Farming Practices and Associated Risk Level of Fish Infections in Wouri Division, Cameroon
Received: 03-Jul-2023 / Manuscript No. JFLP-23-104617 / Editor assigned: 05-Jul-2023 / PreQC No. JFLP-23-104617(PQ) / Reviewed: 19-Jul-2023 / QC No. JFLP-23-104617 / Revised: 24-Jul-2023 / Manuscript No. JFLP-23-104617(R) / Accepted Date: 26-Jul-2023 / Published Date: 31-Jul-2023 DOI: 10.4172/2332-2608.1000431
Abstract
The prevention of diseases in fish is important for both animal welfare and farm productivity. In Cameroon, information related to the origin of infections in farmed fish is scarce. This study aims to assess the farming practices influencing the Risk Level of Fish Infections (RLFI) for a sustainable aquaculture in Wouri Division. A total of 35 farms were audited from March to May 2022 using “snow ball” technique, on-farms observations and face-to face interview-based questionnaire. The determination of the RLFI was based on the Compliance Rate (CR) of biosecurity measures. The results showed that the intensive production system (87.87%) was the most practiced followed by the semi-intensive system (12.13%). Overall, the CR was intermediate (40.52±14.70%) corresponding to a moderate RLFI and significantly higher on nursery farms (48.81±12.44%) followed by nursery + grow-out (47.92±13.63%) and grow-out (35.41±14.02%) farms. No farm recorded a minor RLFI. The CR was insignificantly higher in intensive system (42.09±14.75%) than in semi-intensive system (29.17 ±8.10%) and not affected by the culture facilities. A positive, strong and significant relationship was found between the fishing method, quarantine of new fish and the CR. The government should financially and technically empower fish farmers in biosecurity practices for optimum fish production.
Keywords
Farming practices; Biosecurity; Fish infections; Sustainable aquaculture
Introduction
Diseases have been reported as the main limiting factor in Aquaculture. According to [1], it’s the principal cause of massive deaths of farmed fish resulting in gross economic loss in Cameroon. The number of disease outbreaks and economic losses reflect the immaturity of the fish farming industry and current gaps in aquatic animal health governance. As a result, disease remains an economic and societal challenge. For example, infectious anemia in salmon resulted in estimated annual economic losses of 11 and USD 14 million in Norway and Canada, respectively, from 1998 to 1999 ) [2]. The direct financial loss of USD 420.50 caused by the enteric red plague has been reported in farmed fishes in Cameroon [1]. For the sustainability of the fish farming industry, diseases must then be properly managed with an emphasis on the prevention, a sign of maturation of the fish farming industry. In other words, it is better to act upstream by preventing pathologies through the rigorous implementation of biosecurity measures because downstream, their treatment is technically and financially demanding.
Biosecurity is the application of measures aimed to reduce risk level or the probability of the introduction (external biosecurity) and further spread of pathogens within the farm (internal biosecurity) [3]. The key concept in biosecurity is to avoid transmission, either between farms or within the farm. Hence, biosecurity measures must reduce the infection’s risk level of farms animals or the probability of effective transmission. Therefore, the epidemiology of the diseases to be avoided, particularly of the routes of transmission, the stability of the agent in the environment and the role of fomites and vector should be thoroughly known [4]. Biosecurity is also a strategic approach that integrates and encompasses both policy and regulatory frameworks to analyze and manage risks to prevent the exposure, introduction, transmission and spread of disease on farms. Biosecurity helps to reduce the cost associated with farm diseases, including sub-clinical diseases whose consequences may be invisible, but which have a significant effect on performance, product quality, clientele, quality assurance and fish production costs [2, 3, 5]. A good biosecurity practice may help to improve the animal welfare, farm productivity and may contribute to reducing the use of antibiotics. Knowing of the biosecurity compliance rate can also help to evaluate and the risk level of fish infections as they are negatively correlated or evolve in opposite directions. As the compliance rate increases, the level of risk of fish infections decreases and vice versa.
