Review Article Open Access
Fallopia japonica: Bioactive Secondary Metabolites and Molecular Mode of Anticancer
Mahmoud Zaki El-Readi1,2*, Safaa Yehia Eid1,3, Hiba Saeed Al-Amodi1 and Michael Wink3*
1Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Makkah, K.S.A
2Department of Biochemistry, Faculty of Pharmacy, Al-Azhar University, 71524 Assiut, Egypt
3Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Im Neuenheimer Feld 364, 69120 Heidelberg, Germany
- Corresponding Author:
- Dr. Mahmoud Zaki El-Readi
Department of Clinical Biochemistry
Faculty of medicine, Umm Al-Qura University
Abdia, Makkah, Saudia Arabia
Tel: +966-25270000/ 4347
Fax: +96625270000/4319
E-mail: mzreadi@uqu.edu.sa
Dr. Michael Wink
Institut für Pharmazie und Molekulare Biotechnologie
Universität Heidelberg, Im Neuenheimer Feld 364, 69120 Heidelberg, Germany
Tel: +49 6221 54 4880
Fax: +49 6221 54 4884
E-mail: wink@uni-hd.de
Received Date: September 19, 2016; Accepted Date: October 12, 2016; Published Date: October 17, 2016
Citation: El-Readi MZ, Eid SY, Al-Amodi HS, Wink M (2016) Fallopia japonica: Bioactive Secondary Metabolites and Molecular Mode of Anticancer. J Tradi Med Clin Natur 5:193.
Copyright: © 2016 El-Readi MJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Traditional medicinal plants are a large source of natural anticancer compounds that might serve as leads for the development of novel drugs. In recent years, the scientific community in the Western world has recognized the potential of natural products, used in Traditional Chinese Medicine (TCM). Since ancient times Japanese knotweed (Fallopia japonica), has been utilized in many TCM herbal preparations as anti-cancer agent. F. japonica (FJ) is known to produce a series of bioactive secondary metabolites, including anthraquinones, stilbens, tannins, lignans, anthocyanins, phenethyl alcohols, sterols, and essential oils. Resveratrol (a stilben) and emodin (an anthraquinone) are the major active ingredients of FJ. The anticancer activity of both compounds has various molecular modes of action and mechanisms through their ability to modulate the proliferation, apoptosis, cell cycle, growth factors, protein kinase C (PKC), NF-kappa B (NF-κB), and mitogen-activated protein kinase (MAPK) signaling cascades. This review presents an overview of the secondary metabolites of F. japonica and anticancer activities of the extract and main active principles, resveratrol and emodin. The possible molecular targets and potential chemopreventive effects are discussed.
Keywords
Fallopia japonica; Traditional Chinese Medicine (TCM); Resveratrol; Emodin; Chemopreventive
Introduction
Traditional Chinese medicine (TCM, Zhong-Yi), has a long history of use, with extensive literature and clinical applications covering thousands of years [1,2]. It has been widely accepted that TCM has evolved over the millennia, with a battery of herbal materials to preserve health, as well as treat and prevent illnesses [3,4]. Over the past decades, TCM has been an area of intensive research aiming at developing new drugs for the ever-evolving diseases afflicting mankind [5]. Because the development of new chemical drugs remains time consuming, capitalintensive and risky (i.e., a low rate of success), much more effort has been put into TCM for drug discovery. Now, TCM is a growing means of drug development in China. In 2007, China collected 3563 extracts, 64,715 compositions, and 5000 single compounds from 3000 Chinese herbs, together with about 130 kinds of chemical drugs obtained from either TCM ingredients or their derivatives [6].
Fallopia japonica (Syn. F. japonica Houtt.) is a member of the Polygonaceae family (buckwheat). It is known as Japanese knotweed Pleuropteris zuccarinii, P. japonicum Meissn, Ronse Decraene (Syn. Reynoutria japonica Houtt.), and Polygonum zuccarinii Small [7,8]. The English names for Japanese knotweed include, Huzhang, fleeceflower, Hancock’s curse, elephant ears, donkey rhubarb, sally rhubarb, American bamboo, Japanese bamboo, and Mexican bamboo (though it is not actually a bamboo). Huzhang root extract is a TCM treatment. It is also known as, He Shou Wu, which is used as a blood tonic (herbal preparation). Japanese Knotweed is considered an invasive pest and is a commercial source of resveratrol.
Fallopia japonica (FJ) is a large perennial plant, native to eastern Asia in Japan, China and Korea, this species grows in vast areas throughout the northeastern USA into Canada and Europe, and it was introduced from Japan into the UK in the 19th century [9].
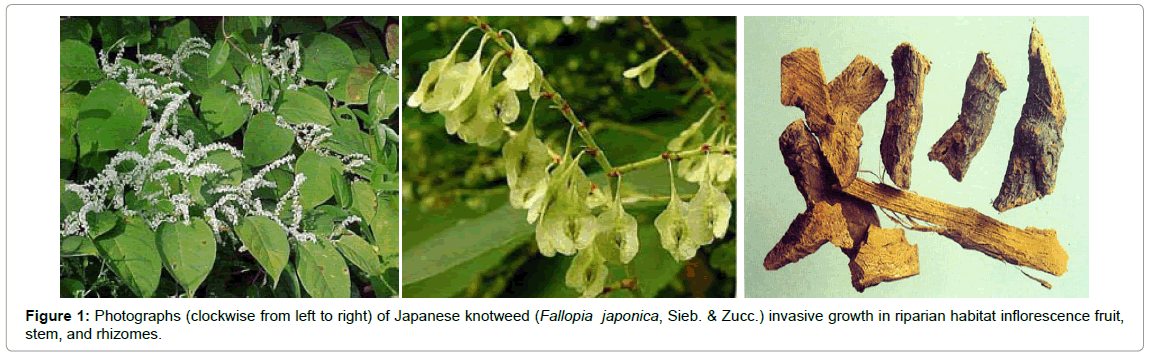
Historically, in 1777, Houttuyn as Reynoutria japonica described Japanese knotweed. Japanese knotweed stems have distinct raised nodes (appearance of bamboo). Stems are a reddish color and have a maximum height of 3–4 m (per growing season). The young stems have a flavor similar to mild rhubarb [10]. In some locations, semi-cultivated Japanese knotweed has been used for food production. Such populations can be controlled to prevent the invasion of sensitive wetland areas and driving out of the native vegetation. Morphological, leaves rang from triangular to heart-shaped, and are 7–14 cm long and 5–12 cm broad, and an entire margin [10]. The flowers are an attractive white color (July–August), and are considered an important source of nectar for honeybees; they yield a nice monofloral honey (bamboo honey, USA) (Figure 1) [11]. Knotweed flowers are mainly male sterile as reported in the UK and North America [12]. Japanese knotweed rhizomes with a distinctive orange interior can extend more than 15–20 m in length and 2 m in depth [10,13]. Fallopia japonica is highly able to produce series bioactive secondary metabolites including anthraquinones, stilbens, tannins, lignans, anthocyanins, phenethyl alcohols, sterols, and essential oils. Interestingly, resveratrol concentration in F. japonica is much higher than that reported in red wine, thus it is an important commercial source of resveratrol, and its scientific name is used in supplement labels. F. japonica also produces emodin. Emodin is used in traditional Chinese herbal medicine as a quality-control index for F. japonica. It has a wide range of pharmacological activities such as anti-bacterial [14], anti-inflammatory [15] immunosuppressive [16], and antitumor activity [17]. In addition, our LC-MS data suggests that the quantity of emodin from total contents of methanolic extract of F. japonica is about 30%. The medicinal importance of rhizomes of Fallopia spp., have been reported, related to resveratrol and emodin [18]. This review presents an overview of the chemical composition of F. japonica and anticancer activity of the extract and main active principles resveratrol and emodin. We highlight the possible molecular targets of their chemopreventive effect, and consider this herb and its active metabolites as novel dietary chemopreventive agents.
The use of Fallopia japonica in traditional medicine
Traditionally, F. japonica has been used in China, Korea, Taiwan, and Japan. The extract from the roots of F. japonica has been used in TCM as a natural laxative, and occasionally as food. It has been recorded that emodin has a mild laxative effect in doses of 20-50 mg per day. F. japonica is a concentrated source of emodin, and is used as a nutritional supplement to regulate the bowels motility.
Therapeutically, the aerial parts, dried root, and rhizome of F. japonica are often used as analgesic, antipyretic, diuretic, and antitussive agents. It can also be used for the treatment of chronic bronchitis, infectious hepatitis, diarrhea, cancer, gallstone, hypertension, atherosclerosis, menoxenia, hyperlipidemia, leucorrhoea, pruritus vulvae of the dampness-heat type, mycotic trichomoniasis, bacterial vaginitis, dysmenorrhea, trauma with blood stasis, snake bites, skin burns, osteomyelitis and allergic inflammatory diseases [19-24]. Methanolic extract of the roots of F. japonica, is used to maintain oral health in Korea, it is shown to reduce the viability of Streptococcus mutans and Streptococcus sobrinus; and inhibit sucrose-dependent adherence, glucan formation, and glycolytic acid production [25].
Bioactive secondary metabolites of Fallopia japonica
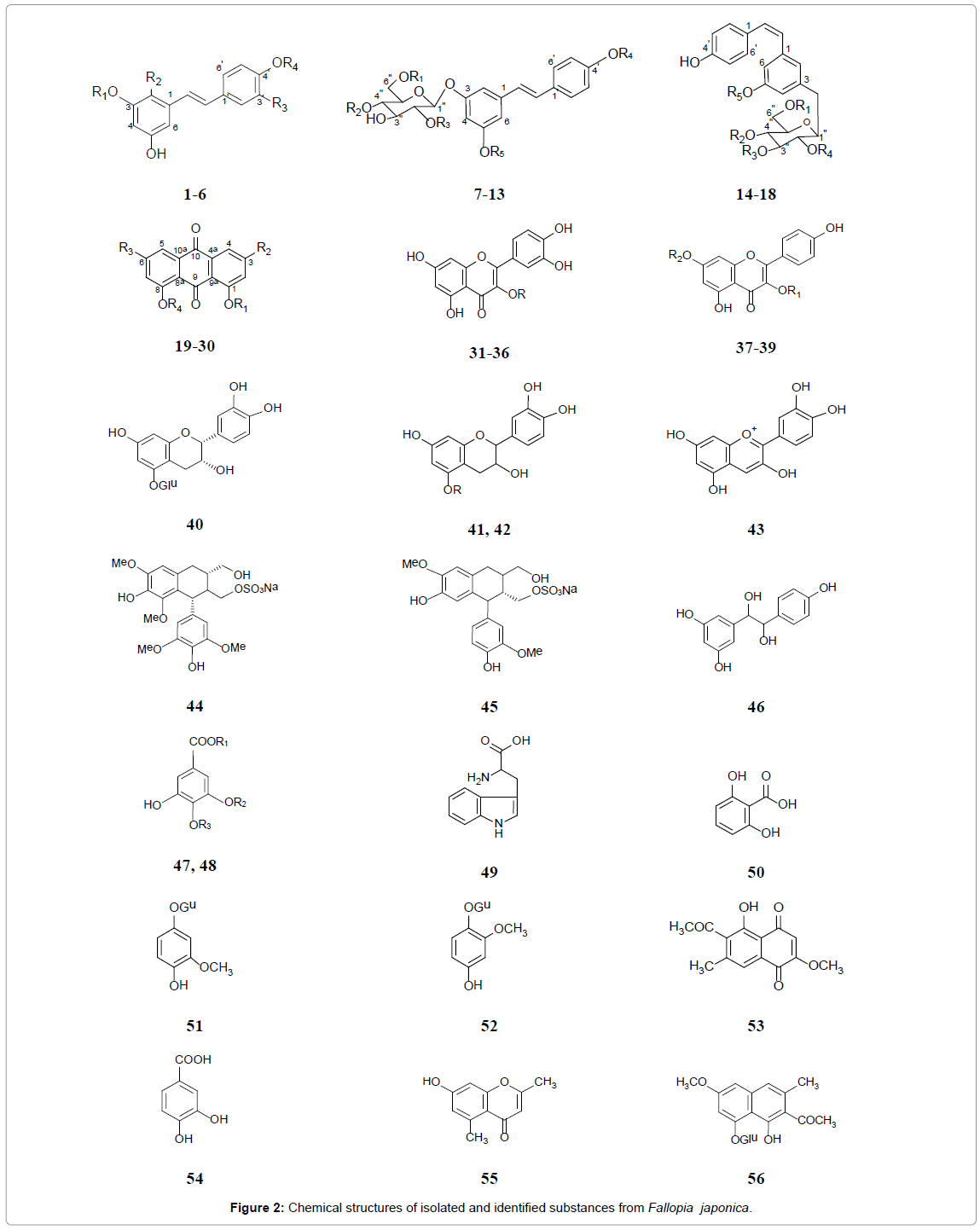
Many chemical components have been reported from this plant (Table 1 and Figure 2). The roots of F. japonica has been reported to contain a large number of stilbens, frequently found as glycosides and sulfates, including resveratrol (trans-3,5,4’–trihydroxystilbene) (1), hydroxyresveratrol (2) resveratroloside (3), piceid (polydatin) (4), piceatannol or astringinin (5), piceatannol glucoside (6), transresveratrol derivatives (7-13) and cis-resveratrol derivatives (14-18) [9,26-28]. Anthraquinones, including emodin and its derivatives (19- 21), anthraglycosides A (22) and B (23), physcion (24), chrysophanol (25), rhein (26), fallacinol (27), citreorosein (28), questin (29) and questinol (30), are important chemical constituents of the rhizome [18, 29-31]. Additionally, phenol glycosides: Quercetin and its glycosides (31-36), kaempferol-3-O-α-L-rhamnoside (37) and apigenin derivatives (38, 39) are found in its roots [32,33]. Furthermore, (−)-epicatechin-5- O-β-D-glucopyranoside (40) [34], (+)-catechin (41) and its glucoside (42) [35], cyanidin (43) [36], (−)-lyoniresinol-2a-sulfate (44) and (+)-isolaricireinol-2a-sulfate (45), 1-(3’,5’-dihydroxyphenyl)-2-(4”-hydroxyphenyl)-ethane-1,2-diol (44), gallic acid and derivative (47, 48), tryptophan (49), 2,6-dihydroxybenzoic acid (50), tachioside (51), isotachioside (52) [35,37], 2-methoxy-6-acetyl-7-methyljuglone (53), protocatechuic acid (54), 2,5-dimethyl-7-hydroxy chromone (55), torachrysone-8-O-β-D-glucoside (56), 7-hydroxy-4-methoxy- 5-methylcoumarine (57), 1-(3-O-β-D-glucopyranosyl-4,5- dihydroxyphenyl)-ethanone (58) [18], 5,7-dimethoxyphthalide (59) [38], and chlorogenic acid (60) [39] have been identified in F. japonica root. A total of 18 volatile compounds were identified in the extract of F. japonica leaves. The major volatile compounds found in the extract of F. japonica leaves are 2-hexenal (61), 3-hexen-1-ol (62), n-hexanal, 1-penten-3-ol and 2-penten-1-ol [40-47].
