prescription sale of naloxone in particular. The fact that the larger proportion of survey respondents reported interest in receiving additional training regarding naloxone distribution and opioid overdose prevention in general demonstrate that there is a big opportunity to further strengthen the role of pharmacies and pharmacy staff members as public health agents who can help reduce opioid overdose deaths.
Concerns relating to over-the-counter distribution of take-home naloxone, (N=3): It was reported in 3 papers [54,72,75], discussed concerns related to over-the-counter distribution of take-home naloxone, with one paper explicitly outlining the factors that influence the likelihood of individuals with opioid dependence as well as their family and friends to purchase naloxone from a pharmacy without a prescription. Concerns raised by pharmacist included cost, nonavailability of naloxone formulations suitable for over-the-counter distribution and time involved in dispensing and educating individuals on the safe use of take-home naloxone.
Concerns raised by pharmacist as anticipated barriers in relation to stocking and dispensing naloxone over-the-counter included cost of existing naloxone formulations. Additionally, the lack of time that will be involved in giving education that goes with take-home naloxone dispensing, lack of training and knowledge of state laws, and lack of reimbursement for the time and resources used for patient education and counselling [54,75].
Stopka and colleagues, indicates that the availability of intramuscular naloxone is vital as it is the last generic choice available at a lower cost however providing options for both intranasal and intramuscular naloxone remains important. Additionally, there is variability in pricing across pharmacies that stock naloxone. Furthermore, while some insurance companies cover the cost of naloxone to individuals, they do not universally cover the cost of naloxone purchased by family members or friends buying it in case it is needed to revive their loved ones and friends in an overdose situation witnessed.
Donovan et al. [72] highlights concerns of individuals who are at risk of opioid overdose and are likely to purchase naloxone from the pharmacy. The main themes that were listed as factors that influence the likelihood of obtaining a pharmacy-based naloxone were the following five factors identified; Individual, interpersonal, social, pharmacy, community and generational level influences associated with pharmacy-based naloxone [72].
Individual level influences were associated with expression of fear and helplessness associated with the use of illicit drugs and therefore some saw the opportunity to obtain naloxone easily to feel empowered to deal with overdose situations[72]. Individuals with opioid use disorder as well as those on high doses of opioid for analgesia also verbalized that their past experiences with pharmacist will influence their likelihood of obtaining naloxone from the pharmacy[72].
Interpersonal level of influence involved how friends and family of individuals at risk of opioid overdose described obtaining naloxone over-the-counter in pharmacies as a form of motivation to offer help to their family members in critical moments. This they explain is to enable them to intervene in an overdose situation. They also viewed the point of purchase as an important source of information about naloxone and harm reduction in general[72].
Pharmacy level influences were associated with the kind of social interactions that individual at risk of opioid overdose have with pharmacist that were frequently described as tensed and mistrustful [72]. This is due to perceived stigma from pharmacist, confusion at the counter, and sometimes frustration from individual about very little training they receive from pharmacist when they purchase naloxone [72]. Many individuals also described their receptiveness to a discussion on naloxone if raised by a pharmacist; otherwise they will not initiate such[72]. Despite this, all patient groups were willing to solicit for and learn about naloxone from their pharmacist[72].
Just as individuals described a sense of responsibility to a family member or friend in equipping themselves with naloxone, many also described strong commitment to overdose prevention among the wider community of people at risk of opioid overdose, hence a community level influence[72]. Participants who had obtained naloxone from a pharmacy reported the need for training in the broader community around how to recognise the signs and symptoms of overdose and the need for knowledge on the accessibility of pharmacy-based naloxone [72]. Society and generational level influences include concerns for the changing fatality of opioid drugs that are available hence viewed as a generational crisis which demands novel but effective interventions such as pharmacy-based naloxone distribution[72].
Feasibility of implementing an over-the-counter distribution of take- home naloxone, (N=4)
Four studies; (N=4; Cressman et al. [37], Pricolo & Nielson [38], Abouk et al. [73], Bachyrycz et al., [74]) provided information around the implementation of over-the-counter dispensing of takehome naloxone and its resultant experienced challenges. Components of program implementation that emerged as barriers included; appropriate formulations of take-home naloxone suitable for overthe- counter dispensing, cost of take-home naloxone kits, training and licensing of pharmacist, legal considerations, pharmacist attitudes toward opioid dependent individual and stocking/availability of takehome naloxone.
Overall feasibility of over-the-counter dispensing of take-home naloxone
Studies assessing the feasibility of over-the-counter distribution of take-home naloxone demonstrate that this model of distribution of naloxone in community pharmacies is acceptable and feasible. As demonstrated in Australia, a proposal to reschedule naloxone put forward by a member of the general public, continued advocacy by health professionals, consumers and academics due to the rising opioid overdose mortality, as well as international moves to expand access to naloxone led to the down scheduling of naloxone by the Australian Therapeutic Goods Administration [38]. This changed the existing S4 status of naloxone to a’ pharmacist only’ S3 medicine, enabling the pharmacist to initiate naloxone supply independently and granted members of the public the ability to request for naloxone [38].
Bachyrycz et al. [74] report that patient at risk of opioid overdose might feel comfortable soliciting for naloxone from a pharmacist. In their study of opioid overdose prevention through pharmacy-based naloxone prescription program, most of the naloxone (89%) was first time prescription, and patient request which was the most common reason for prescription of naloxone rescue kits was 56.4% [74]. A pharmacist prescription due to high dose of prescription opioids was at (28.6%), and a prescription due to a history of opioid misuse /abuse at 15.0% [74].
Abouk and colleagues [73], highlighted the association between state laws facilitating pharmacy distribution of naloxone and risk of fatal overdose. The evaluation concluded that naloxone access laws that granted direct authority to pharmacist were associated with significant reductions in fatal overdose with significant effect size increasing overtime relative to implementation of the naloxone access law [73]. Naloxone access laws granting direct access to pharmacist were estimated to have reduced fatal overdoses which are opioid related significantly at 0.387 (95% CI,0.119-0.656, p=0.007) per 100,000 within 3 or more years after implementation. The estimated resultant increase between indirect authority to dispense was little and insignificant at 0.121 (95% CI 0.014 -0.27; p=.09) [73].
Training and certification/licensing of pharmacist, (N=3)
Three studies [37, 38, 74]; discussed the aspects of training as well as the process of licensing to enable the involvement of pharmacist in over-the-counter dispensing of take-home naloxone.