In Cameroon, some works have been done on biosecurity practices in pig and poultry farms [6-8]. The concept of aquaculture biosecurity is still poorly known, aside from the study carried out by [9] on biosecurity practices in aquaculture farms in the West Region of Cameroon. In the same vein, there is an urgent need to establish a general mapping of biosecurity practices in aquaculture farms throughout the country. A thorough knowledge of the biosecurity compliance and indirectly the risk level of fish infections or the probability of disease transmission within or between farms as well as the determining factors (farming practices) can help decision-making by stakeholders in the aquaculture sector to improve farm productivity.
Farming practices such as “herd size” have been found to be associated with the biosecurity status in pig herds [10, 11]. Additionally, husbandry system, culture duration, pond water source, size of ponds, number of ponds per farm and capture method have been reported to influence the biosecurity score [9]. To our knowledge, no relationship was established between those farming practices and the risk level of fish diseases. Moreover, such factors have not yet been investigated in fish farms of the Littoral Region of Cameroon and especially in Wouri Division which is one of the main fish farming poles of the Country. This study aims to assess the farming practices influencing the risk level of fish infections for a sustainable aquaculture in Wouri Division, Cameroon.
Materials and Methods
Study area
The investigation was conducted from March to May 2022 in 33 fish farms located in Wouri Division in the Littoral Region of Cameroon (Figure 1). The area lies between longitude 9°76’78’’-9°46’4.3’’East of the Greenwish meridian and latitude 3°97’04’’- 3°58’13’’North of the equator. The climate is of equatorial type characterized by a long rainy season running from March to November and a short dry season from December to February. The average annual temperature ranges between 25.5 and 28.9 °C while the rainfall is 3619 mm [12].
Selection of fish farms
The first farm was located with the help of a local inhabitant. The next farms were located using the “snow ball” technique. Indeed, the previously selected farmer indicated the neighboring farm and so on till no new farm could be found within the study area [7, 13]. Farm’s selection process was also based on road accessibility including distance and time to trek to farms, functional status and farmer consent to collaborate in the study [14].
Questionnaire design
The data was collected through personal observation of the researcher and face-to-face interview between the latter and the farm managers using a semi-structured questionnaire divided into two parts namely questions related to the fish farming practices and the biosecurity measures (Table 1) grouped into components (isolation, traffic control and sanitation) [4, 5]. The questionnaire was previously tested in a sub-sample of seven (7) farms in the study area to verify the relevance, clarity, redundancy and consistency of the question and subsequent adjustments made when necessary. The GPS (Global Positioning System) was used to geolocate the farms.
| Biosecurity Components | N° | Biosecurity Measures |
|---|---|---|
| Isolation | 1 | Farm is fenced |
| 2 | Other animals species are absent on the farm | |
| 3 | New fish are quarantined before rearing | |
| 4 | Absence of bushes and trees around farms | |
| 5 | Space for visitors | |
| 6 | Water flow is continuous | |
| 7 | Rearing facilities are layout in derivation | |
| Traffic control | 8 | Visitors not allowed to have contact with water |
| 9 | No exchange of breeding tools between farms | |
| 10 | Water supply tracks protected to trap debris and unwanted aquatic animals | |
| Sanitation | 11 | Use of footbaths |
| 12 | Veterinary intervention | |
| 13 | Incineration of dead fish | |
| 14 | Especial outfit (clean coverall and boots) for staff | |
| 15 | Especial outfit for visitors | |
| 16 | Analysis of water quality | |
| 17 | Diagnosis of fish diseases | |
| 18 | Sanitary lock | |
| 19 | Awareness of biosecurity measures | |
| 20 | Awareness of fish diseases | |
| 21 | Disinfection of breeding tools before use | |
| 22 | Disinfection of breeding tools after use | |
| 23 | Treatment of fish disesases | |
| 24 | Captured fish not put back into water |
Table 1: Biosecurity components and measures studied.