| S. No. | Compounds | Reference |
|---|---|---|
| 1 | Resveratrol (trans-3,5,4’–tetrahydroxystilbene): R1=H, R2=H, R3=H R4=H | [26,41] |
| 2 | Hydroxyresveratrol (trans-2,3,5,4’–tetrahydroxystilbene):R1=H, R2=OH, R3=H, R4=H | [28,42] |
| 3 | Resveratroloside: R1=H, R2=H, R3=H, R4=Glu | [26] |
| 4 | Piceid (polydatin): R1=Glu, R2=H, R3=H, R4=H | [26] |
| 5 | Piceatannol or Astringinin (trans-3,4,3’,5’-tetrahydroxystilbene): R1=H, R2=H, R3=OH, R4=H | [28,42] |
| 6 | Piceatannol glucoside: R1=H, R2=H, R3=OH, R4=Glu | [9] |
| 7 | Sodium and potassium trans-resveratrol-3-O-β-D-glucopyranoside-6''-sulfate: R1=SO3M, R2=H, R3=H, R4=H, R5=H | [27] |
| 8 | Sodium and potassium trans-resveratrol-3-O-β-D-glucopyranoside-4''-sulfate: R1=H, R2=SO3M, R3=H, R4=H, R5=H | [27] |
| 9 | Sodium and potassium trans-resveratrol-3-O-β-D-glucopyranoside-2"-sulfate: R1=H, R2=H, R3=SO3M, R4=H, R5=H | [27] |
| 10 | Sodium and potassium trans-resveratrol-3-O-β-D-glucopyranoside-4'-sulfate: R1=H, R2=H, R3=H, R4=SO3M, R5=H | [27] |
| 11 | Sodium and potassium trans-resveratrol-3-O-β-D-glucopyranoside-5-sulfate: R1=H, R2=H, R3=H, R4=H, R5=SO3M, M=K+ or Na+ | [27] |
| 12 | 2''-O-Galloylpiceid: R1=H, R2=H, R3=3,4,5-trihydroxybenzoyl, R4=H, R5=H | [43] |
| 13 | 6''-O-Galloylpiceid: R1=3,4,5-trihydroxybenzoyl, R2=H, R3=H, R4=H, R5=H | [43] |
| 14 | Sodium and potassium cis-resveratrol-3-O-β-D-glucopyranoside-6"-sulfate: R1=SO3M, R2=H, R3=H, R4=H, R5=H | [27] |
| 15 | Sodium and potassium cis-resveratrol-3-O-β-D-glucopyranoside-4"-sulfate: R1=H, R2=SO3M, R3=H, R4=H, R5=H | [27] |
| 16 | Sodium and potassium cis-resveratrol-3-O-β-D-glucopyranoside-3"-sulfate: R1=H, R2=H, R3=SO3M, R4=H, R5=H | [27] |
| 17 | Sodium and potassium cis-resveratrol-3-O-β-D-glucopyranoside-2"-sulfate: R1=H, R2=H, R3=H, R4=SO3M, R5=H | [27] |
| 18 | Sodium and potassium cis-resveratrol-3-O-β-D-glucopyranoside-5-sulfate: R1=H, R2=H, R3=H, R4=H, R5=SO3M, M=K+ or Na+ | [27] |
| 19 | Emodin: R1=H, R2=CH3, R3=OH, R4=H | [44] |
| 20 | Emodin-8-O-β-D-glucopyranoside: R1=H, R2=CH3, R3=OH, R4=Glu | [44] |
| 21 | Emodin-8-O-β-D-(6'-O-malonyl)-glucoside: R1=H, R2=CH3, R3=OH, R4=(6'-O-malonyl)-Glu | [33] |
| 22 | Anthraglycosides B: R1=Glu, R2=CH3, R3=OH, R4=H | [45] |
| 23 | Anthraglycosides A: R1=Glu, R2=CH3, R3=OCH3, R4=H | [29] |
| 24 | Physcion: R1=H, R2=CH3, R3=OCH3, R4=H | [44] |
| 25 | Chrysophanol: R1=H, R2=CH3, R3=H, R4=H | [46] |
| 26 | Rhein: R1=H, R2=COOH, R3=H, R4=H | [33] |
| 27 | Fallacinol: R1=H, R2=CH2OH, R3=OCH3, R4=H | [18] |
| 28 | Citreorosein: R1=H, R2=CH2OH, R3=OH, R4=H | [18] |
| 29 | Questin: R1=H, R2=CH3, R3=OH, R4=CH3 | [18] |
| 30 | Questinol: R1=H, R2=CH2OH, R3=OH, R4=CH3 | [18] |
| 31 | Hyperin: R=3-O-β-D-Gal | [33] |
| 32 | Avicularin: R=3-O-α-L-Ara | [33] |
| 33 | Reynoutrin: R=3-O-Xyl | [33] |
| 34 | Isoquercitrin (quercetin-3-glucoside): R=3-O-β-D-Glu | [33] |
| 35 | Quercetin-3-glucuronide: R=3-O-β-D-Glc | [33] |
| 36 | Quercitrin (quercetin-3-rhamnoside): R=3-O-α-L-Rha | [33] |
| 37 | Kaempferol-3-O-α-L-rhamnoside: R1=O-Rha, R2=H | [33] |
| 38 | Apiin (apigenin-7-O-[-β-D-Apiofuranosyl-(1→2)-β-D-glucopyranoside]): R1=H, R2=Apio-(1→2)-Glu | [33] |
| 39 | Apigenin-7-O-β-D-glucoside: R1=H, R2=Glu | [33] |
| 40 | (−)-Epicatechin-5-O-β-D-glucopyranoside | [47] |
| 41 | (+)-Catechin: R=H | [35,47] |
| 42 | (+)-Catechin-5-O-β-D-glucopyranoside: R=Glu | [35] |
| 43 | Cyanidin (3,3',4',5,7-pentahydroxyflavylium) | [36] |
| 44 | Sodium (−)-lyoniresinol-2a-sulfate | [35] |
| 45 | Sodium (+)-isolaricireinol-2a-sulfate | [35] |
| 46 | 1-(3',5'-Dihydroxyphenyl)-2-(4"-hydroxyphenyl)-ethane-1,2-diol | [35] |
| 47 | Sodium 3,4-dihydroxy-5-methoxybenzoic acid methyl ester-4-sulfate: R1=Me, R2=Me, R3=SO3Na | [35] |
| 48 | Gallic acid: R1=H, R2=H, R3=H | [35] |
| 49 | Tryptophan | [35] |
| 50 | 2,6-Dihydroxybenzoic acid | [35] |
| 51 | Tachioside | [37,47] |
| 52 | Isotachioside | [37,47] |
| 53 | 2-Methoxy-6-acetyl-7-methyljuglone | [18] |
| 54 | Protocatechuic acid | [18] |
| 55 | 2,5-Dimethyl-7-hydroxy chromone | [18] |
| 56 | Torachrysone-8-O-β-D-glucoside | [18] |
| 57 | 7-Hydroxy-4-methoxy-5-methylcoumarine | [18] |
| 58 | 1-(3-O-β-D-Glucopyranosyl-4,5-dihydroxyphenyl)-ethanone | [35] |
| 59 | 5,7-Dimethoxyphthalide | [37,47] |
| 60 | Chlorogenic acid | [37,47] |
| 61 | The major volatile compound 2-hexenal (74.27%) and | [40] |
| 62 | 3-hexen-1-ol (8.11%) | [40] |
Table 1: Names of isolated and identified substances from Fallopia japonica.
General biological and pharmacological activities of extracts
Pharmacological studies have evaluated several aspects of FJ extract including antioxidant [48], anti-inflammatory activities. FJ has ability to inhibit NF-κB and neutrophil infiltration animal models of edema [49]. Water extracts and essential oil of FJ inhibited growth of several strains of MO and prevented the induction of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by lipopolysaccharide (LPS) [40].
F. japonica increases the burn’s healing through their ability to enhancing the immune system in a dose-dependent manner [50]. Administration of F. japonica, the neutrophil levels, and neutrophilic adhesive rates remained normal in severely burned animals [51]. Moreover, F. japonica treatment maintained normal levels of TNF and adhesive leukocytes in burned rats [52]. Treatment of burn shock animals with F. japonica isolated substance leads to enhance the cardiac and microcirculatory functions (cardiac output, cardiac index, and stroke volume index) and decreases in the number of leukocytes [52-55].
Additionally, the EtOH extract of F. japonica possess antiviral activity against HBV [56], inhibiting several kinds of virus, which highly express the surface antigen of HBV (HBsAg) [57] and inhibit bacterial DNA primase [58].
Furthermore, F. japonica extracts protected the gastric mucosa from the harmful effect stress ulcers and decreased the gastric secretion [59]. In vitro and in vivo, the tannins of F. japonica decreased the activities of the digestive enzymes (trypsin, amylase, and lipase) [60,61]. F. japonica extracts inhibit acyl-co enzyme A–cholesterol acyltransferase activity [23]. F. japonica suppressed the activity of the CNS in mice [59]. Finally, the alcohol extract and compounds of F. japonica possess potent estrogenic activities [45,62].
Anticancer and Chemopreventive Properties
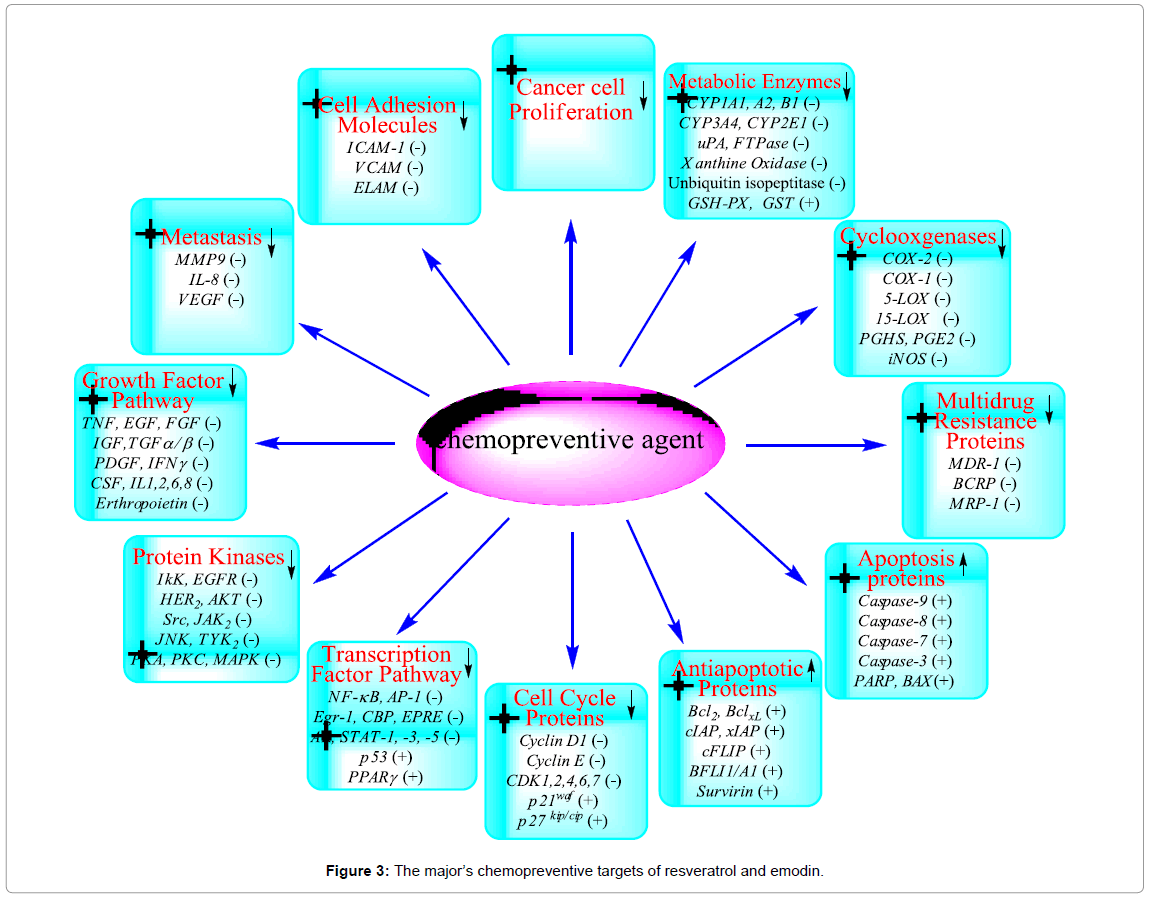
Several environmental carcinogens, inflammatory agents, and tumor promoters activated tumorigenesis process. These carcinogens are known to modulate the transcription factors (e.g., NF-κB, AP-1, p53), anti-apoptotic proteins (e.g., Bcl-2, Bcl-XL, Mcl-2), proapoptotic proteins (e.g., caspases, PARP), protein kinases (e.g., IκK, EGFR, HER2, JNK, MAPK), cell cycle proteins (e.g., cyclins, cyclin-dependent kinases), cell adhesion molecules, ABC-transporters proteins (e.g., MDR1, BCRP, MRP), metabolic enzymes (e.g., CYP1A1, CYP3A4, GST), COX-2, and growth factor signaling pathways [63]. The use of traditional medicine plants is widespread and provides a large source of natural anticancer compounds that might serve as leads for the development of novel drugs. Therefore, there is much interest from investigations of natural anticancer and bioactive compounds for preservation of traditional medicines. The use of naturally occurring products has been a major focus for a long period due to their potential chemotherapeutic activity. Extract of F. japonica is used in nutraceutical products because its resveratrol and emodin content. Both compounds have shown antitumor, antimetastatic, chemopreventive, chemical carcinogenesis-inhibitive, oncogene signal transduction-inhibitive and immunomodulating properties. Extensive pharmacological studies have shown that resveratrol and emodin contribute to the traditional efficacy of F. japonica. The molecular targets of chemopreventive activity of resveratrol and emodin are summarized in Figure 3.