Pricolo and Nielsen [38], identified that most pharmacist in Australia did not feel comfortable educating patients about overdose or on the correct use of naloxone [38]. This is due to the lack of training developed in conjunction with manufacturing companies leaving professional bodies with the responsibility to develop protocols on highly important messages pertaining to sites of administration, timing and doses as well as right needle gauges [38]. The use of multiple source of information without proper consolidation of facts also leads to confusion over key messages [38].
Training for pharmacist involved with over-the-counter dispensing of naloxone is considered erroneous by some pharmacist [37]. For example in New Mexico, in order for a pharmacist to prescribe and dispense naloxone rescue kits to a patient they must complete the NMPHA certification training, consisting of a 4 hour accreditation council for pharmacy education (ACPE) class and gain full understanding of the detailed protocol for naloxone rescue kit prescribing [74]. Maintaining the certification also requires that pharmacist must then complete two hours live (ACPE or equivalent) continuing medical education every two years on the topic of opioid use /abuse [74].
Cost of naloxone rescue kits or take-home naloxone; (N=3).
Currently in Australia when naloxone is sold over-the-counter there is no mechanism to subsidise the cost [38]. As these pharmacies operate as independent businesses, there is a requirement to cover cost of both the medicine and the staff time in counselling and supplying naloxone, both of which is currently covered by the consumer [38]. In New Mexico required state Medicaid programs cover naloxone rescue kits, however for patients who are not covered by Medicaid a naloxone rescue kit might be cost prohibitive [74]. In Canada although nearly all pharmacies in jurisdictions where cost is reimbursed by the provincial government dispensed naloxone free of charge, jurisdictions not covered by government reimbursement were not able to do same [74].
Lack of appropriate take-home naloxone products suitable for over-the-counter dispensing, (N=2)
According to Pricolo and colleagues [38], in some Australian jurisdictions the lack of an appropriately packaged product still prevents pharmacist from supplying naloxone despite the rescheduling. For S3 ’pharmacist only medicines’ it is required as per the TGA (Therapeutic Goods Administration) that the information on the label of the package must enable the consumer to use the medication safely and effectively, be able to readily find the information they need, understand and act on it appropriately and access further information if they require, [38]. There were jurisdiction differences in these requirements in the labelling for pharmacist only medicines however, generally the need for ‘’adequate directions for use’’ to be contained in or on the packaging is required [38].
The availability of user-friendly take-home naloxone formulations was also identified to be a barrier to access during implementation [74]. This study by Cressman and colleagues [74] also identified that more user-friendly formulations example naloxone nasal spray, could improve uptake and demand, reduce the need for intensive training and the risk of needle stick injury as well as increase the efficacy with which the drug is administered [74]. However available injectable forms of naloxone are cheaper than the nasal formulations thus the cost is likely to deter many opioid users as it is not cost effective [74].
Legal considerations, (N=1)
In studying the association between state laws facilitating pharmacy distribution of naloxone and the risk of fatal overdoses, it was evident that, the details of the law itself is important, (Abouk et al, 2019). There was evidence that showed that, states that adopted naloxone access laws granting direct authority to pharmacies to supply naloxone resulted in significant decline of fatal opioid related overdoses, (Abouk et al, 2019). On the other hand, naloxone access laws that did not grant direct access to pharmacist to dispense naloxone appeared not to be associated with decreases in mortality, (Abouk et al, 2019). Therefore, permitting pharmacist to dispense directly under their own authority maximized the potential benefits of the policy [73].
Attitude of pharmacist relating to stocking of take-home naloxone; (N= 1)
There was non-availability of take-home naloxone in community pharmacies in Canada despite its non-prescription status [37]. This was due to many pharmacists perceiving a lack of demand for naloxone relating to perceived stigma among people with opioid use disorder [37]. Additionally, there is the perception that people on high doses of prescribed opioid as analgesia are not at risk of opioid overdose fatality [37].
Discussion
This article is a systematic review that set out to analyse existing literature on over-the-counter distribution of take-home naloxone as a medium of distribution that can enhance easy accessibility to naloxone, a safe and effective opioid antagonist. The main aim of the study was to establish the feasibility and acceptability of over-thecounter distribution of take-home naloxone. The objectives were to determine the willingness, acceptance, attitude and perceptions of both pharmacist and individuals at risk of opioid overdose on over-thecounter distribution of take-home naloxone. Additionally, laws and policies that have been put in place towards enhancing this medium of distribution of take-home naloxone was analysed to ascertain its effectiveness. Literature that covers implementation and evaluation of over-the-counter distribution of take-home naloxone programmes to give an overview of the expected and real challenges experienced by opioid dependents, their families and pharmacist was also included in the analysis for this study.
Findings from this systematic review suggest that, over-thecounter distribution of take-home naloxone is generally an acceptable and feasible intervention among individuals at risk of opioid overdose fatalities and pharmacist. The implementations of over-the-counter distribution of take-home naloxone in countries that have legalized it have registered some successes, as well as, practical challenges. The paragraphs below highlight the findings of this study that further confirms existing literature on the topic under discussion.
Summary of the Findings
The results of the articles were classified into two broad categories after thematic analysis;
1. Studies discussing the acceptability of over-the-counter distribution of take-home naloxone, which indicates that a general increase in the willingness of pharmacist to get involved with over the counter distribution of take-home naloxone, as well as, the willingness of the at risk population to solicit for naloxone through this same medium of distribution from the pharmacist, (N=3; Pricolo and Nielson [38], Donovan et al.[72], Nielsen et al. [54]).
2. Studies discussing the feasibility of over-the-counter distribution of take home naloxne and highlights experienced challenges such as; cost of naloxone, lack of appropriately packaged naloxne products for over-the-counter distribution, training and certification of pharmacist and legal considerations on this route of distribution of naloxone, (N=4; Abouk et al. [73], Bachyrycz et al. [74], Pricolo & Nielson [38], Cressman et al. [37]).
Discussion of the Findings
Willingness to distribute take-home naloxone over-thecounter
The analysis of articles that covered the willingness of pharmacist to involve in take-home-naloxone as an intervention indicate an increased interest in such interventions. Pharmacist have increased interest in engaging in harm reduction interventions that are pharmacy based, an example being over-the-counter distribution of take-home naloxone [75]. Resultantly there has been an increase in the percentage of pharmacies stocking and selling naloxone without prescription in countries that have legalized it. This further extends the findings of recent studies indicating that, providers’ willingness to involve in harm reduction programmes have increased over time from the early 2,000’s to today [6]. This increased willingness is parallel to increasing attention and concern regarding the opioid crisis and confirms the role of pharmacist in the era of the opioid epidemic as indicated in existing literature [6].