Biosecurity scoring system
The linear scoring system was used by assigning 1 and 0 respectively to the implemented biosecurity measure or not. The final score of a farm was the sum of all the values recorded in the farms (0 or 1 per measure). Given that a biosecurity component (isolation, traffic control and sanitation) included several measures, the mean score of a component was obtained by adding up the scores of individual measures. Thereafter, the total score was divided by the total number of measures within the component [6]. The total score recorded by a farm could not exceed 33 points. The linear scoring system was empirically calculated as previously reported by [15-17]. The measures were therefore weighted equally and any biosecurity measure estimated to be less efficient in the transmission and occurrence of a disease since fish may suffer from poor health resulting from the lack of implementing biosecurity measures. The main concern of this study was the importance of implementing biosecurity measures on the health of reared fish and not the risk level generated by each biosecurity measure as it is the case in disease transmission pathways. The weighed scoring systems in the disease transmission pathways should not have the same efficiency as direct contact is likely more risky than indirect contact with less efficiency for transmitting pathogens. The Compliance Rate (CR) of biosecurity measures defined and ranked after [14] was used to determine the risk level of fish infections and to categorize the fish farms (Table 2). The biosecurity compliance rate and the risk level of fish infections are negatively correlated or evolve in opposite directions. In other words, as the compliance rate increases, the level of risk of fish infections decreases and vice versa.
Compliance Rate |
Implementation Level | Biosecurity Practice | Risk Level | Type of Farm |
|---|---|---|---|---|
| [0-25] | Low | Poor | Major | A |
| [25-75] | Intermediate | Intermediate | Moderate | B |
| [75-100] | high | Good | Minor | C |
Table 2: Classification of fish farms based on the compliance rate of biosecurity measures.
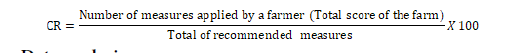
Data analysis
Farming related characteristics and biosecurity compliance rates were subjected to descriptive statistics. The Kruskal-Wallis (K) and Mann-Whitney (U) tests, the analysis of variance (F) were used to assess the effects of the farming practices on the compliance rate of biosecurity measures while the relationship between both variables was determined using the multivariate linear regression model. Data were analyzed using the R software and the significance level (p) was set at 0.05.
Results
Fish farming practices in Wouri Division
The fish farming practices in Wouri Divison are summarized in Table 3. It appears that Clarias gariepinus Burchell, 1822 was the only reared species of fish. The intensive production system (87.87%) and non-integrated fish farming (100%) were the most practiced. Growout was the most common culture phase (66.66%) followed by nursery or fry farming (27.27%) and nursery + grow-out (18.18%). The nonground system was diversified and especially the fastanks (69.69%) was the most represented and used by about 95% of the farms, unlike the ground system (ponds). Only 36.36% of fish farmers practiced quarantine of new fish. The main source of water was boreholes (63.63%) while 87.87% of farms did not treat water before use.
| Farming practices | Variables (N = 33) | Frequencies (%) |
|---|---|---|
| Fish species reared | Clarias gariepinus | 100 |
| Production systems | Intensive | 87.87 |
| Semi intensive | 12.13 | |
| Extensive | 0 | |
| Type of fish farming | Integrated | 0 |
| No integrated | 100 | |
| Culture phases | Fry farming (nursery) | 27.27 |
| Grow-out | 66.66 | |
| Fry farming + grow-out | 18.18 | |
| Types of culture facilities | Ponds | 3.03 |
| Fastanks | 69.69 | |
| Concrete tanks | 6.06 | |
| Fastanks + concrete tanks | 15.15 | |
| Ponds + fastanks + concrete tanks | 6.06 | |
| Number of production cycles per year | 01-Feb | 51.51 |
| >2 | 48.48 | |
| Source of water | River | 3.03 |
| Boreholes | 63.63 | |
| Wells | 30.3 | |
| Camwater1 | 3.03 | |
| Treatment of water before use | Yes | 12.12 |
| No | 87.87 | |
| Use of fertilizer | yes | 6.06 |
| No | 93.93 | |
| Inspection of fish | Yes | 93.93 |
| No | 6.06 | |
| Quarantine of new fish | Yes | 36.36 |
| No | 63.63 | |
| Duration of acclimatization of new fish (days) | No acclimatization | 21.22 |
| 01-Jul | 78.78 | |
| Type of feeds used | Farm-made | 3.03 |
| Manufactured | 72.72 | |
| Farm-made + manufactured | 24.25 | |
| Feed storage | Store | 81.81 |
| Others | 18.18 | |
| Duration of feed storage (weeks) | ≤4 | 78.78 |
| >4 | 21.21 | |
| Workforce on the farm (manpower) | [1-2] | 57.58 |
| [2-7] | 42.42 | |
| n: Number of audited farms; 1: Cameroon water utilities corporation (company in charge of potable water supply in Cameroon) | ||
Table 3: Frequency distribution of farms in Wouri Division according to the farming practices.