Inhibition of cancer cell growth
The extracts of F. japonica inhibited proliferation and induced apoptosis of many cancer cells for example on 0.2 mg/mL concentration in human lung cancer (A549 and H1650) cell lines [64] but not cytotoxic effect on the normal human liver cells [65]. Additionally, methanolic extract of F. japonica was highly effective in multiple oral cancer cell lines due to its inhibitory effect on cell proliferation in KB, HEp-2, and YD-15 cells. Sp 1 protein is an important target of anticancer drugs development because its expression is very high in several cancer cell lines and solid tumor. F. japonica induced apoptosis, and reduced the expression level of Sp1 protein (transcriptional level) in HEp-2 and YD-15 cells and decreased Sp1 promoter activity [66- 70]. Furthermore, ethyle acetate extract and its fractions exhibited anti-proliferative activities in KB cells differentially more than aqueous extract, dependent on the amount of emodin [71]. However, it has been reported that methanolic extract of F. japonica increases cell proliferation in breast cancer cell lines MCF-7 (at 30 and 100 μg/mL) because its estrogenic activity [45].
Resveratrol, a stilbene contained in F. japonica extract, has been reported as a biologically active compound. Resveratrol exhibited anti-proliferative effects through various molecular mechanisms on different cancer cells [72-78]. These anti-proliferative effects of resveratrol is mainly because its interaction with replication enzymes e.g. DNA polymerase and ribonucleotide reductase (by scavenge the essential tyrosyl radical) [79,80]. Resveratrol also possesses inhibitory effects on each stages of carcinogenesis process (initiation, promotion, and progression [81].
Since the clinical use of two anthraquinones, mitoxantrone and daunorubicin, for cancer treatment began 25 years ago, anthraquinones and anthraquinone derivatives, such as emodin from F. japonica, have been reported to possess anticancer activity [82,83]. These anthraquinones are usually potent inhibitors of topoisomerase II in DNA, and some also induce apoptosis in cancer cells [84]. Anthraquinones target DNA damage by mutation, their intercalation action usually leads to frame-shift mutations. This mutation leads to change in the amino acid sequence of a protein, influences promoters, and other regulatory sequences in gene code, resulting in cell death [85,86]. Emodin has been reported to inhibit proliferation in breast, lung, cervical, colorectal, and prostate cancers cells In vitro [87-91]. Emodin has been reported to exhibit anti-proliferative effects in lung, breast and pancreatic cancer and sensitize these cells to chemotherapeutic agents mainly by inhibiting HER-2/neu overexpression [92-96]. Relevant to its antiproliferative effects, emodin is also known to inhibit tyrosine phosphorylation of protein tyrosine kinases, p56lck and HER-2/neu [44]. Emodin also displays an over 25-fold differential cytotoxicity against ras transformed bronchial epithelial cells to the normal human bronchial epithelial cells [90]. By studying the SAR of emodin and comparing its activity to another anthraquiniones found that the C1 and C3 position of emodin structure is important for anti-tumor function (Figure 2) [97].
However, emodin evokes less or no cytotoxic effect in several normal cells, including: HBL-100 cells derived from normal human breast tissue, human fibroblast like lung WI-38 cells, and three primary cultured normal rat cells [98]. These data suggest that cancer cells more sensitive to emodin-induced cytotoxicity than normal cells. Emodin’s specificity towards malignant cells might be due to its targeting on some oncogene signaling transductions, which are constitutively active or amplified in cancer cells. We demonstrated the potential of cytotoxic effect of FJ extract related to emodin, which showed significant cytotoxicity more than resveratrol or polydatin on five different cell lines including MDR and sensitive cells.
Inhibition of cytochrome P450 and other metabolic enzymes (anti-initiation or anti-xenobiotic effects)
The function of most cytochrome P450 superfamily (CYP) enzymes is to catalyze the oxidation of organic substances. The substrates of CYP enzymes include metabolic intermediates such as lipids and steroidal hormones, as well as xenobiotic substances such as drugs and other toxic chemicals like those found in smoking and gasoline. CYPs are the major metabolic enzymes involved in drug bioactivation or detoxification, representing for ∼75% of the total metabolism. They are catalyzed by cytochromes P450 is a monooxygenase reaction. For example, monoxygenase (cytochromes P450 1A1, 1A2 and 1B1) can insert one oxygen atom into an organic substrate (RH) while the other oxygen is reduced [99]. Inhibiting the monooxygenation reaction is the molecular target of anticancer activity of natural plant SMs.
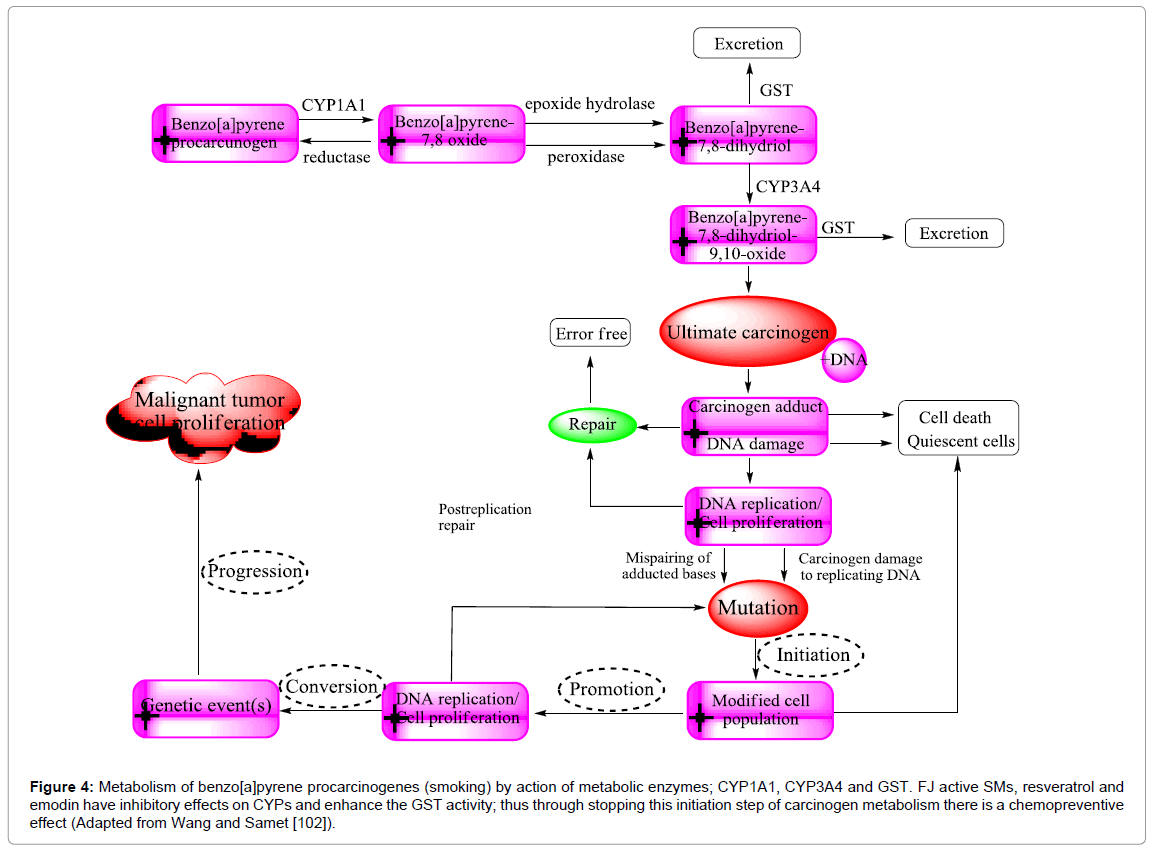
F. japonica antimutagenicity is believed to cause the inhibitory action of resveratrol, and emodin on CYP1A1. Resveratrol, by very different mechanisms of action displays numerous properties. However, its anti-initiation effects are related to its anti-xenobiotic abilities through Phase I cytochromes and Phase II detoxifying enzymes (Figure 4). The aryl hydrocarbon receptor (AhR) is modulated the carcinogen activation pathway by activation of CYP1A1 and CYP1A2 enzymes in microsomes. Different carcinogens are targeted by certain CYP: The classic hydrocarbon carcinogen; 7,12-dimethyl-benz(a)anthracene (DMBA) is activated by CYP1A1, CYP1A2, and CYP1B1 enzymes. For example, tobacco smoke is a core problem in at least eight different kinds of cancer because it contains high amounts of aryl hydrocarbon and dioxin receptor (AhR) ligands such as anthracenes and benzo(a) pyrene (BaP) [100]. The mutagenesis caused by peroxidative strand breakage or covalent adduct formation of DNA is considered a major mechanism in tobacco-related carcinogenesis. Adduct formation is essentially caused by monooxygenases, which catalyzed oxidation of tobacco smoke compounds (inert molecules) e.g. BaP into diol-epoxy derivatives (highly mutagenic) (Figure 4). Consequently, free radicals responsible for oxidative DNA damage are released [101]. BaP itself is an agonistic ligand of the AhR, the principal inducer of CYP1A transcription.
Figure 4: Metabolism of benzo[a]pyrene procarcinogenes (smoking) by action of metabolic enzymes; CYP1A1, CYP3A4 and GST. FJ active SMs, resveratrol and emodin have inhibitory effects on CYPs and enhance the GST activity; thus through stopping this initiation step of carcinogen metabolism there is a chemopreventive effect (Adapted from Wang and Samet [102]).
Resveratrol is a competitive antagonist for AhR and blocks the conversion of the AhR forms (cytosolic ligand bound ◊ nuclear DNAbinding) resulting in inhibition of AhR-mediated transactivation of CYP genes. In addition, resveratrol inhibit the interaction between AhR and the transcriptional complex and subsequent free radical production, leading to cellular and DNA damage [102-104]. Resveratrol is also directly inhibited CYP1A1 and CYP1A2 human liver cells in dose dependent manner [105,106]. Moreover, resveratrol is inhibitor of CYP3A4 (irreversible) and of CYP2E1 (noncompetitive reversible) [106]. It has been demonstrated that the inhibitory effects of resveratrol on substrate-oxidation reaction that catalyzed by CYP3A4 and CYP3A5 is depend on the time and NADPH concentration [107,108].
Chen et al. reported that the resveratrol has therapeutically effect on hypercholesterolaemia. They demonstrated significant decreasing in the level of all parameters of lipid profile in mice that fed with a hypercholesterolaemic diet and resveratrol (200 mg/kg/day) for 8 weeks [109]. The underlying mechanism of the anti-dyslipidaemic effect of resveratrol is the ability of resveratrol to modulate the enzyme expression and activity of cholesterol 7a-hydroxylase (CYP7A1). CYP7A1 has an important role in conversion of cholesterol into 7-ahydroxycholesterol and subsequently eliminated from plasma and excreted as cholic acid in bile [109].
The anti-inflammatory role of resveratrol in humans via downregulating proinflammatory conditions or by inhibiting LDL oxidation need more studies. Previously studies were designed in triple-blind, randomised, placebo-controlled trial in 75 patients consuming resveratrol-enriched grape extract, grape extract alone, or placebo for at least 6 months. Resveratrol-enriched grape extract induced a significant decrease in the low-density lipoprotein (LDL) cholesterol, apoB, oxidised LDL and oxidized LDL/apoB ratio compared with placebo and grape extract groups [110,111] Considering the homogenous consumption of statins by all individuals enrolled in the three groups, these data revealed impressive results: Resveratrol reduces hypercholesterolaemia, and, more importantly, it reduces the overall burden of oxidation of lipids and thus can be safely administrating in the primary prevention of cardiovascular disease in association with statins [112].
Emodin can also suppress the mutagenicity of carcinogens that are metabolically activated by this enzyme, like heterocyclic amines (food carcinogens) i.g indole type (Trp-P-2), quinoline type (2-amino- 3-methylimidazo[4,5-f] quinoline) and the pyridine type (2-amino-1- methyle-6-phenylimidazo[4,5-b]pyridine) [113]. This may means that the chemical structure of emodin is responsible for the destroying effects on CYP1A1 and CYP1A3 protein [114]. In addition, emodin produced oxidative stress, which resulted in extreme down-regulation of CYP protein expression as reported in previous studies. Anthraquinone inhibited incorporation of amino acids to protein in mouse neoplastic cells [115,116].
Moreover, emodin effect on rat liver microsomes exhibits phosphorylation CYP1A1 protein (inactive) and inhibition of monooxygenase activity (CYP1A1-dependent) [117,118]. Therefore, the direct interaction of hydroxyanthraquinone and CYPs leads to inhibition of monooxygenase activity (Figure 4). However, Wang et al. reported that emodin has the ability to induce CYP1A1 and CYP1B1 in human lung cancer cells at both gene (mRNA) and protein levels [119]. Binding of the emodin to an aryl hydrocarbon (Ah) receptor, a transcriptional factor of CYP1A1 and CYP1B1 genes are the possible mechanism involved [120]. Emodin also increases monooxygenase activities by benzo[a]pyrene and 7-ethoxyresorufin, which are substrates of CYP1A1 and CYP1B1 [121,122]. We demonstrated that F. japonica, and its active SMs; resveratrol, emodin, and polydatin significantly inhibit CYP3A4 in dose dependent manner and suppress gene expression (data unpublished), suggesting the predominately F. japonica extract inhibition of CYP3A4 related to resveratrol effect as CYP3A4 inhibitor.
Glutathione-S-transferase (GST) is a phase II metabolic enzyme. It is involved in carcinogenesis and resistance of cancer cells to oxidative stress and chemotherapeutic drugs. In vitro and in vivo, resveratrol has been shown to induce phase II of metabolism; detoxification enzymes mainly UDP-glucuronosyltransferase (UGT), glutathione- S-transferase (GST), and quinone reductase activities through modulation of the mitogen-activated protein kinase (MAPK) pathway [123,124]. It is capable of metabolically detoxifying drug/carcinogens, thus Phase II metabolic enzymes exert antimutagenic action [125]. On the other hand, emodin decreases TNF-α and TPA-induced GSTP1- 1 gene expression through inhibiting NF-κB and AP-1 binding onto GSTP1-1 promoter in K562 and U937 leukemia cells [126]. This could contribute to a reduction of incidences of glutathione-related drug resistance in human cancers.