The above finding is in line with existing literature on the involvement of pharmacist in various levels of opioid dependence treatment. In many countries’ pharmacists are considered the most accessible health care providers due to their unique location in the communities especially in rural areas and are essential in helping to reduce the mortality and morbidity of the opioid overdose crisis [76]. Pharmacist is notably involved in numerous care activities that are related to curbing the opioid crisis [76]. For instance, in the United States of America, one of the roles of pharmacist is the screening of potential opioid dependents and referring them to specialist treatment [77]. Several harm reduction programmes are also rolled out in pharmacies such as, sterile syringe provision through the needle exchange programme and naloxone prescribing and distribution under other forms of distributive arrangements [74, 77, 78]. Additionally, pharmacist in many states in the United States of America are also actively involved in other opioid use disorder treatment namely; opioid overdose education and naloxone distribution programmes (OEND), medication assisted treatment (MAT), opioid substitution therapies (OST) and supervising consumption rooms [76]. Study shows that, pharmacists who are more willing to be involved in over-the counterdistribution of take-home naloxone are those that are already involved in providing other harm reduction programmes [79].
Additionally, literature shows that individuals at risk of opioid overdose fatalities are also willing to solicit for naloxone from the pharmacist, considering it is some form of empowerment to prevent their own overdose and to intervene in an overdose situation witnessed [72]. Over-the-counter distribution of take-home naloxone would also allow family members and friends to access the medication[72]. Various levels of motivation that can influence the purchase of pharmacy-based naloxone including individual, interpersonal, community and societal influences have been enumerated[72]. Nonetheless, experienced challenges from programme evaluations highlights existing barriers to this novel mode of distribution of take-home naloxone. Existing laws that regulate the storage, distribution and usage of naloxone products continues to pose as a barrier to its accessibility [73]. Whereas some countries have taken steps to effect changes in these laws to enable widespread availability of naloxone, the high cost of the medication as well as the lack of appropriate packaging that fits an over-thecounter status defeats the purpose of rescheduling [37, 38, 74]. Despite the increased willingness on the part of pharmacist to be involved in this intervention, there is generally low level of confidence due to lack of knowledge around naloxone and effective use among the atrisk population [74]. Existing training and certification programmes that could bridge this knowledge gap for pharmacist are considered erroneous, [37, 38, 74]. This influences stocking of naloxone, as well as, enabling appropriate engagement with individuals who are identified by pharmacist to be at risk of opioid overdose fatalities [74]. These identified challenges during programme implementation are fully discussed in later paragraphs of this article.
The increased interest among pharmacist in involvement in harm reduction programmes coupled with, the willingness of the at-risk population to solicit for naloxone from pharmacies offers a window of opportunity to rigorously incorporate interventions such as overthe- counter distribution of take-home naloxone. This will lead to an increased accessibility to naloxone and decrease the rising statistics of opioid overdose fatalities.
Cost of naloxone rescue kits or take-home naloxone
This review found that the cost involved in distributing take-home naloxone over-the-counter remains a major barrier. The variability in coverage of take-home naloxone by insurance, price of available takehome naloxone formulations and lack of reimbursement of time and resources spent by pharmacist in educating patients during naloxone purchase leads to it being cost prohibitive [75]. This variability in cost is due to governmental decision making through policies that affect the pricing of naloxone products, the need to cover cost of production by pharmaceutical manufacturers and consideration of other factors at wholesale and retail pharmacy level that influences pricing [37]. This finding on cost as a prohibitive factor in the implementation of takehome naloxone is in tune with existing literature [37].
The literature on the demographics of drug users indicates that, although illicit drug is used by people of all socioeconomic strata, drug-related morbidity and mortality are disproportionately higher Cost of naloxone rescue kits or take-home naloxone This review found that the cost involved in distributing take-home naloxone over-the-counter remains a major barrier. The variability in coverage of take-home naloxone by insurance, price of available takehome naloxone formulations and lack of reimbursement of time and resources spent by pharmacist in educating patients during naloxone purchase leads to it being cost prohibitive [75]. This variability in cost is due to governmental decision making through policies that affect the pricing of naloxone products, the need to cover cost of production by pharmaceutical manufacturers and consideration of other factors at wholesale and retail pharmacy level that influences pricing [37]. This finding on cost as a prohibitive factor in the implementation of takehome naloxone is in tune with existing literature [37]. The literature on the demographics of drug users indicates that, although illicit drug is used by people of all socioeconomic strata, drug-related morbidity and mortality are disproportionately higher among the lower socioeconomic status group [80]. Thus people who are prone to opioid dependence and prone to opioid overdose are usually of the low socio economic status and this is one of the reasons for which the cost of naloxone may prohibit the likelihood of accessing this form of harm reduction strategy. Studies indicate a link between opioid addiction and socioeconomic status, where the abuse of both prescription and illegal opioids may be highest among the poor. For instance, in the United States of America, statistics from the centres for disease control and prevention states that, as of 2011 the rate of overdose deaths from opioid prescription drugs were highest in states with higher poverty level.
The possible explanation for this prevalence amongst the poor includes, inadequate follow up care for low income patients being treated for chronic pain at publicly funded clinics, self-medication for the stress and depression associated with poverty, limited access to opioid treatment programmes and rehabilitation facilities. Substance misuse, mental illness and homelessness has also been shown to cooccur making this more complex a situation to deal with [80]. Most frequently, the lack of health insurance and or financial resources is cited as a barrier to accessing treatment for addiction in national surveys conducted in the United States of America [80]. In this context of the complexity of the addiction problem and the socioeconomic status of individuals at risk of opioid overdose, the cost of take-home naloxone products will pose a barrier to increase its accessibility to this group of people who need it most.
For instance, according to the 2018 data collection on prices of naloxone products in Europe by the European Monitoring Centre for Drugs and Drug Addiction, the price of a single-dose generic injectable naloxone varied between €2 and €3 resulting in a cost of between €5 and €10 per overdose kit, which typically contains two ampoules and syringes [81]. Naloxone kits containing a syringe pre-filled with 2ml of naloxone solution providing up to five individual doses (for use during one emergency) were reported to cost between €35 and €45 [81]. When a mucosal atomizer device is provided in combination with naloxone the price per kit increases by about EUR €5 [81]. During the nasal naloxone trial in France, the product came in a pack containing four nasal spray devices delivering 0.9mg/0.1ml each and the medication was provided for free by the pharmaceutical company that produced them during the trial, however a price of €100 per pack following the trial was anticipated [81]. A new nasal spray delivering 1.8mg of naloxone with two nasal spray as device per pack (for administration in one emergency) is marketed at variable prices ranging from €23 to €53 [81].