Frequency distribution of farms according to the compliance rate of biosecurity components
The frequency distribution of farms according to the compliance rate of biosecurity components is summarized in Table 4. Overall, the compliance rate (40.52±14.70%) was intermediate corresponding to a moderate risk level of fish infections and significantly higher for the isolation component (60.17 ± 19.81%) followed by traffic control (53.53 ± 25.87%) and sanitation (27.70 ± 19.70%). No farm was at a minor risk level of contamination. The compliance rate was low and intermediate in 18.18% and 81.81% of farms respectively. No farm showed a good biosecurity practice.
| Biosecurity Components | Compliance rate (%) | F | p | |||
|---|---|---|---|---|---|---|
| [0-25] | [25-75] | [75-100] | M ±SD (Min-Max) | |||
| Isolation | 1 (3.03) | 27 (81.81) | 5 (15.15) | 60.17±19.81 (14.28-100) | 22.73 | <0.0001* |
| Trafic Control | 2 (6.06) | 27 (81.81) | 4 (12.12) | 53.53±25.87 (0-100) | ||
| Sanitation | 13 (39.39) | 20 (60.60) | 0 (0) | 27.70±19.70 (0-64.28) | ||
| Overall | 6 (18.18) | 27 (81.81) | 0 (0) | 40.52±14.70 (16.67-75) | ||
| Number of farms (% of farms); M: Mean; SD: Standard deviation; Min: minimum; Max: maximum; *: Significant | ||||||
Table 4:Frequency distribution of farms according to the compliance rate of biosecurity components.
Effect of the production system on the compliance rate of biosecurity practices
The biosecurity practices (Figure 2) were insignificantly (U = 27; p = 0.09) more applied in intensive system (42.09±14.75%) than in semi-intensive system (29.17 ±8.10%). In other words, the risk level of fish infections was higher in the semi-intensive system. Whatever the production system, the biosecurity component related to the isolation recorded the highest (p < 0.05) compliance rate followed by the traffic control and sanitation.
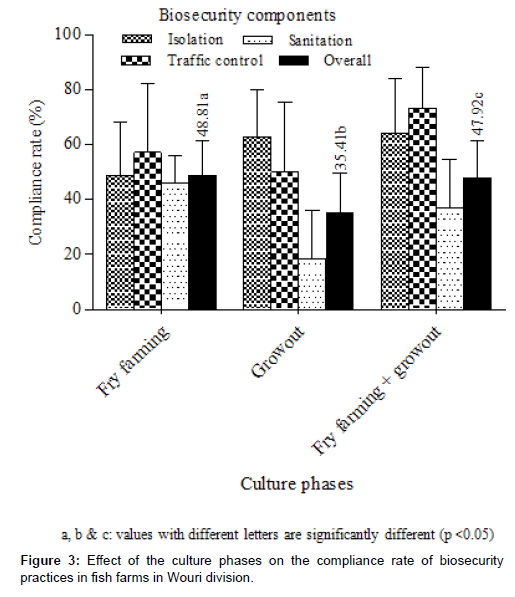
Effect of the culture phases of fish on the compliance rate of biosecurity practices
The effect of the culture phases of fish on the compliance rate of biosecurity practices is highlighted in Figure 3. Regardless of the biosecurity components, compliance rates were intermediate and significantly (K = 6.76; p = 0.034) higher (48.81±12.44%) on farms practicing nursery (fry farming) followed by nursery + grow-out (47.92±13.63%) and grow-out (35.41±14.02%) farms. All three categories of farms were at moderate risk of pathogens contamination. Whether in nursery or nursery + grow-out farms, the most observed components were in decreasing order, traffic control, isolation and sanitation. This trend was not noted in farms practicing only grow-out although sanitation was always the least observed (18.57±17.40%).