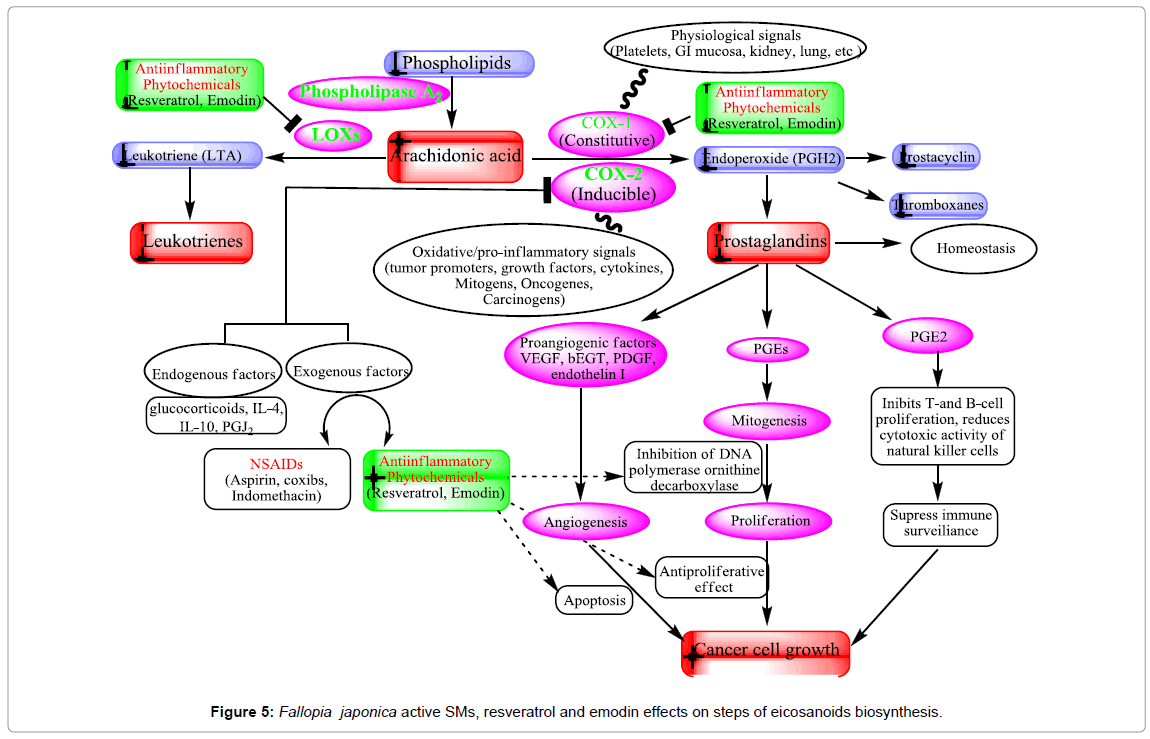
Inhibition of COX-2 (anti-promotion effects)
Cyclooxygenase (COX) is an enzyme that is responsible for the formation of important biological mediators called prostanoids, including prostaglandins, prostacyclin, and thromboxane. Lipooxygenase (LOX) enzyme is responsible for formation of leukotrines. The inflammation process is mainly related to the activities of COX- 2 and lipooxygenase (LOX) enzymes. Pharmacological inhibition of COX can provide relief from the symptoms of inflammation and pain. Non-steroidal anti-inflammatory drugs (e.g aspirin and ibuprofen), target inhibition of COX. The ability of resveratrol to inhibit the NF- κB pathway (IκB kinase inhibition) is linked to its chemopreventive activity [127]. The transcription nuclear factor kappa B (NF-κB) and activated protein-1 (AP-1) regulate the action of these enzymes. These compounds enhance tumor growth by acting on cell proliferation, angiogenesis, and immunosuppression (Figure 5). Cyclooxygenases inhibitors are considered valuable therapeutic agents against cancer [128]. Resveratrol has a chemopreventive effect on various cancer models unrelated to AhR activation and xenobiotic metabolism. Resveratrol inhibits the constitutive cyclooxygenase-1 (COX-1) but not the inducible COX-2 and inhibits COX-2 activity as well as COX-2 gene expression, which is important in promoting tumorigenesis [129- 131]. Down regulation of the COX-2 gene is resulting in the inhibition of protein kinase C (PKC) [129].
Recent, emodin studies show potent dose dependent inhibition of COX-2 and NO• through its direct inhibition of iNOS enzyme activity, and suppression of iNOS protein without affecting macrophage viability and causing 65–68% reduction of oedema volume at 40 mg/kg [132,133]. In addition, emodin is a potent inhibitor of LPSinduced NO production and iNOS gene expression. The mechanisms of inhibition of iNOS by emodin is inhibited NF-κB activation [134]. Moreover, expression of iNOS and the COX-2 protein was inhibited by emodin in LPS-activated RAW 264.7 cells, and PGE2 production was reduced [135]. These results indicate that emodin can prevent the initiation stage of cancer through its ability to inhibition of COX-2 and inflammatory mediators (Figure 5).
Inhibition of multidrug resistance proteins
Multidrug resistance is the phenomenon of tumor resistance to chemically and functionally unrelated anticancer drugs, and is the one of the most formidable challenges in the field of cancer chemotherapy. The first mediator of MDR to be characterized at the molecular level was P-glycoprotein (P-gp/MDR1 or ABCB1), and there were many ATP-binding cassette (ABC) proteins including MRP1, and BCRP involved in MDR [136,137]. ABC-transporters mediate resistance to different classes of chemotherapeutic drugs including: vinblastine, vincristine, daunorubicin, doxorubicn, colchicine, paclitaxel, and etoposide, by actively extruding the drugs from the cells to lower the intracellular concentrations. Actually, there are few studies dealing with modulation of MDR with F. japonica and its active metabolites. Recent studies reported that, resveratrol has inhibitory effects on P-gp efflux function and increased the accumulation of P-gp substrates (rhodamine 123 and daunorubicin) in a concentration-dependent in KB-C2 cells [138]. In addition, resveratrol down-regulated Bcl- 2 and MDR1 genes and hence synergistically enhance the cytotoxic effect of combined chemotherapeutic agents leading to overcome the multidrug-resistant of KBv200 cells. Resveratrol can reverse multidrug resistance in KBv200 cells. The potential mechanism may be by inhibiting the multidrug-resistant gene expressions and/or promoting cell apoptosis [139].
Resveratrol also induced apoptotic death in doxorubicin-resistant AML cells, and it was shown to inhibit the efflux function and expression of an MRP1 gene [140]. Moreover, resveratrol can enhance the sensitivity of CNE2 cells to chemotherapeutic drugs under hypoxia. The potential mechanism is partly attributed to inhibiting the gene expressions of HIF-1 alpha, MDR1 and MRP1 [141].
On the other hand, co-treatment with emodin could remarkably enhance chemosensitivity of SGC996 cells when compared to cisplatin, carboplatin or oxaliplatin treatment alone. The mechanisms may be attributed to reduction of glutathione levels, and downregulation of multidrug resistance-related protein 1 (MRP1) expression in SGC996 cells. Furthermore, in vivo experiments on mice show that emodin/ cisplatin co-treatment decreased the tumor size by increasing apoptosis and down regulating MRP1 expression [142]. In addition, emodin/ cisplatin co-treatment increases ROS level and enhanced sensitivity of resistance cells, as compared to cisplatin-only treatment with little effect on normal cells. Emodin/cisplatin co-treatment inhibited the tumor growth in vivo by down regulated MDR1 expression and increased drug accumulation [143].
We evaluated the effect of F. japonica and its active principle resveratrol, emodin, and polydatin on MDR colon and leukemia cell lines and we found that the tested substance and extract show significant inhibition of efflux function and down regulation of MDR1, MRP1, and BCRP genes. Moreover, the effect of co-treatment of the MDR cell lines with FJ extract, or its active compounds with doxorubicin, remarkably enhance the chemosenstivity of resistance cells, especially leukemia cell lines.
Induction of apoptosis (anti-progression effects)
Apoptosis is a programed cell death, which is a fundamental process in the developmental and homeostatic maintenance of complex biological systems. A disregulation or failure of normal apoptotic processes will contribute to transformation of cells and provide a growth advantage to cancer cells. Apoptosis is characterized by cell shrinkage, chromatin condensation, DNA fragmentation, and the activation of specific cysteine proteases known as caspases. There are two pathways mediated by caspase-3; extrinsic pathway (receptorand caspase-8–mediated) and the intrinsic pathway (mitochondrial release of cytochrome c and activation of caspase-9) [144]. Apoptosis and necrosis are distinguished by the initiation of cell death from the outside and inside the cell (mitochondria), respectively [145].
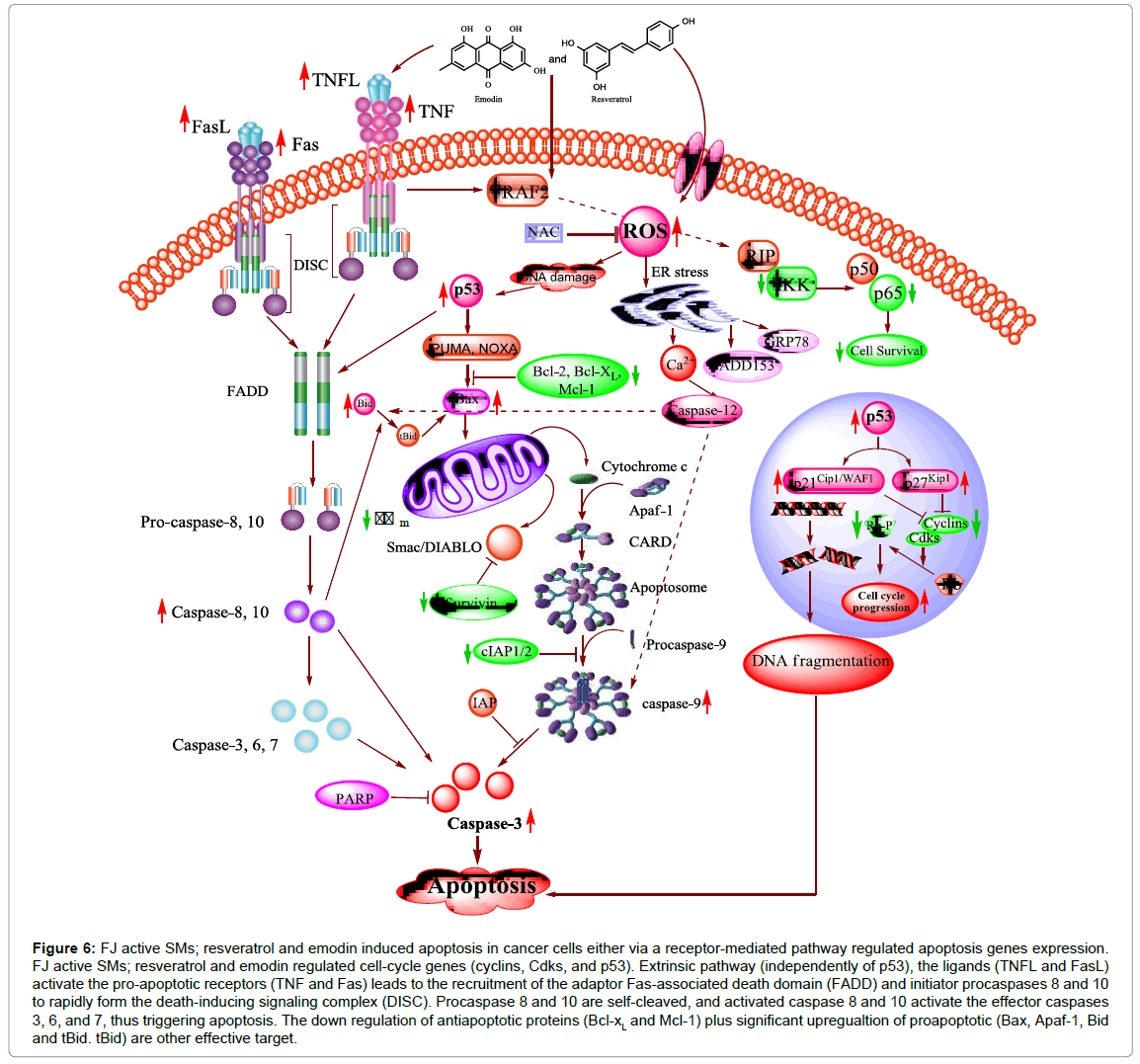
Many studies has concerned the apoptotic effect of F. japonica and its active SMs. Lin et al. reported that A549 cells treated with F. japonica extracts showed chromatin condensation, nuclear fragmentation, and vacuolization of cytoplasm. In addition, FJ methanolic extract induced apoptosis by PARP cleavage and the activation of caspase 3 (64). Ex vivo and in vivo studies have confirmed the apoptotic effect of resveratrol [146]. In numerous cell types, resveratrol has been observed inducing apoptosis through different pathways (Figure 6) [147-149].
Figure 6: FJ active SMs; resveratrol and emodin induced apoptosis in cancer cells either via a receptor-mediated pathway regulated apoptosis genes expression. FJ active SMs; resveratrol and emodin regulated cell-cycle genes (cyclins, Cdks, and p53). Extrinsic pathway (independently of p53), the ligands (TNFL and FasL) activate the pro-apoptotic receptors (TNF and Fas) leads to the recruitment of the adaptor Fas-associated death domain (FADD) and initiator procaspases 8 and 10 to rapidly form the death-inducing signaling complex (DISC). Procaspase 8 and 10 are self-cleaved, and activated caspase 8 and 10 activate the effector caspases 3, 6, and 7, thus triggering apoptosis. The down regulation of antiapoptotic proteins (Bcl-xL and Mcl-1) plus significant upregualtion of proapoptotic (Bax, Apaf-1, Bid and tBid. tBid) are other effective target.
Anti-leukemic activity in mice and human cell lines of resveratrol by inducing apoptosis [150] however, resveratrol has not any apoptotic effects on normal cell even at higher concentrations [151,152]. Resveratrol has been shown to induce apoptosis by activating death receptors (Fas and TNF) pathways [147,148,153]. This extrinsic pathway activates intracellular caspases (effectors mediators), thus the apoptotic effects of resveratrol cytotoxicity block by caspase inhibitors.
On the other hand, apoptosis could be a potential general mechanism, providing a mechanistic basis for the antiproliferative and anti-neoplastic effects of emodin (Figure 6). A number of studies have demonstrated that emodin is capable of inducing apoptotic cell death in various cancer cells through both mechanisms [94,154- 157]. Emodin is activated the caspase-3 cascade independent or dependent on ROS [158]. Emodin (quinone structure) is highly redox active molecules can form semiquinone radicals and reactive oxygen species (superoxide anion radical, hydrogen peroxide, and hydroxyl radical). The generation of ROS may contribute to mitochondrial damage, reduction of mitochondrial transmembrane potential, release of cytochrome c and Smac, and subsequent caspase activation and apoptosis [159].