In addition to the cost being a barrier, cover of cost by insurance that sometimes exist for known opioid users does not extend to family members and friends who purchase naloxone to help with a witnessed overdose situation. However, literature indicates that people likely to witness an overdose are opioid dependents themselves, their friends and families [45,46]. The other group of people who are likely to witness an overdose are those whose work brings them into contact with people who are likely to overdose namely; healthcare workers, police, emergency service workers, people providing accommodation to people who use drugs, peer education and outreach workers. In some countries, this latter group of people are covered by laws that allow them to acquire naloxone and administer them when needed at no cost [48,81]. Unfortunately, this does not cover family members and friends who are likely to be the first to witness an overdose situation before summoning for help from first responders indicated above.
Given that individuals at risk of opioid overdose fatalities particularly illicit opioid users face complex socio-economic challenges especially financial constraints, there is the need for comprehensive cost cover, insurance and reimbursement procedure, to enhance the uptake of naloxone in such novel interventions aimed at increasing its accessibility. Additionally, cover for cost should include close family members and friends who are the most likely group of people to witness an overdose situation.
Lack of appropriate take-home naloxone products suitable for over-the-counter dispensing
One of the findings of this review was the non-availability of naloxone formulations suitable for over-the-counter dispensing. Naloxone in the form of nasal spray is deemed suitable for overthe- counter dispensing as it makes education on effective use less complicated, as well as resulting in less risk associated with needle prick injuries, however it is expensive [37]. Additionally, the lack of appropriately packaged naloxone kits conforming with standardized ‘pharmacist only’ medicines and labels that contain the necessary information to the user defeats the purpose of rescheduling of naloxone to an over-the-counter status in countries who have legalized this form of intervention [38].
Generally, an over-the-counter drug is considered safe and effective for use by the general public without a prescribers’ authorization [82]. Globally over-the-counter drugs are an important class of medications as they reduce the burden on prescribers and healthcare facilities on the need to attend to a range of ailments that patients can otherwise solicit medications over-the-counter for adequately and safely [83]. The necessary characteristics for an over-the-counter drug include; an acceptable safety margin for the drug, low misuse and abuse potential of the drug under conditions of widespread availability, not requiring supervision by a healthcare professional for its use and adequate labelling, with emphasis on standardized labelling that provides the consumer with vital information [82]. The information necessary to be displayed include, the product’s active ingredient, the purpose of the product, the uses or indications for the product, specific warnings including contra-indications and when there is the need to consult a doctor or a health professional. Additionally, the dosage instructions and the product’s inactive ingredients are supposed to be listed in case of allergies and sensitivities [82].
Existing formulations of naloxone products in the form of takehome naloxone may not fully meet the criteria for over-the-counter distribution and there maybe the need for modifications for it to fit this status. Currently, naloxone is available in injectable formulations for intramuscular, intravenous and subcutaneous administration [84]. Although not approved by the food and drugs authority in certain countries, the practice of using an atomization device to deliver the injectable solution through the nasal route has also been reported and studied [84-86].
From the year 2016, the nasal spray formulation of naloxone, a needleless device that delivers a fixed intranasal dose of naloxone was approved for use by countries like Canada, France and the United States of America [81]. The nasal spray was intended for use safely by two main user populations; lay people (a patients, family friend or other family member of a known or suspected opioid overdose victim) and authorized first responders (fire fighters, paramedics, emergency medical technicians or police officers) who are likely to be called out to an opioid overdose situation after being witnessed by the first category of witnesses as mentioned above [84, 87]. The nasal spray formulation is intended to be more convenient for laypersons as it could potentially decrease the risk associated with the use of needles such as needle stick injuries and exposure to blood-borne virus [84, 87]. However, the effectiveness of the intranasal formulations maybe be limited in individuals with nasal abnormalities [84, 87]. Additionally, the cost of intranasal naloxone is estimated to be approximately four times higher than a take-home naloxone kit that contains naloxone and other kit components such as syringes, gloves, mask and others [87].
Even though naloxone is proven to be a safe drug to use with low abuse potential, its safety of use without adequate training or supervision in relation to injectable formulations may inhibit its status as an over-the-counter medication. The intranasal formulations that could possibly reduce this associated risk of needle stick injury have concurrent limitations regarding its route of absorption in addition to cost as a major hindrance.
Training and certification / Licensing of pharmacist
One of the challenges experienced during implementation of take home naloxone programmes identified in this study is that, the process of training and licensing of pharmacist to equip them with adequate knowledge and authority to feel confident and qualified to engage in this novel mode of distribution of naloxone is considered too elaborate [37]. The erroneous process of licensing and certification as well as lack of collaboration between manufacturing companies and professional bodies to roll out uniform and appropriate training protocols is identified as a major difficulty [38,74].This leads to continued trend in the perceived stigma experienced by individuals with opioid dependence from pharmacist [37].
However literature shows that, pharmacists with specialty training and certification in opioid overdose treatment are able to work in fully integrated interdisciplinary care teams to design, implement, monitor, and modify evidence-based pharmacotherapy-centred opioid use disorder care plans [76]. Research has shown that pharmacists can effectively engage patients with opioid use disorder to improve their treatment outcomes given that they have the requisite training and certification that allows them to confidently engage in these form of service provision [28, 52, 88]. In turn, patients have reported positive attitudes when a pharmacist was included in their opioid use disorder treatment care [79, 89]. There have been calls for pharmacists to advocate for the ability to be independent opioid use disorder treatment providers through provider status coupled with waiver training that currently only physicians, physician assistants, and nurse specialists may obtain [76].
There is the need for collaboration between manufacturing companies and supervisory professional bodies to develop effective training resources and to develop certification and licensing procedures that are less complicated and yet up to standard and can be more easily acquired as a prerequisite for engagement in other novel models of treatment such as over-the-counter distribution of takehome naloxone. The existence of less cumbersome and long training, certification and licensing procedures will also provide the opportunity for pharmacist to gain an in-depth knowledge and better understanding of issues related to opioid dependency and related complications like overdose deaths. This will resultantly have a positive impact on issues related to perceptions of pharmacists in relation to stocking naloxone products in their pharmacies.
Legal considerations
The findings in this systematic review on legal issues in relation to take-home naloxone and its distribution over-the-counter conform to existing concerns raised in other studies. In view of the opioid crisis and calls by the international community on the need to make naloxone accessible, some countries have formulated and implemented policies and legislations that allows pharmacist to dispense naloxone under their own authority without the need for prescription [73]. These legislations have recorded decrease in opioid overdose fatalities in areas where they are being utilized [38,73]. Evidence shows that although naloxone access laws have the potential to improve naloxone access and save lives, the details of the law itself matters, in that, in detail only laws that gave pharmacist the ability to dispense under their own authority reported significant decreases in overdose deaths [73].