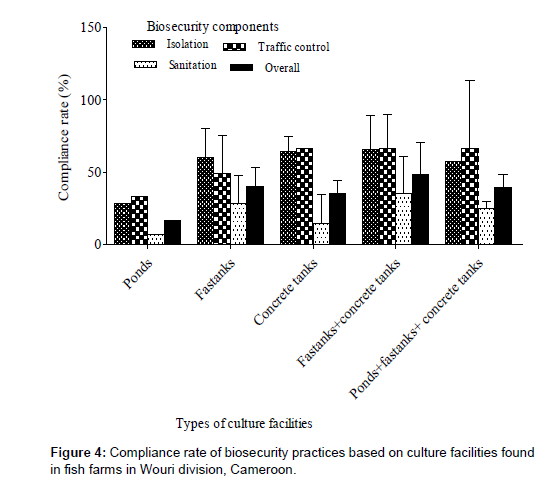
Compliance rate of biosecurity practices based on culture facilities
The compliance rate according to culture facilities (Figure 4) showed that biosecurity practice was poor (16.67±0%) on farms where ponds were the only culture facility and intermediate on farms with other types of facilities. The biosecurity compliance rate was not significantly (K = 3.54; p = 0.473) affected by the type of culture facilities. Overall, the traffic management component was the most observed followed by isolation and sanitation but without significant difference.
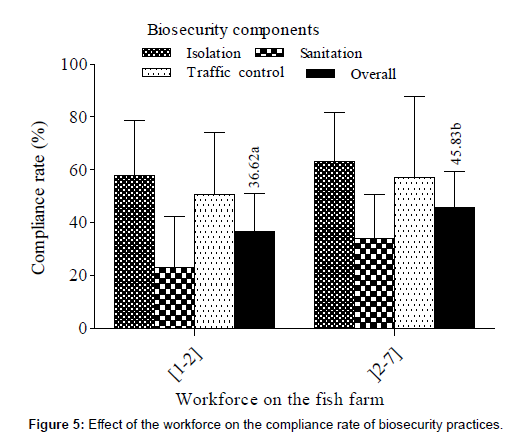
Effect of the workforce on the compliance rate of biosecurity practices
The increase of human resources had a positive effect on the compliance rate (Figure 5). Indeed, a significant higher (U=78.50; p = 0.048) compliance rate was recorded in farms with more than two workers.
Relationship between the farming practices and the compliance rate of biosecurity measures
The multivariate linear regression of the farming practices influencing the compliance rate of biosecurity measures (Table 5) showed a positive, strong (R2= 0.917) and significant relationship between the protection of farms from wild animals, fishing method, quarantine of new fish and the compliance rate. In contrast, the latter was negatively, strongly and significantly associated with the acclimatization of new fish.
| Farming Practices | Regression Coefficient | p | R2 | Constant |
|---|---|---|---|---|
| Production system | -2.105 | 0.613 | ||
| Culture phases | -3.953 | 0.488 | ||
| Protection of farm against wild animals | 7.598 | 0.033* | ||
| Quarantine of new fish | 15.414 | 0.000* | MR2= 0.917 | 41.402 |
| Acclimatization of new fish | -14,481 | 0.003* | AR2= 0.839 | |
| Fishing methods | 12.719 | 0.048* | ||
| Fishing frequencies | -0.481 | 0.588 | ||
| Treatment of water before use | 8.265 | 0.148 | ||
| Types of culture facilities | -1.456 | 0.952 | MR2=0.513 | 35.463 |
| Number of culture facilities | 0.644 | 0.159 | AR2= 0.220 | |
| Type of feeds | -12.881 | 0.258 | MR2=0.451 | 45.859 |
| Feeding frequencies | -0.695 | 0.135 | AR2= 0.268 | |
| Storage of fish | -1.389 | 0.18 | ||
| MR2: Multiple R-squared; AR2: Adjusted R-squared ; R2: Determination coefficient; p: Error probability; *: Significant | ||||
Table 5: Multivariate linear regression result of fish farming practices influencing the biosecurity compliance rate in Wouri Division, Cameroon.