Suppression of anti-apoptotic proteins
The therapeutic value of chemotherapeutic agents is largely dependent on their ability to trigger the anti-apoptotic molecules. The Bcl-2 protein family contains anti-apoptotic (Bcl-2, Bcl-XL, and Mcl-1) and proapoptotic (Bax, Bak) proteins and they are wellcharacterized regulators of apoptosis. F. japonica active SM; resveratrol and emodin, trigger Bcl-2 and Bax modulation, mitochondrial dysfunction, mitochondrial cytochome c release, caspase activation and consequently leads to apoptosis (Figure 6) [149,155,156,160-163].
Direct caspase inhibitors are participants in different survival signaling pathways, the inhibitors of apoptosis protein (IAP) family such as XIAP (X-linked inhibitor of apoptosis) and surviving are important to the control of drug resistance and cell proliferation in different cancer types [164-166]. F. japonica methanolic extract, resveratrol, and emodin decreased expression of XIAP and survivin mRNA, protein, and its transactivation, suggesting that F. japonica inhibits survivin expression through the down-regulation of Sp1 to induce apoptosis in several cancer cells [71,167]. Surprisingly, survivin expression induced by gemcitabine could be inhibited when combined with emodin treatment [93]. Its downstream target, surviving, mediates apoptoitc cell death, indicating that the inhibitory effects of F. japonica and its franctions on oral cancer cell proliferation are due to emodin in F. japonica [71].
Cell Cycle Arrest
The cell cycle, or cell-division cycle, is a series of events that takes place in a cell leading to its division and duplication (replication). In eukaryotes, the cell cycle can be divided into two brief periods: interphase (during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA) and the mitosis (M) phase (during which the cell splits itself into two distinct cells, often called “daughter cells”). The cell cycle consists of four distinct phases: G1 phase, S phase (synthesis), G2 phase (collectively known as interphase) and M phase (mitosis). Activation of each phase is dependent on the proper progression and completion of the previous one. Cells that have temporarily or reversibly stopped dividing are said to have entered a state of quiescence called G0 phase. A dysregulation of the cell cycle components may lead to tumor formation. Cells contain various pathways designed to protect them from the genomic instability, or toxicity that can result when their DNA is damaged. Checkpoint proteins that control the normal passage of cells through the cell cycle play a pivotal role in this response. There are several cell cycle checkpoints, which are used by the cell to monitor and regulate the progress of the cell cycle [168]. The two main checkpoints are the G1/S checkpoint (rate-limiting step and restriction point) and the G2/M checkpoint [169]. Tumor cells frequently loose checkpoint controls and this facilitates the development of the tumor [170]. Thus, one of the important approaches for cancer chemotherapy is to regulate cellcycle progression [169].
Resveratrol produces a differentiation of human promyelocytic cells (HL-60 line) and decreases tumor growth in rat models [146,171]. The effect of resveratrol on cell cycle arrest (G2/M transition and G0/ G1 phase) gradually decreases the anti-apoptotic oncoprotein Bcl- 2 expression and subsequently undergoes apoptosis [172,173]. In addition, resveratrol induces antiproliferation and arrests the S phase at low concentrations (30-60 μM), but high concentrations do not induce S phase accumulation in human histiocytic lymphoma U937 cells. Removal of resveratrol from the culture medium stimulates U937 cells to reenter the cell cycle synchronously, as judged by the expression patterns of cyclin E, A and by fluorescent activated cell sorting analysis [174]. This data demonstrates that resveratrol causes S phase arrest and reversible cell cycle arrest. Thus, resveratrol provides an important new cell cycle blocker as well as a cancer chemopreventive agent. Hsieh et al. found that resveratrol suppress of cell growth through cell cycle arrest at S- and G2-phases [72,175].
On the other hand, the effect of the main FJ anthraquinone, emodin, on the G2/M cell cycle has been demonstrated on various cancer cells, including v-ras-transformed and hepatoma cells [90,98]. Elevations of p53 and p21 expression were suggested as a common mechanism involved in this induced G2/M cell-cycle arrest [98]. Similarly, G1/S cell-cycle arrest was found in human breast, colon carcinoma cells upon treatment with emodin [88,176].
G2/M phase arrest was observed with increased protein levels in the cell cycle regulatory genes; cyclin A, cyclin B, Chk2, Cdk2, and P27 and decreased protein levels in Cdc25c and P21 in hepatoma cells; Huh7, Hep3B, and HepG2 after time courses of emodin treatment [177]. Furthermore, treatment of cancer cells with various concentrations of emodin led to cell cycle arrest at G0/G1 and G2/M increased the Bax, p21, and Chk2 expression but inhibited Bcl-2, cyclin B1 and Cdc2 [163].
Apoptosis in HL-60 cells was efficiently induced by emodin in a dose dependent manner and cells were arrested at G0/G1. The expressions of Akt, p-Akt, IκB-α, p- IκB-α, p65, p-p65, mTOR and p-mTOR in Akt signal pathway was downregulated after emodin treatment [178]. However, G0/G1 phase cell population increased and G2/M phase cells decreased in HL-60/ADR cells after treatment with emodin [179].
Transcription factor pathway
The transcription factor is a protein that binds to specific DNA sequences, thereby controlling the transcription of genetic information from DNA to mRNA [180]. Transcription factors perform this function alone, or with other proteins in a complex, by promoting (as an activator), or blocking (as a repressor) the recruitment of RNA polymerase to specific genes [181]. Many transcription factors, especially some that are oncogenes or tumor suppressors, help regulate the cell cycle. Due to their important roles in development, intercellular signaling, and cell cycle, some human diseases have been associated with mutations in transcription factors [182]. Many transcription factors are either tumor suppressors or oncogenes, and, thus, mutations or aberrant regulations of them is associated with cancer.
NF-κB transcription factors
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls the transcription of DNA. Conversely, the modulation of NF-κB has been impact role in cancer development. Active form of NF-κB increase the expression of genes that responsible on cell proliferation, cell survival and prevent apoptosis. Apoptosis is increased by deficiency in NF-κB expression due to its regulates the anti-apoptotic genes and caspases [183].
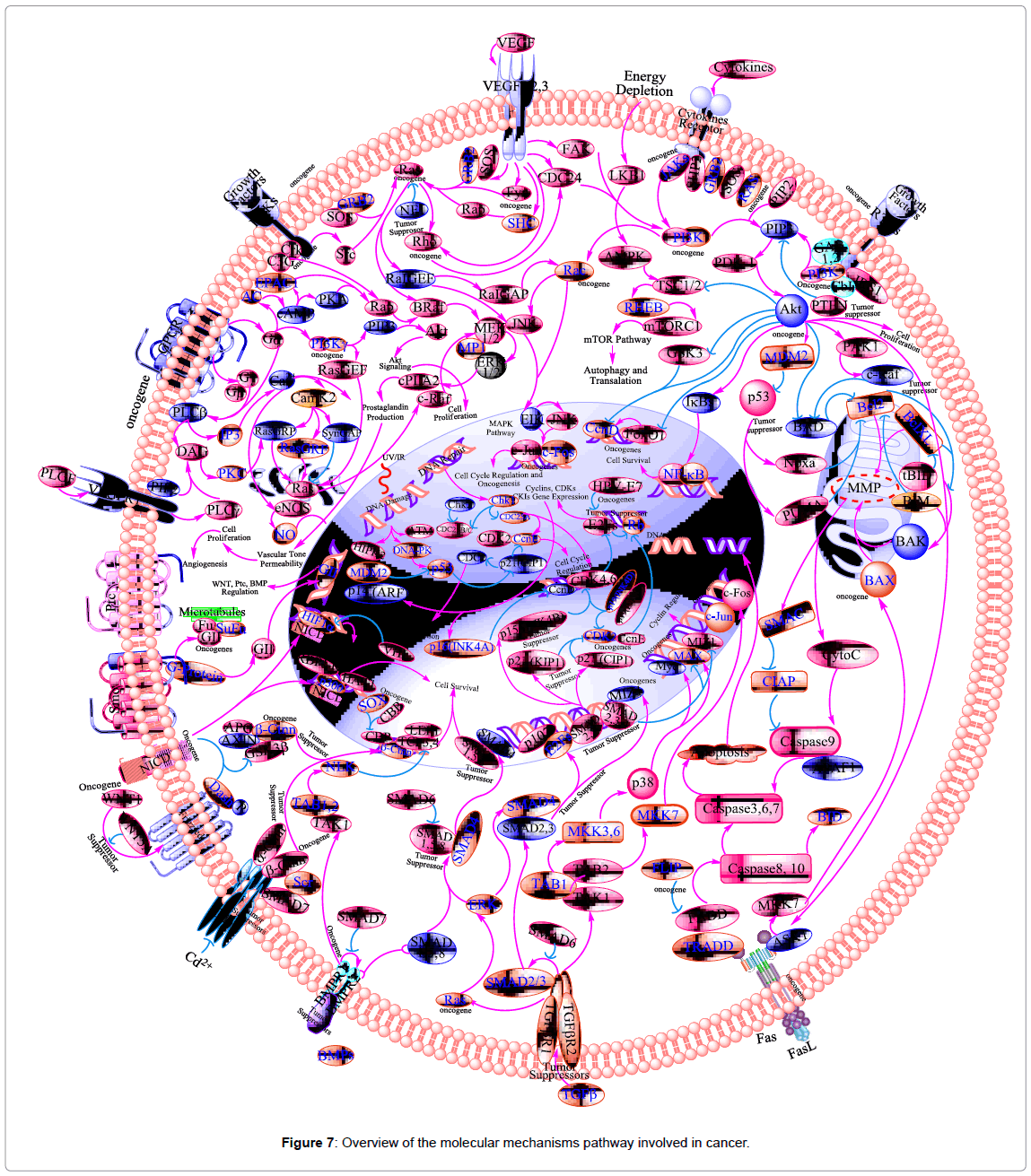
In cancer cells there is NF-κB in active form because mutations in genes encoding the NF-κB transcription factors themselves or in IκB genes that control NF-κB activity. Inhibition of NF-κB in tumor cells leads to prevent the proliferation and stop the cell resistance to the anti-tumor agents. Thus, NF-κB has much interest in drug discovery research as a target for anti-cancer agents [184]. Block of NF-κB is considered as an important target for many natural products that have been discovered to use as anti-oxidants, anti-cancer, and antiinflammatory agents [185]. Thus, NF-κB is considered as one of the main molecular targets of chemopreventive phytochemicals (Figure 7), as a transcription factor involved in multiple cellular processes, including cytokine gene expression, cellular adhesion, apoptosis, and metastasis [186]. Free radicals, inflammatory stimuli, cytokines, carcinogens, tumor promoters, endotoxins, g-radiation, ultraviolet (UV) light, and X-rays, activate NF-κB. In nucleus, active NF-κB induces >200 genes that have a role in suppress apoptosis and induce cellular transformation, proliferation, invasion, metastasis, chemoresistance, radio-resistance, and inflammation.
Resveratrol and emodin mediate the anti-inflammatory, cell growth modulatory and anticarcinogenic effects may be by block any one or more steps in the NF-κB signaling pathway, such as the signals that activate the NF-κB signaling cascade, translocation of NF-κB into the nucleus, DNA binding of the dimers, or interactions with the basal transcriptional machinery [187,188]. Resveratrol suppressed TNF-induced phosphorylation of the p65 subunit, induced oncogenic H-Ras that blockaded of IκB kinase activity, and blocked the expression of mRNA-encoding monocyte chemoattractant protein-1 of NF-κB and NF-κB-dependent reporter gene transcription [127,188- 190]. Therefore, the NF-κB suppression that targeted by resveratrol is essential for induction the cell cycle arrest and apoptosis in cancer cells.
Emodin has similar immunosuppressive and anti-inflammatory effects. Many researchers are interested in how emodin may regulate NF-κB signaling pathways. Kumar et al. reported that emodin inhibits NF-κB activation induced by TNF. This inhibition is not due to its chemical modification of NF-κB subunits, but its suppressive effect of degradation of IκB, an inhibitory subunit of NF-κB molecules [159]. In addition, down-regulation of IκK-γ, which is essential in phosphorylating IκB-α by emodin treatment lead to subsequently inhibit NF-κB [191]. Protein tyrosine kinases (PTK), reactive oxygen species, proteases IκKs, RIP, NIK, TRAF-2 play a critical role in regulating NF-κB activation [192]. Moreover, there is a positive association between cellular ROS and cytotoxic efficacy of anti-cancer drugs, and NF-κB is involved in this relationship [193-195].
On the other hand, manipulation of cellular redox state and NF-κB activation may initiate a novel approach to improving chemotherapeutic efficacy [196,197]. TNF-induced NF-κB activation, ΙκB degradation, ROS generation, mitochondrial- and caspasedependent apoptosis pathways are mediated by treatment of human cancer cells by emodin [20,155,156,159]. Emodin inhibiting AP-1 and NF-κB signaling pathways lead to suppressing MMP-9 expression and inhibits the invasiveness of cancer [198].
Tumor Suppressor Gene p53
Tumor suppressor gene p53 is an important molecule in the process of apoptosis and cell cycle arrest. Many tumor cells evade apoptosis and cell-cycle arrest via mutation of p53. In response to stress stimuli, such as DNA damage, p53 is stabilized, which leads to its nuclear translocation and transactivation of many target genes (e.g., p21, Bax, CD95). In certain cells, activation of p53 leads to activation of p21 (tumor suppressor gene) which inhibits the cyclin-cdk complex, cell cycle arrest (G1 phase) and apoptosis (Figure 7).
There are many studies reported the role of p53 in resveratrolinduced apoptosis [199-201]. Resveratrol increased expression of p53 and suppression of cell progression through the S- and G2-phases of the cell cycle [175].
In addition, resveratrol modulate the cell cycle regulating genes (e.g. cyclins, Cdks, p53, and hyperphosphorylated Rb) leads to a reversible cell arrest (S phase) of the vascular smooth muscle cell (VSMC) [202]. Narayanan et al. reported that resveratrol downregulated PSA, androgen receptor (AR) co-activator ARA 24, and NF- κB p65 in androgen sensitive prostate cancer cells (LNCaP). The downregulation of these genes related with activation of p53-responsive genes (e.g. PIG 7, p21 Cip1/WAF1, p300/CBP, and Apaf-1) [75,200].