Literature available indicates that, the successful implementation of take-home naloxone programmes involves widespread distribution of the medication [90,91], however take-home naloxone programmes still face practical implementation problems due to regulatory hurdles in most countries [15]. Naloxone has been classified as an over-thecounter medication since 1996 as well as Canada, Australia and France where it is now possible to obtain some forms of naloxone in pharmacies without a prescription [51,81]. In the United States of America pharmacies in most states can issue naloxone based on standing orders and do not require specific prescription [81]. Except for these countries mentioned above, take-home naloxone can only be obtained from a pharmacy with a prescription [15,51,81].
Several strategies have been employed by different countries in terms of legislations to improve distribution and access to take-home naloxone:
• In Italy naloxone distribution started as a form of local initiatives in 1991, however the reclassification of naloxone in 1996 as an over-the-counter drug permitted a wider range of providers involved in the distribution of the medication [81]. The most targeted individuals for naloxone training and distribution programmes were people who use drugs and their peers, as well as, harm reduction staff and outreach workers [81]. There has been significant increase in the development of training materials, training delivery to targeted population and the number of naloxone kits distributed [81].
• To solve the problem of bottle necks, France enlarged the range of providers that can give naloxone in 2017 [81]. There was an extension of providers from the hospital setting only to drug treatment centres and community-based harm reduction agencies [81]. Thus, there is no requirement for a prescription for the acquisition of naloxone from these agencies. From June 2019, naloxone in prefilled syringes can be obtained from pharmacies without prescription [81].
• In the United Kingdom, naloxone has been recognised since 2005 as an emergency medication to which broad access (under ‘’duty of rescue’’ obligations) should be ensured. While naloxone remains a prescription only drug and cannot be sold over-the-counter, it can be lawfully administered as a life saving measure by any member of the public [81]. Since 2015, the United Kingdom updated its ‘’Human Medicines Act’ that has allowed staff at drug treatment agencies to give out take-home naloxone without a prescription to individual who may need it to save a life.
• In Denmark since 2018, patients in opioid dependent treatment have been able to receive a prescription for the medication which allows the cost to be reimbursed by health insurance [81].
• In response to significant increases in opioid overdose related deaths in the United States of America, special efforts to simplify and improve naloxone availability through pharmacy dispensing were made [51]. In the United States of America many states have changed their policies and legislation, for example in 2017, 44 states permitted naloxone to be prescribed to a person with whom the prescriber does not have a patient relationship (third party prescribing) and state laws authorised the lay administration of naloxone [51]. Pharmacy naloxone dispensing is strongly encouraged and most states have laws to protect healthcare professionals who prescribe and dispense naloxone from civil and criminal case, as well as Good Samaritan laws to protect people who administer naloxone or call for help during an opioid overdose emergency [51,92].
• In Canada the prescription status of naloxone was changed in March 2016 to increase access to naloxone, thereby allowing pharmacies to be able to dispense nasal naloxone to those in need and emergency responders also being able to use it without a prescription [92,93].
Statistics available show that, there have desired decreases achieved in opioid overdose deaths in countries that have laws granting pharmacist authority to dispense naloxone through interventions such as take-home naloxone, for instance Italy [81]. Comparatively, in countries where there is some form of laws that allows dispensing arrangements in place but does not allow pharmacist to dispense under their own authority there are still high numbers of opioid overdose situations being recorded, for example United Kingdom [81]. Deductively, whiles it is important for policies and legislations to be formulated to curb the opioid crisis, the extent to which the laws affect the production, distribution and usage of naloxone should be taken into consideration.
Limitations
Limitations of studies used for this review Over-the-counter distribution of take-home naloxone is a new intervention, as such not many studies were found on this topic, and thus the results presented in this systematic review may not cover all the issues that may appear in future. The few articles that were identified and articles analysed for this systematic review had some limitations as follows:
• For most of the studies, data on naloxone dispensing was collected by self-reports from pharmacists, pharmacy managers and pharmacy staff, therefore, recall bias is possible. Additionally, questions on estimated sales of naloxone are not as strong as actual data on sales and there is the likelihood of either an underestimation or overestimation of the statistics on sales.
• Studies that centred on naloxone access laws were limited by the number of countries that have passed these laws and therefore have evidence of its effectiveness.
• Articles selected were qualitative studies and the analyses maybe a representation of the beliefs of a small subset of the population of interest which limits the generalizability of study results.
• Due to necessary limits on the length and nature of online data collection, a detailed exploration of the reasons behind attitudes and perceptions of study participants is not always possible. However, most of the studies in this review used online data collection process.
Limitations of this systematic review
This systematic review has some limitations that need to be addressed in a future attempt of this topic. The sample size is relatively small as only seven articles met the inclusion criteria. These studies were also limited to United State of America, Canada and Australia, thus limiting the generalizability of the results of this study. The ability for database searches to retrieve relevant articles depends on how accurately a study is categorised by its keywords. The keywords for this study were narrowed to opioid overdose deaths and over-the-counter distribution of take-home naloxone and may have limited the number of relevant articles that were retrieved.
The articles used in the analysis focused solely on feedback from patients and pharmacist who is involved with take-home naloxone interventions, therefore the views of the general population on such programme implementation may not have been captured. The search for articles in other sources of data, for instance websites of organisations and individuals who may be involved with substance use disorder treatment by virtue of other activities other than being the at-risk population or professionals in the frontline of service delivery maybe be necessary. This form of a wider search of other websites may have generated results on opinion of the general public on this form of intervention.
The research methods used in the studies used for tis systematic review are weak in strength and low in quality. Broadly, study methods were surveys (online and telephone based), semi-structured interviews and programme evaluations. The variations in the methodology and analytical methods used in the articles identified for this systematic review did not allow for a detailed assessment of the efficacy of overthe- counter distribution of take-home naloxone.
Future direction
To strengthen the results of studies on this topic, there is the need for further research into this novel intervention. Specifically, on-going quality monitoring and evaluation procedures should be a core component of over-the-counter distribution of naloxone programme implementation. This will help rigorous assessment of the effectiveness as well as challenges with this form of intervention. Data on the effectiveness of this intervention will allow the expansion of programmes to other countries appropriately. Additionally, future programme implementation can be amended, and strategies enhanced based on results that highlights challenges with ongoing programmes.
Conclusion
The results of this systematic literature review conducted indicate that over-the-counter distribution of take-home naloxone is acceptable and feasible. However, the challenges experienced during implementation have varying implications for the various stakeholders that are involved in the provision of this intervention, that is, law and policy makers, pharmacist and individuals who are at risk of opioid overdose deaths as well as their close friends and families. Coordination across agencies to address key barriers to widespread access to naloxone supply is critical to maximise the potential of this life saving medication.