Discussion
The fish farming practices in Wouri Divison revealed Clarias gariepinus Burchell, 1822 to be an excellent candidate for fish farming for several reasons. These include high growth rate, reduced respiratory requirements, adaptation to various farming systems, resistance or hardiness to diseases [18] and possibility of high stocking density [19]. In addition, broodstock produce large quantities of eggs and sperm. This species of fish accepts a wide variety of artificial feeds and tolerates poor environmental conditions. The flesh is highly appreciated by a large segment of Cameroonians and is therefore easily marketed. The high representation (87.87%) of farms practicing the intensive production system compared to the semi-intensive system suggests that the proximity of the study area to the Institute of Fisheries and Aquatic Sciences (ISH) at Yabassi, University of Douala-Cameroon would have contributed to the training of fish farmers in modern production techniques. Indeed, 53.85% of farmers who received training in fish farming came from that school whose purpose is to train fisheries engineers. Contrary to the present study, 84.3% of the farms practicing the extensive production system have been reported in the in the West Region of Cameroon because of the high cost and the unavailability of quality manufactured feed and the lack of mastery of production techniques [9].
The above-ground system, especially fastanks (69.69%) was the most represented (95% of farms) while the ground system (ponds) was of concern to only 3.03% of the producers. The reason being the scarcity of large land areas required by the ground system. This observation contrasts with [20] and [21] who obtained 97.5 and 82.6% respectively of the farms with ponds built in derivation given that land areas are available. Fry farming or nursery phase was practiced only by 27.27% of farms unlike the grow-out phase (66.66%) probably because fry are more vulnerable to diseases hence the risk of loss of production by fish farmers.
Overall the moderate risk level of fish infection (CR = 40.52±14.70%) recorded during this study is below the expected minor level (high CR). The similar value was reported by [22] in fish farms in Côte d’Ivoire while a major risk level (low CR) was obtained by [6, 9, 23, 24]. The explanations provided by these authors were the ignorance of farmers due to the lack of training, the inadequate or lack of application of the appropriate measures against disease transmission and occurrence in their farms, the lack of knowledge and understanding, lack of communication, time, and audit programs of biosecurity, potential risks and economic constraint. The most observed biosecurity component was isolation followed by traffic control and sanitation because isolation measures appear to be inexpensive and less constraining.
The biosecurity practice although overall intermediate was more observed (p = 0.090) in the intensive system (42.09±14.75%) compared to the semi-intensive system (29.17±8.10%). In other words, the semi-intensive system was more prone to infections probably due to the improved rearing techniques in the intensive system to reduce mortalities. The management of the farm and particularly the hygiene rules are more accurate in the intensive system as a result of the new infrastructural technology used as well as the advanced and easily controllable production techniques.
Regarding the effect of culture phases on biosecurity practice, the compliance rate was intermediate and significantly higher during the nursery phase in the hatcheries (48.81±12.44%) compared to the growout phase (35.41±14.02%). Thus, the risk of infection of fish was lower during fry farming. This observation would depend on the sensitivity and zoo technical delicacy of the fry production phase. Indeed, fry are more vulnerable to diseases than other developmental stages because of the weakness of their immune systems [25]. Fish acquire adaptive immunity with age, which would justify the decrease in compliance with hygiene measures during fish grow-out. The fear of production loss would have led fish farmers to emphasize barrier measures in the hatchery.