On the other hand, the modulation of p53 and p21 pathway is the underlining mechanism of anti-proliferative effects of emodin [203]. In addition, emodin induced accumulation of p53 in HepG2/C3A cells with a resultant increase in p21 expression and cell cycle arrest [98]. This action might be through emodin’s inhibitory effect on the COP9 signalosome (CSN) associated kinases CK2 and PKD [204,205]. CSN is a multimeric protein kinases complex associated with CK2 and PKD, which can be phosphorylate p53, and subsequently degrade by the Ub/26S proteasome system [206]. Moreover, there is a positive association between cellular ROS and cytotoxic efficacy of anti-cancer drugs [194,195].
Reactive oxygen species that initiated the ATM-p53-Bax signaling pathway considered as mode of emodin action to induce apoptosis in human lung adenocarcinoma A549 cells [94]. Furthermore, Emodin increased the protein levels of p53 as response to reactive oxygen species (ROS) production in a dose-dependent manner [207]. In addition, the change in the expression of p53 pathway is associated with IGF-2 pathway leads to apoptosis in BCap-37 cells that treated [208]. Emodin induced apoptosis in the C6, HepG2/C3A, prostate LNCaP, and vascular smooth muscle cells by decreased the expression of AR and PSA, increased of Bax/Bcl-2 ratio, and increased the expression of p53, p21, Fas, caspase-3 and -9 [98,209,210].
Suppression Protein Kinases
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them (phosphorylation). Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction [211].
The human protein kinase family is divided into the following groups: AGC kinases (e.g., PKA, PKC and PKG protein kinases); the calcium/calmodulin-dependent protein kinases (CAMK); CK1- the casein kinase 1 group (CMGC) (e.g., CDK, MAPK, GSK3 and CLK kinases); the homologs of yeast Sterile 7, Sterile 11, and Sterile 20 kinases (STE); the tyrosine kinases (TK); and the tyrosine-kinase like group of kinases (TKL) [211]. The MAPK pathway has received increasing attention as a target molecule for cancer. In addition, Rb and the E2F transcription factors regulate cell cycle (G1/S-phase). Tumor suppressor protein p16 is a member of the cyclin-dependent kinase 4 (CDK4 or INK4) inhibitor protein families (Figure 7). It functionally competes with cyclin-D for association with cyclin-dependent kinase 4/6 (CDK4/6) and keeping CDK4/6 inactive. The cyclin-D/CDK4 complex is known to phosphorylate the retinoblastoma (Rb) protein at mid-G1 phase, which is thought to be a critical step in the progression through G1 into S-phase of the cell cycle [212]. The Rb-E2F/DP pathway is an important contributor to resveratrol-mediated cell cycle arrest and apoptosis [213]. It has been reported that resveratrol inhibits phosphorylation of Rb, down-regulation of expression of E2F transcription factors, and induces of the Cdk inhibitor (p21Cip1/ WAF1) resulted in cell cycle arrest (S-phase and G1/S-phase) in cancer cells [190,213]. Furthermore, in hormone-insensitive DU145 prostate cancer cells, resveratrol modulated mitogen-activated protein kinase [MAPK, extracellular signal-regulated kinase 1/2 (ERK1/2)], and stimulated c-fos and c-jun expression [214].
Emodin was found to act similarly to antiestrogens, capable of inhibiting estrogen-stimulated growth and DNA synthesis, and the phosphorylation of Rb protein [96]. In addition, there is high synergy of As/IFN-α with emodin treatment with regard to the inhibition of proliferation and induction of cell death of HTLV-I–transformed T cells. There is a potent antiproliferative effect on tumor cells, which was linked to the accumulation of hypophosphorylated Rb and G0/G1 arrest. Emodin reduces the level of phosphorylated Rb and induces G1 cell-cycle arrest in HTLV-I–transformed cells [157].
Protein kinase CKII has essential role in cell proliferation, transformation, and differentiation process [215,216]. Unlike most of the Ser/Thr protein kinases, whose substrates contain consensus sequences generally determined by basic and prolyl residues, substrates of CKII share unique phosphoacceptor sites specified by clusters of acidic residues [215,216]. Yim et al., reported that emodin is specific inhibitor of CKII. They unexpectedly found that emodin acts as a competitive inhibitor of CKII with respect to ATP, with an IC50 value of 2 mM [217].
Structurally, emodin fit to penetrate and coupling with the active site of CKIIa, block the ATP binding as well as it close the hydrophobic pocket between the N-terminal and the C-terminal lobes. This leads to its inhibition of the binding of the natural co-substrates of CKII, in a competitive manner [218]. The importance of hydroxyl group 3 in anchoring emodin with CKII through polar interactions is further proven by the observation that the two analogues of emodin, 1,8- dihydroxy-anthraquinone and chrysophanic acid, both lacking this group, did not show obvious inhibitory effect on CKII activity [219].
In addition to protein kinase CKII, emodin also shows an inhibitory effect on some other kinases. Among them, emodin acts as a modest inhibitor of PKC [217,220]. The mitogen-activated protein kinase (MAPK) signaling pathways play a central role in regulating cell proliferation, apoptosis, and migration [221]. The MAPK members consist of three major classes; the c-jun N-terminal kinases (JNKs), the extracellular signal-regulated proteins kinase (ERKs) and p38 (Figure 7) [198,222].
Growth factors pathway
Growth factors are proteins that bind to receptors on the cell surface, with the primary result of activating cellular proliferation and/or differentiation. Some of the growth factors implicated in carcinogenesis are: epidermal growth factor (EGF), platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), transforming growth factors (TGF)-α and -β, erythropoietin (Epo), insulin-like growth factor (IGF), interleukin (IL)-1, 2, 6, 8, tumor necrosis factor (TNF), interferon-γ (INF-γ) and colony-stimulating factors (CSFs) (Figure 7) [223]. Resveratrol modulates growth factors (e.g. EGF, TGF-α, TGF-β, and FJ) lead to decrease in cell migration, adhesion, and stop the cell proliferation in much cancer cell line [224-228]. Furthermore, resveratrol exhibited activation of mitogen-activated protein kinase [MAPK, extracellular signal-regulated kinase 1/2 (ERK1/2)], nuclear translocation of Ser15-phosphorylated p53, and p53-dependent apoptosis and stimulated c-fos and c-jun expression in hormone-insensitive DU145 prostate cancer cells.
On the other hand, emodin decreased the level of LIGHT-induced generation of ROS, as well as the expression of CCR1, CCR2, and ICAM-1 and the production of IL-8, MCP-1, TNF-alpha, IL-6, IκBalpha, and the phosphorylation of the p38 MAPK [229]. In addition, emodin induces gene expression profiling changes in p53, JAK2/ STAT3, and IGF-2 pathways lead to mitochondrial induction of apoptosis in myeloma and BCap-37 cells [208,230].
In vitro, emodin stimulated VEGF-α, inhibited basic fibroblast growth factor-induced proliferation and migration dose-dependently in human umbilical vein endothelial cells (HUVECs). It suppress cyclin D1 and E expression (G0/G1 arrest) and retinoblastoma protein phosphorylation inhibits proliferation, migration of HUVECs (231). In vivo, emodin strongly suppresses VEGF-α-induced angiogenesis through inhibition of phosphorylation of KDR/Flk-1 of the Matrigel plug in mice [231]. In addition, inhibition of phosphatidylinositol 3-kinase (PI3K) is considered as molecular target of emodin that leads to inhibition of EGF-induced migration in various human cancer cell lines [232]. In addition, treatment of EC cell with emodin inhibits expression of ICAM-1, ELAM-1, and VCAM-1 [159].
Anti-metastasis
The high mortality rates associated with cancer are caused by the metastatic spread of tumor cells from the original site. There are at least four inter-related biological events required for tumor metastasis: Angiogenesis, cell adhesion, cell invasion (extracellular matrix degradation and cell migration), and cell proliferation. Angiogenesis is a process of blood vessel formation, it is plays an important role in cell growth. Tumor angiogenesis actually starts with cancerous tumor cells releasing molecules that send signals to surrounding normal host tissue. These signaling upregulated different genes in the host tissue that, in turn, make proteins to encourage growth of new blood vessels. More than a dozen different proteins (e.g., FGF, EGF, GC-SF, IL-8, PDEGF, TGFα, TNF, VEGF), as well as several smaller molecules (e.g., adenosine, PGE), have been identified as angiogenic factors released by tumors as signals for angiogenesis. Among these molecules, VEGF and βFGF appear to be the most important for sustaining tumor growth (Figure 7). VEGF and βFGF are produced by many kinds of cancer cells and by certain types of normal cells. Chemopreventive phytochemicals; resveratrol, and emodin have been found to target these pathways [233].
Resveratrol effects on angiogenesis have been studied [175,233- 243]. Lin et al. reported that resveratrol suppressed the angiogenesis process that induced by VEGF in human umbilical vein endothelial cells [238]. Resveratrol suppressed of MMP-9 mRNA expression and PMA-mediated activation of JNK and PKC-α [234]. Bruder et al. reported the disruption of ROS dependent Src kinase activation is the underlying mechanism of the inhibition of VEGF-induced angiogenesis by resveratrol [237]. Resveratrol upregulated tissue type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) gene transcription in endothelial cells [239,240].
Similarly, one critical aspect of the anti-cancer activity of emodin is its inhibitory effect on cancer metastasis. Emodin potentially interferes with tumor metastasis progression at several pivotal points. Cell surface adhesion proteins (ICAM-1, VCAM-1, and ELAM-1) that regulated by NF-κB are inhibited by emodin [159,244]. The cholesterol content in membrane of cancer cells is higher than normal cells. Emodin decrease cholesterol content in cell membrane and disrupt the lipid raftsassociated integrin-signaling pathway [245]. Emodin inhibits EGFinduced cell migration in various cancer cells [232]. Emodin inhibits the PI3K-mediated Cdc42 and Rac1 activation and suppresses the p21- activated kinase (PAK) complex [232]. Similar to resveratrol, emodin inhibits MMP-9, AP-1 and NF-κB signaling pathways resulting in inhibit the invasiveness cancer cells [198,246-248].
Conclusion
From above mention studies that described in this review, we can conclude the traditional use of Fallopia japonica in many anticancer formulas relates to its ability to produce active SMs, especially resveratrol and emodin. F. japonica active SMs, resveratrol and emodin, have great potential in the prevention and therapy of a wide variety of tumors. F. japonica active SMs, resveratrol and emodin have antiproliferative effects through the induction of apoptosis in various cell lines: leukemia and breast, prostate, colon, pancreas, hepatic carcinomas. Lastly, resveratrol and emodin have potential for treating inflammatory disorders other than cancer; through their action as antiinflammatory, they can effect in different molecular targets, which are involved in chemotherapy approaches. To conclude, F. japonica should be considered for used in cancer therapy due to its SMs multifactoral effect; large scales clinical studies of the extract should be encouraged.
References
- Xu J, Yang Y (2009) Traditional Chinese medicine in the Chinese health care system. Health Policy 90: 133-139.
- Xiao X, Xiao P, Wang Y (2009) Some key issues about scientific research on traditional Chinese medicine. China J Chinese Materia Medica34: 119-123.
- Lu AP, Jia HW, Xiao C, Lu QP (2004) Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol 10: 1854-1856.
- Matsumoto C, Kojima T, Ogawa K, Kamegai S, Oyama T, et al. (2008) A Proteomic Approach for the Diagnosis of 'Oketsu' (blood stasis), a Pathophysiologic Concept of Japanese Traditional (Kampo) Medicine. Evid Based Complement Alternat Med 5: 463-474.
- Li WF, Jiang JG, Chen J (2008) Chinese medicine and its modernization demands. Arch Med Res 39: 246-251.
- Deng ZL (2007) Application of new techniques in the innovative research of Chinese herbal medicine. Chin Pharm 16: 58-59.
- Gleason HA, Cronquist A (1963) Manual of vascular plants of Northeastern United States and adjacent Canada. Princeton, Van Nostrand.
- Rhoads AF, Klein WM (1993) The vascular flora of Pennsylvania: annotated checklist and atlas. American Philosophical Society, Philadelphia.
- Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, et al. (2000) Isolation and identification of stilbenes in two varieties of Polygonum c uspidatum. J Agric Food Chem 48: 253-256.
- Child L, Wade PM (2000) The Japanese knotweed manual: the management and control of an invasive alien weed. Packard Publishing Ltd.
- Hollingsworth ML, Hollingsworth PM, Jenkins GI, Bailey JP, Ferris C (1998) The use of molecular markers to study patterns of genotypic diversity in some invasive alien Fallopia spp (Polygonaceae). Mol Ecol 7: 1681-1691.
- Beerling DJ, Bailey JP, Conolly AP (1994) Fallopia japonica (Houtt.) Ronse Decraene.J Ecol 82: 959-979.
- Seiger LA, Merchant HC (1997) Mechanical control of Japanese knotweed (Fallopia japonica [Houtt.] Ronse Decraene): Effects of cutting regime on rhizomatous reserves. Nat Areas J 17: 341-345.
- Basu S, Ghosh A, Hazra B (2005) Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: isolation of emodin and physcion as active antibacterial agents. Phytother Res 19: 888-894.
- Kitano A, Saika S, Yamanaka O, Ikeda K, Okada Y, et al. (2007) Emodin suppression of ocular surface inflammatory reaction. Invest Ophthalmol Vis Sci 48: 5013-5022.
- Kuo YC, Tsai WJ, Meng HC, Chen WP, Yang LY, et al. (2001) Immune reponses in human mesangial cells regulated by emodin from Polygonum hypoleucum Ohwi. Life Sci 68: 1271-1286.
- Wang XD, Gu LQ, Wu JY (2007) Apoptosis-inducing activity of new pyrazole emodin derivatives in human hepatocellular carcinoma HepG2 cells. Biol Pharm Bull 30: 1113-1116.
- Kimura Y, Kozawa M, Baba K, Hata K (1983) New Constitutents of Roots of Polygonum cuspidatum. Planta Med 48: 164-168.
- Shan T, Wang Y, Wu T, Guo J, Liu J, et al. (2008) Porcine adipose triglyceride lipase complementary deoxyribonucleic acid clone, expression pattern, and regulation by resveratrol. J Anim Sci 86: 1781-1788.
- Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, et al. (2003) Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol 473: 117-125.
- Zhao RZ, Liu S, Zhou LL (2005) Rapid Quantitative HPTLC Analysis, on One Plate, of Emodin, Resveratrol, and Polydatin in the Chinese Herb Polygonum cuspidatum. Chromatographia 61: 311-314.
- Zhou Z, Miwa M, Nara K, Wu B, Nakaya H, et al. (2003) Patch establishment and development of a clonal plant, Polygonum cuspidatum, on Mount Fuji. Mol Ecol 12: 1361-1373.