Naloxone’s status as a prescription medication in many countries continues to pose as a major barrier to its access. Attempts made by various countries to amend naloxone access laws are an effort to make the medication available to the recipient with the same or nearly same ease as an over-the-counter medication. While there are several avenues for the Food and drugs Authorities in various countries to move some formulations of naloxone to an over-the-counter status, the process requires a significant amount of investment of resources in the form of time and capital. There should be dedicated funding from Government and private sector organizations to undertake the necessary research for data that will provide concrete evidence base for Food and Drug Authorities to confidently switch naloxone to an overthe- counter status via existing or amended regulations. For countries considering rescheduling, working collaboratively with commercial producers of naloxone may facilitate the availability of an appropriately packaged product.
The cost of naloxone products continues to limit efforts made towards making the medication accessible. There is the need for strategies to help reduce the burden of cost on the at risk-population and their families. Governmental and private sector collaboration should consider programs that ensure naloxone products intended for distribution are financially accessible to both individual drug users and agencies. This will allow easy uptake of the medication for direct use by opioid dependents or agencies that are involved in the provision of harm reduction interventions who may need to purchase the medication for the purposes of re-distribution to at risk population. There should be requirement by both public and private health insurance plans to cover naloxone at little or no cost to the recipient, both when the medication is meant for the individual drug user procuring it or for a family member or peer. Moreover, if an overthe- counter version of naloxone becomes available, the government should ensure that it remains financially accessible by requiring that private and public insurance continue to cover it on the same basis as prescription medication. There should be a comprehensive and easily accessible data-base on the details of individuals who are dependent on opioids and their close family members who are likely to procure naloxone for the purpose of intervening in an overdose situation.
There is the need for professional bodies and other agents involved with naloxone production to collaboratively develop and implement less cumbersome but effective training and licensing programmes that will allow pharmacist to gain the requisite knowledge to enable them feel confident to get involved in over-the-counter distribution of takehome naloxone. There will be increased awareness on the burden of the opioid epidemic and the need for increased accessibility to naloxone through this training programme, which will in turn have a positive effect on attitudes towards stocking of the medication in pharmacies. There is the need for the development of consistent messaging and best practice guidelines for pharmacies who are new to naloxone supply. Pharmacy-based harm reduction interventions are a potential foundation and catalyst for pharmacist to play a more central role as facilitators and advocates for strategies towards curbing the opioid epidemic. In many countries, pharmacies are a major point of opioid use disorder treatment interventions and co-locating naloxone access alongside such provision is in line with pharmacy harm reduction services and generally supportive attitudes of pharmacists towards harm reduction.
Over-the-counter distribution of take-home naloxone is an effective novel intervention that will increase the accessibility of the lifesaving medication to resultantly reduce the rate of opioid overdose deaths. Whiles this harm reduction strategy may not be the total solution to the issues of accessibility of naloxone, it will contribute to the aim of reducing the rising statistics of opioid overdose fatalities.
Acknowledgement
My profound gratitude to Dr. Anna Williams for her unwavering support, Dr. Frank Baning (my personal motivator), and Mr. Martin McIntyre (my professional mentor), for their support and encouragement. Both authors contributed equally.
Conflict of Interest
None
References
- Meyer JS and Quenzer LF (2005) Psychopharmacology; drugs, the brain and behaviour. Sinauer Associates, Sunderland, Massachusetts. 11: 248-272.
- Narita M, Funada M, Suzuki T (2001) Regulations of opioid dependence by receptor types. Pharmacology and therapeutics 89:1-15.
- Kling MA, Carso RE, Borj R (2000) Opioid receptor imaging with positron emission tomography and [(18)F]cyclotoxy in long-term methadone-treated former heroin addicts. J Pharmacol Exp Ther 295: 1070-1076.
- Robinson TE, Berrigde KC (2001) Incentive sensitization and addiction. Addiction 96: 103-114.
- Bruton L, Lazo L, Parker K (2005) Goodman & Gilman’s; The pharmacological basis of therapeutics. New York, McGraw-Hill 590, USA.
- Behar M, Bagnulo R, Coffin PO (2018) Acceptability and Feasibility of Naloxone prescribing in primary health care settings: A systematic Review. Prev Med 114: 79-88.
- Lewanowitch T, Miller JH, Irvin R (2006) Reversal of morphine, methadone and heroin induced affects in mice by naloxone methiodite. Life Sciences 78: 682-688.
- Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S (2006) Prescribing Naloxone to Actively Injecting Heroin Users: A Program to Reduce Heroin Overdose Deaths. J Addict Dis 25: 89-96.
- UNODC (2018) World drug report 2018: Drug related mortalities. Press Release, USA.
- IASP (2019) IASP Statement on OPIOIDS. USA.
- European Monitoring Center for Drug DA (2017) United Kingdom Drug Report 2017.
- Blumberg H, Dayton HB, George M, Rapaport DN (1961) N-allyinoroxymorphine: A potent narcotic antagonist. Fed Proc 20: 311.
- Lewenstein MJ, Fishman JJ (1966) Morphine derivative: US patent 3. 254: 088.
- Garfield E (1983) The 1982 John Scott award goes to Jack Fishman and Harold Blumberg for synthesis and investigation of Naloxone. Essays Inf Sci 6:121-130
- WHO (2011) Comparative Table of Core and complementary Medicines on Who Essential Medicines List from 1977-2011?.
- Akil H, Mayer DJ, Liebesland JC (1976) Antagonism of Stimulation -produced analgesia by naloxone, a narcotic antagonist. Science Direct 191: 961-962.
- Berglow P (2018) Commentary; The opioid overdose epidemic; evidence-based interventions. Am J Addict 27: 605-607.
- Strang J, Farrell M (1992) Harm Minimization for drug misusers. Bio Med J 304: 1127.
- Strang J, Darke S, Hall W, Farrell M, Ali S R (1996) Heroin overdose: The case for take-home naloxone. Bio Med J 312: 1435 -1436.
- Donald R, Campbell ND, Strang J (2017) Twenty years of take-home naloxone for the prevention of overdose deaths from Heroin and other opioids - conception to maturation. Drug Alcohol depend 178: 176-187.
- United Nations Office on Drugs and Crime (2013) Opioid overdose: preventing and reducing opioid overdose mortality.
- WHO (2013) Opioid Overdose: Preventing and Reducing Opioid Overdose Mortality.
- Marsdern J, Stillwell G, Jones H, Cooper A, Eastwood B, et al. (2017) Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction 112: 1408-1418.