Although the compliance rate was higher during nursery phase, this is still problematic as the norm recommends a good biosecurity practice especially in hatcheries and not an intermediate practice as is the case in this study. These observations are due to the financial constraints raised by 57.57% of the fish farmers. The lack of aquacultural training, ignorance or poor application of biosecurity measures may also be mentioned [23, 24, 26]. This intermediate value of the compliance rate could be an explanation for the mortality rates of up to 100% observed in the Wouri Division farms and especially in hatcheries. As for the effect of the type of culture facilities on the observance of hygiene measures, the biosecurity practice was poor (16.67±0%) in the pond farms and intermediate in the other types of infrastructure. In fact, unlike ponds, rearing practices and biosecurity measures are more accurate, easily applicable and controllable in above-ground systems like fastanks and Water Recycle System (WRS) because of the more advanced technology.
The multivariate linear regression of the farming practices influencing the biosecurity practices showed a positive, strong and significant relationship between the protection of farms from wild animals, fishing method, quarantine of new fish and the compliance rate of biosecurity measures. This could be explained by the fact that some measures (fenced farm, no exchange of fishing materials with other farms, disinfection of farming equipment after use, quarantine of new fish) were more applied than others. This type of relationship between farming practices and compliance rate was expected since the implementation of standard biosecurity measures (use of foot bath, veterinary visit, and management of dead fish, wearing of protective clothing by employees, special clothing for visitors, water quality analysis and treatment of fish diseases) was limited by high costs. Fishing method has been reported to be positively associated with the compliance rate of biosecurity measures in the West Region of Cameroon [9]. A negative, strong and significant association was establish between the compliance rate of biosecurity measures and the acclimatization of new fish. Fish farmers seem to replace quarantine with acclimatization, which is not a hygiene measure per se as there may be healthy carriers in the batch of new fish imported into the farm.
Though the increase of manpower had a positive effect on the compliance rate, the risk level of fish infections remained moderate because the manpower was mainly composed of family members some of whom lacked experience [9].
The government should build the capacity of fish farmers in aquaculture biosecurity through seminars and funding for fish farming projects. Good biosecurity practice will in the long term allow certification of farms and will thus guarantee the quality assurance of aquaculture products.
Conclusion
Overall, the risk level of fish diseases in Wouri Division was moderate i.e. intermediate compliance rate of biosecurity measures and varied according to the farming practices. A positive and significant relationship was found between protection of farms from wild animals, fishing method, quarantine of new fish and the compliance rate. The latter was negatively, strongly and significantly associated with the acclimatization of new fish. The intermediate biosecurity practice in fish farms may be responsible for the frequently reported epizootics. The government should financially and technically empower fish farmers in biosecurity practices for optimum fish production.
Conflict of Interest
The authors have no conflict of interest declare.
References
- Fonkwa G, Nack J, Awah-Ndukum J, Yamssi C, Tomedi EM, et al. (2022) First report of enteric red plague of Oreochromis niloticus (Cichlidae) and Cyprinus carpio (Cyprinidae) reared in Cameroon: mortality rate, risk factors and financial loss. RALF 9: 323-335.
- FAO (2019) Committee for Inland Fisheries and Aquaculture of Africa. Fish diseases and environmental constraints, eighteenth conference session Bamako, Mali 1-9.
- Alarcon VL, Allepuz A, Mateu E (2021) Biosecurity in pig farms: a review. Porc Health Manag 7: 1-15.
- FAO, WHO, World Bank (2010) Good practices for biosecurity in the pig sector-Issues and options in developing and transition countries. In: FAO Animal Production and Health Paper No 169. Food and Agriculture Organization of the United Nations/World Organisation for Animal Health/World Bank, Rome, Italy.
- Arthur JR, Baldock CF, Bondad-Reantaso MG, Perera R, Ponia B, et al. (2008) Pathogen risk analysis for biosecurity and the management of live aquatic animal movements. Diseases in Asian Aquaculture 6: 21-52.
- Kouam MK, Moussala JO (2018) Assessment of factors influencing the implementation of biosecurity measures on pig farms in the Western Highlands of Cameroon (Central Africa). Vet Med Int.
- Kouam MK, Manjeli J, Moussala JO (2019) Management and biosecurity practices on pig farms in the Western Highlands of Cameroon (Central Africa). Vet Med Sci 6: 82-91.
- Tatfo KFDP, Bouelet NIS, Medoua NG, Kansci G (2021) Biosecurity Practices and Characteristics of Poultry Farms in Three Regions of Cameroon. J World's Poult Res 11: 64-72.