- Park CS, Lee YC, Kim JD, Kim HM, Kim CH (2004) Inhibitory effects of Polygonum cuspidatum water extract (PCWE) and its component resveratrol on acyl-coenzyme A-cholesterol acyltransferase activity for cholesteryl ester synthesis in HepG2 cells. Vascul Pharmacol 40: 279-284.
- Choi J, Conrad CC, Malakowsky CA, Talent JM, Yuan CS, et al. (2002) Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. Biochim Biophys Acta 1571: 201-210.
- Song JH, Kim SK, Chang KW, Han SK, Yi HK, et al. (2006) In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch Oral Biol 51: 1131-1140.
- Jayatilake GS, Jayasuriya H, Lee ES, Koonchanok NM, Geahlen RL, et al. (1993) Kinase inhibitors from Polygonum cuspidatum. J Nat Prod 56: 1805-1810.
- Xiao K, Xuan L, Xu Y, Bai D (2000) Stilbene glycoside sulfates from Polygonum cuspidatum. J Nat Prod 63: 1373-1376.
- Pervaiz S (2003) Resveratrol: from grapevines to mammalian biology. FASEB J 17: 1975-1985.
- Yang F, Zhang T, Ito Y (2001) Large-scale separation of resveratrol, anthraglycoside A and anthraglycoside B from Polygonum cuspidatum Sieb. et Zucc by high-speed counter-current chromatography. J Chromatogr A 919: 443-448.
- Zhang W, Jia Y, Huang Q, Li Q, Bi K (2007) Simultaneous Determination of Five Major Compounds in Polygonum cuspidatum by HPLC. Chromatographia 66: 685-689.
- Huang H, Zhang J, Chen G, Lu Z, Wang X, et al. (2008) High performance liquid chromatographic method for the determination and pharmacokinetic studies of oxyresveratrol and resveratrol in rat plasma after oral administration of Smilax china extract. Biomed Chromatogr 22: 421-427.
- Huang KC (1998) The pharmacology of Chinese herbs. CRC Press.
- Huang WY, Cai YZ, Xing J, Corke H, Sun M (2008) Comparative analysis of bioactivities of four Polygonum species. Planta Med 74: 43-49.
- Cui CB, Tezuka Y, Nakano H, Tamaoki T, Park JH (1992) Constituents of a fern, Davillia mariesii Moore. Isolation and identification of a novel norcarotane sesquiterpene glucoside, a chromone glucuronide, and two epicatechin glycosides. Chem Pharm Bull 40: 2035-2040.
- Xiao K, Xuan L, Xu Y, Bai D, Zhong D (2002) Constituents from Polygonum cuspidatum. Chem Pharm Bull 50: 605-608.
- Yoshitama K, Nishino H, Ozawa H, Sakatani M, Okabe Y, et al. (2006) Distribution pattern of anthocyanidins and anthocyanins in polygonaceous plants. J Plant Res 100: 143-149.
- Xiao K, Xuan LJ, Xu YM, Bai DL (2003) Studies on the chemical constituents of Polygonum cuspidatum. Chin Pharm J 38: 12-14.
- Sun MX, Li X, Liu WY, Xiao K (2009) 5,7-Dimethoxy-isobenzofuran-1(3H)-one. Acta Crystallogr Sect E Struct Rep Online 65: o2146.
- Sano K, Sanada S, Ida Y, Shoji J (1991) Studies on the constituents of the bark of Kalopanax pictus Nakai. Chem Pharm Bull 39: 865-870.
- Kim YS, Hwang CS, Shin DH (2005) Volatile constituents from the leaves of Polygonum cuspidatum S. et Z. and their anti-bacterial activities. Food Microbiology 22: 139-144.
- Nonomura S, Kanagawa H, Makimoto A (1963) Chemical constituents of polygonaceous plants. i. studies on the components of ko-j o-kon (Polygonum cuspidatum sieb. et zucc.). Yakugaku Zasshi 83: 988-990.
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, et al. (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24: 2783-2840.
- Lee JP, Min BS, An RB, Na MK, Lee SM, et al. (2003) Stilbenes from the roots of Pleuropterus ciliinervis and their antioxidant activities. Phytochemistry 64: 759-763.
- Jayasuriya H, Koonchanok NM, Geahlen RL, McLaughlin JL, Chang CJ (1992) Emodin, a protein tyrosine kinase inhibitor from Polygonum cuspidatum. J Nat Prod 55: 696-698.
- Matsuda H, Shimoda H, Morikawa T, Yoshikawa M (2001) Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg Med Chem Lett 11: 1839-1842.
- Park JH, Lee CK (2000) The encyclopedia of medicinal plants. Shinil Books, Seoul.
- Lin HW, Sun MX, Wang YH, Yang LM, Yang YR, et al. (2010) Anti-HIV activities of the compounds isolated from Polygonum cuspidatum and Polygonum multiflorum. Planta Med 76: 889-892.
- Pan YM, Zhang XP, Wang HS, Liang Y, Zhu JC, et al. (2007) Antioxidant potential of ethanolic extract of Polygonum cuspidatum and application in peanut oil. Food Chem 105: 1518-1524.
- Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK (2008) Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J Inflamm 5: 1.
- Lou Z, Huang W, Liu J (1995) Effects of Chinese herbs on impaired lymphocyte functions after thermal injury in mice. Chinese J Surg 33: 571-573.
- Wang Y, Zhao K, Wu X (1994) The prognostic implication of determination of activation of leucocyte in severe burns. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 10: 286-289.
- Wu KY (1992) Effect of crystal No 4 of Polygonum cuspidatum on microcirculatory disturbances during burn shock. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 8: 133-135.
- Zhu ZJ (1989) Effect of crystal No. 4 of Polygonum cuspidatum on the restoration of pulse pressure and microcirculatory perfusion during shock. Zhonghua Yi Xue Za Zhi 69: 279-81.
- Zhao KS (1989) Effect of Polygonum cuspidatum No 4 on the enhancement of cardiac function during burn shock. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 5: 275-278.
- Luo S, Luo L (1994) Effect of crystal no. 4 of polygonum cuspidatum on the viability of island flaps with venous stasis: an experimental study. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi 10: 222-225.
- Chang JS, Liu HW, Wang KC, Chen MC, Chiang LC, et al. (2005) Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antiviral Res 66: 29-34.
- Zheng HZ, Dong ZH, She J (1998) Huzhang, Rhizoma Polygonum cuspidatim. In: Modern Study of Traditional Chinese Medicine. Beijing.
- Hegde VR, Pu H, Patel M, Black T, Soriano A, et al. (2004) Two new bacterial DNA primase inhibitors from the plant Polygonum cuspidatum. Bioorg Med Chem Lett 14: 2275-2277.
- Lin MH, Hsu SY (1987) Studies on pharmacological effects of various extracts of Polygonum cuspidatum S. et Z. Tai-wan Yao Hsueh Tsa Chih 39:42-53.
- Horigome T, Kumar R, Okamoto K (1988) Effects of condensed tannins prepared from leaves of fodder plants on digestive enzymes in vitro and in the intestine of rats. Br J Nutr 60: 275-285.
- Lehninger AL, Nelson DL, Cox MM (1993) Principles of biochemistry. 2nd ed. New York, NY: Worth Publishers.
- Zhang Y, Liu Y, Wang T, Li B, Li H, et al. (2006) Resveratrol, a natural ingredient of grape skin: antiarrhythmic efficacy and ionic mechanisms. Biochem Biophys Res Commun 340: 1192-1199.
- Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71: 1397-1421.
- Lin YW, Yang FJ, Chen CL, Lee WT, Chen RS (2010) Free radical scavenging activity and antiproliferative potential of Polygonum cuspidatum root extracts. J Nat Med 64: 146-152.
- Feng L, Zhang LF, Yan T, Jin J, Tao WY (2006) Studies on active substance of anticancer effect in Polygonum cuspidatum. Zhong Yao Cai 29: 689-691.
- Zannetti A, Del Vecchio S, Carriero MV, Fonti R, Franco P,et al. (2000) Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Res6:1546-1551.
- Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, et al. (2002) Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer 2: 35.
- Wang L, Wei D, Huang S, Peng Z, Le X, et al. (2003) Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res 9: 6371-6380.
- Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, et al. (2004) Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int J Oncol 25: 461-468.
- Yao JC, Wang L, Wei D, Gong W, Hassan M, et al. (2004) Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res 10:4109-4117.
- Shin JA, Shim JH, Jeon JG, Choi KH, Choi ES, et al. (2011) Apoptotic effect of Polygonum Cuspidatum in oral cancer cells through the regulation of specificity protein 1. Oral Dis 17: 162-170.
- Schneider Y, Vincent F, Duranton B, Badolo L, Gossé F, et al. (2000) Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett 158: 85-91.
- Alkhalaf M (2007) Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur J Cancer Prev 16:334-341.
- Hsieh TC, Wu JM (1999) Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines.Exp Cell Res 249: 109-115.
- Mitchell SH, Zhu W, Young CY (1999) Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res 59: 5892-5895
- Hsieh TC, Wu JM (2000) Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer Res 20:225-8.
- Atten MJ, Attar BM, Milson T, Holian O (2001) Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C-mediated mechanism. Biochem Pharmacol 62: 1423-1432.
- Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, et al. (2002) Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis 23: 1327-1333.
- Sun NJ, Woo SH, Cassady JM, Snapka RM (1998) DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J Nat Prod 61: 362-366.
- Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O (1998) Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett 421: 277-279.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218-220.
- Berg SL (1996) Status of new anthrapyrazole and pyrazoloacridine derivatives. Crit Rev Oncol Hematol 22: 79-87.
- Shenkenberg TD, Von Hoff DD (1986) Mitoxantrone: a new anticancer drug with significant clinical activity. Ann Intern Med 105: 67-81
- Bellosillo B, Colomer D, Pons G, Gil J (1998) Mitoxantrone, a topoisomerase II inhibitor, induces apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol 100: 142-146.
- Van Wyk BE, Wink M (2004) Medicinal plants of the world : an illustrated scientific guide to important medicinal plants and their uses. 1st ed. Portland, Or.: Timber Press.
- Wink M (2007) Molecular modes of action of cytotoxic alkaloids: from DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids Chem Biol 64: 1-47.
- Chang CJ, Ashendel CL, Chan TCK, Geahlen RL, McLaughlin J (1999) Oncogene signal transduction inhibitors from Chinese medicinal plants. Pure and Applied Chemistry 71:1101-1104.
- Zhang L, Chang CJ, Bacus SS, Hung MC (1995) Suppressed transformation and induced differentiation of HER-2/neu-overexpressing breast cancer cells by emodin. Cancer Res 55: 3890-3896.
- Cha TL, Qiu L, Chen CT, Wen Y, Hung MC (2005) Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res 65: 2287-2295.
- Chan TC, Chang CJ, Koonchanok NM, Geahlen RL (1993) Selective inhibition of the growth of ras-transformed human bronchial epithelial cells by emodin, a protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun 193: 1152-1158.
- Kim HR, Kim K, Lee KH, Kim SJ, Kim J (2008) Inhibition of casein kinase 2 enhances the death ligand- and natural kiler cell-induced hepatocellular carcinoma cell death. Clin Exp Immunol 152: 336-344.
- Cai J, Niu X, Chen Y, Hu Q, Shi G, et al. (2008)Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia 10:41-51.
- Guo Q, Chen Y, Zhang B, Kang M, Xie Q, et al. (2009) Potentiation of the effect of gemcitabine by emodin in pancreatic cancer is associated with survivin inhibition. Biochem Pharmacol 77: 1674-1683.
- Lai JM, Chang JT, Wen CL, Hsu SL (2009) Emodin induces a reactive oxygen species-dependent and ATM-p53-Bax mediated cytotoxicity in lung cancer cells. Eur J Pharmacol 623: 1-9.
- Zhang L, Hung MC (1996) Sensitization of HER-2/neu-overexpressing non-small cell lung cancer cells to chemotherapeutic drugs by tyrosine kinase inhibitor emodin. Oncogene 12: 571-576.
- Zhang L, Lau YK, Xi L, Hong RL, Kim DS, et al. (1998) Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. c Oncogene 16: 2855-2863.
- Kuo YC, Sun CM, Ou JC, Tsai WJ (1997) A tumor cell growth inhibitor from Polygonum hypoleucum Ohwi. Life Sci 61: 2335-2344.
- Shieh DE, Chen YY, Yen MH, Chiang LC, Lin CC (2004) Emodin-induced apoptosis through p53-dependent pathway in human hepatoma cells. Life Sci 74: 2279-2290.
- Danielson PB (2002) The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 3: 561-597.
- Boyle P (1997) Cancer, cigarette smoking and premature death in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki. Lung Cancer 17:1-60.
- Park JY, Shigenaga MK, Ames BN (1996) Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA 93: 2322-2327.
- Wang SS, Samet JM (1997) Tobacco smoking and cancer: the promise of molecular epidemiology. Salud Publica Mex 39: 331-345.
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, et al. (1999) Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol 56: 784-790.
- Ciolino HP, Yeh GC (1999) Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol 56: 760-767.
- Ciolino HP, Daschner PJ, Yeh GC (1998) Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res 58: 5707-5712.
- Piver B, Berthou F, Dreano Y, Lucas D (2001) Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol Lett 125: 83-91.
- Chang TK, Yeung RK (2001) Effect of trans-resveratrol on 7-benzyloxy-4-trifluoromethylcoumarin O-dealkylation catalyzed by human recombinant CYP3A4 and CYP3A5. Can J Physiol Pharmacol 79: 220-226.
- Chan WK, Delucchi AB (2000) Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci 67: 3103-3112.
- Chen Q, Wang E, Ma L, Zhai P (2012) Dietary resveratrol increases the expression of hepatic 7alpha-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis 11: 56.
- Tome-Carneiro J, Gonzalvez M, Larrosa M, Garcia-Almagro FJ, Aviles-Plaza F, et al. (2012) Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res 56: 810-821.
- Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, et al. (2012) One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol 110: 356-363.
- Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, et al. (2014) Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods 6: 11-32.
- Sun M, Sakakibara H, Ashida H, Danno G, Kanazawa K (2000) Cytochrome P4501A1-inhibitory action of antimutagenic anthraquinones in medicinal plants and the structure-activity relationship. Biosci Biotechnol Biochem 64: 1373-1378.
- Soucek P (1999) Cytochrome P450 destruction by quinones: comparison of effects in rat and human liver microsomes. Chem Biol Interact 121: 223-236.
- Duncan RF, Hershey JW (1987) Translational repression by chemical inducers of the stress response occurs by different pathways. Arch Biochem Biophys 256: 651-661.