- Bird SM, Fischbacher CM, Graham L, Fraser A (2015) Impact of opioid substitution therapy for Scotland’s prisoners on drug related deaths soon after release. Addiction 110: 617-624.
- Clark AK, Wilder CM, Winstanley EL (2014) A Systematic Review of Community Opioid Overdose Prevention and Naloxone Distribution Programs. J Addict Med 8:153-163.
- Oslen AM, David LS, Dietze P (2015) Independent evaluation of the implementing of expanded Naloxone Availability in the ACT (1-ENAACT) Program, 2011-2014. Canberra ACT, USA, 2015.
- McDonald, R, Strang J (2016) Are Take Home Naloxone programmes effective; systematic review utilising application of the BRADFORD Hill criteria. Addiction 111:1177-1187.
- Green TC, Dauria EF, Bratberg J Davis CS, Walley AY (2015) Orienting patients to greater opioid safety: models of community pharmacy-based naloxone. Harm reduct J 12:25.
- McAuley A, Lindsay G, Woods M, Louttit D (2010) Responsible management and use of a personal take-home naloxone supply: A pilot project. Drugs Educ Prev Policy 17: 388-399.
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ (2015) Opioid Overdose Prevention Programs Providing Naloxone to Laypersons - United States, 2014. Morb Mortal Wkly Rep 64: 631-635.
- Walley AY, Xuan Z, Hackman HH, Emily Q, Sarah R, et al. (2013) Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. Bio Med J 346: f174.
- Paone D, Heller D, Olson C, Kerner B (2010) Illicit Drug Use in New York City.
- Bennett AS, Bell A, Tomedi L, Hulsey EG, Kral AH (2011) Characteristics of an overdose prevention, response, and naloxone distribution program in Pittsburgh and Allegheny County, Pennsylvania. J Urban Health 88: 1020-1030.
- Enteen L, Bauer J, Rachel ML, Eliza W, Emalie H, et al. (2010) Overdose prevention and naloxone prescription for opioid users in San Francisco. J Urban Health 87:931-941.
- Wilson JD, Spicyn N, Matson P, Alvanzo A, Feldman L (2016) Internal medicine resident knowledge, attitudes, and barriers to naloxone prescription in hospital and clinic settings. Subs Abus 37: 480-487.
- Mayer S, Manning V, Williams A, Loaring J, Strang J (2011) Impact of training for healthcare professionals on how to manage an opioid overdose with naloxone: Effective, but dissemination is challenging. Int J Drug Policy 22: 9-15.
- Cresmann AM, Mazereeuw G, Guan Q, Wenting J, Gomes T, et al. (2017) Availability of naloxone in Canadian pharmacies: a population-based survey. CMAJ Open 5:E779-E784.
- Pricolo A, Nielsen S (2018) Naloxone Rescheduling in Australia: Processes, Implementation and Challenges with supply of naloxone as a ‘pharmacist only’ over-the-counter medicine. Drug Alcohol Rev 37: 450-453.
- Strang J, Kelleher M, Best D, Mayet S, Manning V (2006) Emergency naloxone for heroin overdose should it available over the counter?. Bio Med J 333: 614-615.
- Alcorn T (2014) American embraces treatment for opioid drug overdose. Lancet 383: 1957-1958.
- Sporer KA, Kral AH (2007) Prescription naloxone: a novel approach to heroin over-dose prevention. Ann Emerg Med 49: 172-177.
- Doyon S, Aks SE, Schaeffer S (2014) Expanding access to naloxone in the United States. American journal of clinical toxicology. Clin Toxicol (Phil) 52: 989-992.
- OSF (2013) Widening the Net of Naloxone Prescribers-Standing order model.
- Tobin KE, Davey MA, Latkin CA (2005) Calling emergency medical services during drug overdose: an examination of individual, social and setting correlates. Addiction 100: 397-404.
- Darke S, Ross J, Hall W (1996) Overdose among heroin users in Sydney, Australia: II. Responses to overdose. Addiction 91: 413-417.
- Lagu T, Anderson BJ, Stein M (2006) Overdoses among friends: Drug users are willing to administer naloxone to others. J Subst Abuse Treat 30:129-133.
- Lenton SR, Hargreaves KM (2000) Should we conduct a trial of distributing naloxone to Heroin users for peer administration to prevent fatal overdose. Med J Aust 173: 260-263.
- WHO (2014) Community Management of Opioid overdoses. USA.
- Strang J, McDonald R, Campbell ND (2017) Twenty years of take-home naloxone for prevention of overdose death from heroin and other opioids-conception to maturation: a review. Drug alcohol depend 178: 176-187.
- CCSA, (2016). CCENDU Bulletin − the Availability of Take-Home Naloxone in Canada.
- EMCDDA (2016) Insights: Preventing Overdose Deaths from Heroin and other opioids; Pre-provision of emergency Naloxone (take-home-naloxone). EMCDDA, Lisbon.
- Meyerson BE, Agley J, Davis A, Jayawardene W, Hoss A, et al. (2018) Predicting pharmacy naloxone stocking and dispensing following a state-wide standing order, Indiana 2016. Drug Alcohol Depend 188:187-192.
- Public Health England (2016) Widening the Availability of Naloxone.
- Nielsen S, Menon N, Larney S, Farrell M, Degenhardt L (2016) Community pharmacist knowledge, attitudes and confidence regarding naloxone for overdose reversal. Addiction 111: 2177-2186.
- Strang J, McDonald R (2016) Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill Criteria. Addiction 111: 1177-1187.
- Kelensky T, Walley AY (2017) Opioid overdose prevention and naloxone rescue kits: What we know and what we don’t know: Review. Addict Sci Clin Pract 12:4.
- Lewis RC, Vo HT, Fishman M (2017) Intranasal naloxone and related strategies for opioid overdose intervention by non-medical personnel; a review. Subst Abuse Rehabil 18: 79-95.
- Lambdin BH, Zibbel J, Wheeler E, Kral AH (2008) Identifying gaps in the implementation of naloxone programs for laypersons in the United States. Int J Drug Policy 52:52-55.
- Gunn AH, Smothers ZP, Schramm-Sapyta N, Freiermuth CE, MacEachern M, et al. (2018) The emergency department as an opportunity for naloxone distribution: A systematic review. West J Emerg Med 19:1036-1042.
- Coffin PO, Sullivan SD (2013) Cost effectiveness of distributing naloxone to heroin users for lay overdose in Russian cities. J Med Econ 16: 1051-1060.
- UNODC (2012) Resolution 55/7.