- Ngueguim DF, Kouam MK, Miegoue E, Tiogue CT, Feumba AK, et al. (2020) Socioeconomic Characteristics and Biosecurity Measures of Fish Farms in the West Region of Cameroon. Asian J Res Anim Vet Sci 6: 4-19.
- Laanen M, Persoons D, Ribbensetal S (2013) Relationship between biosecurity and production /antimicrobial treatment characteristics in pig herds. Vet J 198: 508-512.
- Backhans A, Sjölund M, Lindberg A, Emanuelson U (2015) Biosecurity level and health management practices in 60 swedish farrow-to-finish herds. Acta Vet Scand 57.
- Feumba R (2015) Hydrogeology and aquifer vulnerability assessment in the Besseke watershed (Douala, Cameroon). Doctorate/PhD thesis, University of Douala 254.
- Delaunay S, Tescar R, Oualbego A, Vom BK, Lançon J (2008) Cotton cultivation does not disrupt traditional sorghum seed exchanges. Agriculture Notebooks 17: 189-194.
- Racicot M, Vaillancourt JP (2009) Evaluation of biosecurity measures in poultry farms in Quebec by video surveillance and main errors made. Bulletin of the French Veterinary Academy 162: 265-272.
- Can MF, Altug N (2014) Socioeconomic implications of biosecurity practices in small-scale dairy farms. Vet Q 34: 67-73.
- Gelaude P, Schlepers M, Verlinden M, Laanen M, Dewulf J (2014) Biocheck. UGent: A quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult Sci 93: 2740-2751.
- Maduka CV, Igbokwe IO, Atsanda NN (2016) Appraisal of chicken production with associated biosecurity practices in commercial poultry farms located in Jos, Nigeria. Scientific.
- Edea OG, Hinvi LC, Abou Y, Gbangboche AB (2019) Biological and zootechnical characteristics of the African catfish Clarias gariepinus Burchell, 1822. Euro Sci J 15: 54-88.
- Więcaszek B, Krzykawski S, Antoszek A, Kosik J, Serwotka P (2010) Morphometric characteristics of the juvenile North African catfish Clarias gariepinus (Burchell, 1822) from the heated water aquaculture. EJPAU 13.
- Tiogue C, Bibou A, Kenfack A, Et Tchoumboue J (2020) Socio-economic and technical characteristics of fish farms in the Department of Mbam and Inoubou. Int J Biol Chem Sci 14: 983-1000.
- Ntsama ISB, Tambe BA, Takadong JJT, Nama GM, Kanscica G (2018) Characteristics of fish farming practices and agrochemicals usage therein in four regions of Cameroon. Egypt J Aquat Res 44: 145-153.
- Kone M, Cisse M. Ouattara M, Et Fantodji A, (2012) Compliance state of biosecurity measures in fish farming of three regions of Ivory Coast (Sub-Saharan zones). J Anim Plant Sci 16: 2288-2296.
- Ricou J (2006) Biosecurity Guide. In. Epflecublens CH-1015 Lausanne, Switzerland: Faculty of Life Sciences 19.
- Obosi K, Agbeja YE (2015) Assessing the level of aquaculture biosecurity regulations compliance in Ibadan, Nigeria. DJHC 2: 12-19.
- Gilles S, Dugue R, Slembrouck J (2001) African catfish fry production manual. Research Institute for Development. Editions Maisonneuve and Larose, Parsi 126.
- Boutin R (2001) On-farm biosecurity: a “must” for all farms. In: Quebec Agriculture and Agrifood Reference Center 2875.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Georges F, Georgette MJ, Amidou KN, Junior KDF, Jacques N, Frangin TMC, Julius AN, Minette TE, Joseph T (2023) Farming Practices and Associated Risk Level of Fish Infections in Wouri Division, Cameroon. J Fisheries Livest Prod 11: 431. DOI: 10.4172/2332-2608.1000431
Copyright: © 2023 Georges F, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 901
- [From(publication date): 0-2023 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 682
- PDF downloads: 219