- Papastergiou J, Folkins C, Li W, Zervas J (2014) Community Pharmacist-Administered Influenza Immunization Improves Patient Access to Vaccination. Can Pharm J (Ott) 147: 359-365.
- Willis E, Rivers P, Gray LJ, Davies M, Khunh K (2014) The Effectiveness of Screening for Diabetes and Cardiovascular Disease Risk Factors in A Community Pharmacy Setting. PLoS One 9: e91157.
- Lindsey L, Husband A, Nazar H, Todd A (2015) Promoting the Early Detection of Cancer: A Systematic Review of Community Pharmacy-Based Education and Screening Interventions. Cancer Epidmiol 39: 673-681.
- Bleake BE, Dillman NO, Corneliu D, Ward JK, Burson SC, et al. (2014) Heart Failure Assessment At The Community Pharmacy Level, A Feasibility Pilot Study. J Am Pharm Assoc 54: 634-641.
- Taitel M, Cohen E, Duncan I, Pegus C (2011) Pharmacist as Providers: Targeting Pneumococcal Vaccinations to High Risk Populations. Vaccine 29: 8071-8076.
- Anderson C, Blenkinsopp A, Amstrong M (2004) Feedback from Community Users on the Contribution of Community Pharmacy to Improving the Public’s Health: A Systematic Review of the Peer Reviewed and Non-Peer Reviewed Literature 1990-2002. Health Expect 7: 191-202.
- Ayorinda AA, Porteous T, Sharma P (2013) Screening for Major Diseases in Community Pharmacies: A Systematic Review. Int J Pharm Pract 21: 349-361.
- Duvivier H, Gustafson S, Greutman M, Jangchup T, Harden KA, et al. (2017) Indian Health Service Pharmacist Engaged in Opioids Safety Initiatives and Expanding Access to Naloxone. J Am Pharm Assoc 57: S135-S140.
- Gopalakrishnan S, Ganeshkuma P (2013) Systematic Reviews And Meta-Analysis: Understanding The Best Evidence In Primary Healthcare. J Family Med Prim Care 2: 9-14.
- Thomas J, Harden A (2008) Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 8: 1471-2288.
- Donovan E, Bratberg CP, Baird JP, Burstein J, Walley D, et al. (2019) Beliefs associated with pharmacy-based Naloxone: A qualitative study of Pharmacy -Based Naloxone purchasers and People at risk for Opioid overdose. J Urban Health 96:367-378.
- Abouk R, Paula RL, Powell D (2019) Association between state laws facilitating pharmacy distribution of naloxone and risk of fatal overdose. JAMA Intern Med 179: 805-811.
- Bachyrycz A, Shrestha S, Bleake BE, Tinker D, Bakhireva LN (2017) Opioid overdose prevention through pharmacy-based naloxone prescription program: Innovations in healthcare delivery. Subst Abus 38:55-60.
- Nielsen S, Hout Van MC (2016) What is known about community pharmacy supply of naloxone? A scoping review. Int J Drug Policy 32: 24-33.
- Bratberg JP, Smothers ZPW, Collins K, Erstood B, Ruiz Veve J, et al. (2019) Pharmacist and the opioid crisis: A narrative review of Pharmacists practice roles. J am Coll Clin Pharm 1: 1-7.
- Reynolds V, Causey H, McKee J, Reinstein V, Muzuk A (2017) The role of pharmacist in the opioid epidemic: An examination of pharmacist-focused initiatives across the United States and North Carolina. NC Med J 78: 202-205.
- Sawangjit R, Khan TM, Chiyakunapruk N (2017) Effectiveness of pharmacy-based needle/syringe exchange programme for people who inject drugs: a systematic review and meta-analysis. Addiction 112: 236-247.
- Thornton JD, Lyvers E, Scott VGG, Droibedi N (2017) Pharmacists’ readiness to provide naloxone in community pharmacies in west Virginia. J Am Pharm Assoc 57: 512-518.
- Galea S, Vlahov D (2002) Socioeconomic determinants and health of drug users, socioeconomic status; homelessness and incarceration. Public health Rep177: s135-145.
- EMCDDA (2019) Take home naloxone-topic overview.
- Simoens S, Lubeaun M, Verbeke K, Aerchot VA (2009) Patient experiences of over-the-counter medicine purchase in Flemish community pharmacies. Pharm World Sci 31: 450-457.
- Wieringa J, Reber K, Leeflang P (2015) Improving pharmacy store performance: the merits of over-the-counter drugs. Eur J Mark 49: 1276-1299.
- Elzey MJ, Fuclin J, Edwards ES (2017) Take Home naloxone treatment for opioid emergencies: a comparison of routes of administration and associated delivery systems. Expert Opin Drug Deliv 14: 1045-1058.
- Kerr D, Kelly AM, Dietze P, Jolley D, Barger B (2009) Randomised controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction 104: 2067-2074.
- Glaser A, Arakaki D, Dietze P, Patrick I, Walker T, et al. (2005) Randomized trial of intramuscular versus intranasal naloxone in prehospital treatment for suspected opioid overdose. Med J Aust 182: 427-429.
- Peprah K, Frey N (2017) Intranasal and Intramuscular naloxone for opioid overdose in the prehospital setting: a review of comparative clinical and cost effectiveness, and guidelines. CADTH Rapid Response Reports.
- ASHP (2016) ASHP Statement on pharmacist’s role in substance abuse treatment, education and assistance. Am J Health Syst Pharm 73: e267-e270.
- Bounthavong M, Harvey MA, Wells DL, Popish SJ, Himstreet J, et al. (2017) Trends in Naloxone prescriptions prescribed after implementation of a national academic detailing service in the veterans’ health administration; A preliminary analysis. J Am Pharm Assoc 57: S68-S72.
- Bird SM, McAuley A, Perry S, Hunter C (2016) Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction 111: 883-891.
- Bird SM, McAuley A (2019) Scotland’s national naloxone programme. Lancet: 393: 316-318.
- Davis CS, Ruiz S, Glynn P, Picariello G, Walley AY (2014) Expanded access to naloxone among fire fighters, police officers and emergency technicians in Massachusetts. Am J Public Health 104: e7-e9.
- Rando J, Broering D, Oslon JE, Marco C, Evans SB (2015) Intranasal naloxone administration by police first responders is associated with decreased opioid overdose deaths. Am J Emerg Med 33: 1201-1204.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Karikari M, Baxey ES (2022) Feasibility and Acceptability of Over-The- Counter Distribution of Take-Home Naloxone: A Systematic Review. J Addict Res Ther 13: 464. DOI: 10.4172/2155-6105.100464
Copyright: © 2022 Karikari M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1450
- [From(publication date): 0-2022 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 1161
- PDF downloads: 289