- Castiglione S, Paggi MG, Delpino A, Zeuli M, Floridi A (1990) Inhibition of protein synthesis in neoplastic cells by rhein. Biochem Pharmacol 40: 967-973.
- Hao NJ, Huang MP, Lee H (1995) Structure-activity relationships of anthraquinones as inhibitors of 7-ethoxycoumarin O-deethylase and mutagenicity of 2-amino-3-methylimidazo[4,5-f]quinoline. Mutat Res 328: 183-191.
- Marczylo T, Arimoto-Kobayashi S, Hayatsu H (2000) Protection against Trp-P-2 mutagenicity by purpurin: mechanism of in vitro antimutagenesis. Mutagenesis 15: 223-228.
- Wang HW, Chen TL, Yang PC, Ueng TH (2001) Induction of cytochromes P450 1A1 and 1B1 by emodin in human lung adenocarcinoma cell line CL5. Drug Metab Dispos 29: 1229-1235.
- Whitlock JP Jr (1999) Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol 39: 103-125.
- Ryan DE, Levin W (1990) Purification and characterization of hepatic microsomal cytochrome P-450. Pharmacol Ther 45: 153-239.
- Shimada T, Gillam EM, Sutter TR, Strickland PT, Guengerich FP, et al. (1997) Oxidation of xenobiotics by recombinant human cytochrome P450 1B1. Drug Metab Dispos 25: 617-622.
- Szaefer H, Cichocki M, Brauze D, Baer-Dubowska W (2004) Alteration in phase I and II enzyme activities and polycyclic aromatic hydrocarbons-DNA adduct formation by plant phenolics in mouse epidermis. Nutr Cancer 48: 70-77.
- Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, et al. (2003) Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res 523-524: 163-72.
- Prochaska HJ, Santamaria AB (1988) Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem 169: 328-336.
- Duvoix A, Delhalle S, Blasius R, Schnekenburger M, Morceau F, et al. (2004) Effect of chemopreventive agents on glutathione S-transferase P1-1 gene expression mechanisms via activating protein 1 and nuclear factor kappaB inhibition. Biochem Pharmacol 68: 1101-1111.
- Holmes-McNary M, Baldwin AS Jr (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 60: 3477-3483.
- O'Byrne KJ, Dalgleish AG (2001) Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 85: 473-483.
- Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, et al. (1998) Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem 273:21875-21882.
- MacCarrone M, Lorenzon T, Guerrieri P, Agrò AF (1999) Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur J Biochem 265: 27-34
- Hudson TS1, Hartle DK, Hursting SD, Nunez NP, Wang TT, et al. (2007) Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res 67: 8396-8405.
- Ghosh S, Das Sarma M, Patra A, Hazra B (2010) Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J Pharm Pharmacol 62: 1158-1166.
- Gautam R, Karkhile KV, Bhutani KK, Jachak SM (2010) Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumex nepalensis. Planta Med 76: 1564-1569.
- Chen YC, Yang LL, Lee TJF (2000)Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-[kappa]B activation. Biochem Pharmacol 59:1445-1457
- Wang CC1, Huang YJ, Chen LG, Lee LT, Yang LL (2002) Inducible nitric oxide synthase inhibitors of Chinese herbs III. Rheum palmatum. Planta Med 68: 869-874.
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, et al. (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39: 361-398.
- Boumendjel A, Boutonnat J, Robert J (2009) ABC transporters and multidrug resistance. Hoboken, N.J.: John Wiley & Sons.
- Nabekura T, Kamiyama S, Kitagawa S (2005) Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem Biophys Res Commun 327: 866-870.
- Quan F, Pan C, Ma Q, Zhang S, Yan L (2008) Reversal effect of resveratrol on multidrug resistance in KBv200 cell line. Biomed Pharmacother 62: 622-629.
- Kweon SH, Song JH, Kim TS (2010) Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem Biophys Res Commun 395: 104-110.
- Quan F, Zhang SQ, Bai YX, Yao XB, Li HH, et al. (2009) Resveratrol increases sensitivity of CNE2 cellsto chemotherapeutic drugs under hypoxia. J Chin Int Med 7: 952-957.
- Wang W, Sun YP, Huang XZ, He M, Chen YY, et al. (2010) Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem Pharmacol 79: 1134-1140.
- Huang XZ, Wang J, Huang C, Chen YY, Shi GY, et al. (2008) Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther7: 468-475.
- Fischer U, Schulze-Osthoff K (2005) Apoptosis-based therapies and drug targets. Cell Death Differ 12: 942-961.
- Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309-1312.
- Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM (1999) Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun 254: 739-743.
- Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S (1998) Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 92: 996-1002.
- Dorrie J, Gerauer H, Wachter Y, Zunino SJ (2001) Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res 61:4731-4739.
- Tinhofer I, Bernhard D, Senfter M, Anether G, Loeffler M, et al. (2001) Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl-2. FASEB J 15: 1613-1615.
- Gautam SC, Xu YX, Dumaguin M, Janakiraman N, Chapman RA (2000) Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant 25: 639-645.
- Hsieh TC, Burfeind P, Laud K, Backer JM, Traganos F, et al. (1999) Cell cycle effects and control of gene expression by resveratrol in human breast carcinoma cell lines with different metastatic potentials. Int J Oncol 15: 245-252.
- Gehm BD, McAndrews JM, Chien PY, Jameson JL (1997) Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci 94: 14138-14143.
- Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, et al. (2003) Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem 278: 41482-41490.
- Wang C, Wu X, Chen M, Duan W, Sun L, et al. (2007) Emodin induces apoptosis through caspase 3-dependent pathway in HK-2 cells. Toxicol 231: 120-128.
- Su YT, Chang HL, Shyue SK, Hsu SL (2005) Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol 70: 229-241.
- Jing X, Ueki N, Cheng J, Imanishi H, Hada T (2002) Induction of apoptosis in hepatocellular carcinoma cell lines by emodin. Jpn J Cancer Res 93: 874-882.
- Brown M, Bellon M, Nicot C (2007) Emodin and DHA potently increase arsenic trioxide interferon-alpha-induced cell death of HTLV-I-transformed cells by generation of reactive oxygen species and inhibition of Akt and AP-1. Blood 109: 1653-1659.
- Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, et al. (2002) Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol 64: 1713-1724.
- Kumar A, Dhawan S, Aggarwal BB (1998) Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene 17: 913-918.
- Su JL, Lin MT, Hong CC, Chang CC, Shiah SG, et al. (2005) Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis 26:1-10.
- Huang Z, Chen G, Shi P (2008) Emodin-induced apoptosis in human breast cancer BCap-37 cells through the mitochondrial signaling pathway. Arch Pharm Res 31:742-748.
- Heo SK, Yun HJ, Park WH, Park SD (2008) Emodin inhibits TNF-alpha-induced human aortic smooth-muscle cell proliferation via caspase- and mitochondrial-dependent apoptosis. J Cell Biochem 105: 70-80.
- Lin SY, Lai WW, Ho CC, Yu FS, Chen GW, et al. (2009) Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res 29:327-335.
- Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR (2007) Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer 120:2344-2352.
- Caldas H1, Jaynes FO, Boyer MW, Hammond S, Altura RA (2006) Survivin and Granzyme B-induced apoptosis, a novel anticancer therapy. Mol Cancer Ther 5: 693-703.
- Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E (2008) Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis 13: 259-271.
- Zhang L, Lau YK, Xia W, Hortobagyi GN, Hung MC (1999) Tyrosine kinase inhibitor emodin suppresses growth of HER-2/neu-overexpressing breast cancer cells in athymic mice and sensitizes these cells to the inhibitory effect of paclitaxel. Clin Cancer Res 5:343-353.
- Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274: 1664-1672.
- Ortonne JP (2004) Redefining clinical response in psoriasis: targeting the pathological basis of disease. J Drugs Dermatol 3: 13-20.
- Deane NG, Parker MA, Beauchamp RD (2005) Cell proliferation: a matter of time and place. Surgery 138: 1-7.
- Jang M, Pezzuto JM (1999) Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res 25: 65-77.
- Park JW, Choi YJ, Jang MA, Lee YS, Jun DY, et al. (2001) Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett 163: 43-49.
- Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, et al. (1999) Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett 140: 1-10.
- Park JW, Choi YJ, Suh SI, Baek WK, Suh MH, et al. (2001) Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 22:1633-1639.
- Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM (1999) Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res 59:2596-2601.
- Kamei H, Koide T, Kojima T, Hashimoto Y, Hasegawa M (1998) Inhibition of cell growth in culture by quinones. Cancer Biother Radiopharm 13: 185-188.
- Hsu CM, Hsu YA, Tsai Y, Shieh FK, Huang SH, et al. (2010) Emodin inhibits the growth of hepatoma cells: finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells. Biochem Biophys Res Commun 392: 473-478.
- Zheng HY, Hu JD, Zheng ZH, Huang LY, Chen YY, et al. (2007) Emodin induces leukemic HL-60 cells apoptosis probably by inhibiting Akt signal pathway. Yao Xue Xue Bao 42: 1142-1146
- Chen YY, Zheng HY, Hu JD, Zheng ZH, Zheng J, et al. (2007) Inhibitory effects of emodin on drug-resistant HL-60/ADR cell proliferation and its induction of apoptosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 15: 955-960.
- Nebert DW (2002) Transcription factors and cancer: an overview. Toxicology 182: 131-141.
- Lee TI, Young RA (2000) Transcription of eukaryotic protein-coding genes. Annu Rev Genet 34: 77-137.
- Sykiotis GP, Bohmann D (2010) Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal 3: re3.
- Sheikh MS, Huang Y (2003) Death receptor activation complexes: it takes two to activate TNF receptor 1. Cell Cycle 2: 550-552.
- Escárcega RO, Fuentes-Alexandro S, García-Carrasco M, Gatica A, Zamora A (2007) The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol) 19: 154-161.
- Mantovani A, Marchesi F, Portal C, Allavena P, Sica A (2008) Linking inflammation reactions to cancer: novel targets for therapeutic strategies. Adv Exp Med Biol 610: 112-127.
- Bharti AC, Aggarwal BB (2002) Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol 64: 883-888.
- Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745-756.
- Manna SK, Mukhopadhyay A, Aggarwal BB (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 164: 6509-6519.
- Bhat KP, Pezzuto JM (2002) Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci 957: 210-229.
- Kim YA, Lee WH, Choi TH, Rhee SH, Park KY, et al. (2003) Involvement of p21WAF1/CIP1, pRB, Bax and NF-kappaB in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int J Oncol 23: 1143-1149
- Li HL, Chen HL, Li H, Zhang KL, Chen XY, et al. (2005) Regulatory effects of emodin on NF-kappaB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int J Mol Med 16: 41-47.
- Liu ZG (2005) Molecular mechanism of TNF signaling and beyond. Cell Res 15: 24-27.
- Jang JH, Surh YJ (2003) Potentiation of cellular antioxidant capacity by Bcl-2: implications for its antiapoptotic function. Biochem Pharmacol 66: 1371-1379.
- Kurosu T, Fukuda T, Miki T, Miura O (2003) BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in B-cell lymphoma cells. Oncogene 22:4459-4568.
- Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, et al. (2003) Scavenging system efficiency is crucial for cell resistance to ROS-mediated methylglyoxal injury. Free Radic Biol Med 35: 856-871.
- Baldwin AS (2001) Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 107: 241-246
- Mayo MW, Baldwin AS (2000) The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta 1470: 55-62.
- Huang Q, Shen HM, Ong CN (2004) Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol 68: 361-371.
- Lu J, Ho CH, Ghai G, Chen KY (2001) Resveratrol analog, 3,4,5,4'-tetrahydroxystilbene, differentially induces pro-apoptotic p53/Bax gene expression and inhibits the growth of transformed cells but not their normal counterparts. Carcinogenesis 22: 321-328.
- Narayanan BA, Narayanan NK, Re GG, Nixon DW (2003) Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int J Cancer 104: 204-212.
- Shih A, Davis FB, Lin HY, Davis PJ (2002) Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab 87: 1223-1232.
- Haider UG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM (2003) Resveratrol increases serine15-phosphorylated but transcriptionally impaired p53 and induces a reversible DNA replication block in serum-activated vascular smooth muscle cells. Mol Pharmacol 63: 925-932.
- Huang Q, Lu G, Shen HM, Chung MC, Ong CN (2007) Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev 27: 609-630.
- Fullbeck M, Huang X, Dumdey R, Frommel C, Dubiel W, et al. (2005) Novel curcumin- and emodin-related compounds identified by in silico 2D/3D conformer screening induce apoptosis in tumor cells. BMC Cancer 5: 97.
- Uhle S, Medalia O, Waldron R, Dumdey R, Henklein P, et al. (2003) Protein kinase CK2 and protein kinase D are associated with the COP9 signalosome. EMBO J 22: 1302-1312.
- Bech-Otschir D, Kraft R, Huang X, Henklein P, Kapelari B, et al. (2001) COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J 20: 1630-1639
- Wang X, Zou Y, Sun A, Xu D, Niu Y, et al. (2007) Emodin induces growth arrest and death of human vascular smooth muscle cells through reactive oxygen species and p53. J Cardiovasc Pharmacol 49: 253-260.
- Huang Z, Chen G, Shi P (2009) Effects of emodin on the gene expression profiling of human breast carcinoma cells. Cancer Detect Prev 32: 286-291.
- Wang XF, Ge JB, Sun AJ, Xu DL, Wang KQ (2006) Involvement of p53-dependent pathway in the antiproliferative activity of emodin in human smooth muscle cell. Zhonghua Xin Xue Guan Bing Za Zhi 34: 44-49.
- Kuo TC, Yang JS, Lin MW, Hsu SC, Lin JJ, et al. (2009) Emodin has cytotoxic and protective effects in rat C6 glioma cells: roles of Mdr1a and nuclear factor kappaB in cell survival. J Pharmacol Exp Ther 330: 736-744.
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912-1934.
- Sherr CJ (2004) Principles of tumor suppression. Cell 116: 235-246.
- Adhami VM1, Afaq F, Ahmad N (2001) Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun 288: 579-585.
- Shih A, Zhang S, Cao HJ, Boswell S, Wu YH, et al. (2004) Inhibitory effect of epidermal growth factor on resveratrol-induced apoptosis in prostate cancer cells is mediated by protein kinase C-alpha. Mol Cancer Ther 3: 1355-1364
- Unger GM, Davis AT, Slaton JW, Ahmed K (2004) Protein kinase CK2 as regulator of cell survival: Implications for cancer therapy. Curr Cancer Drug Targets4: 77-84
- Olsten ME, Litchfield DW (2004) Order or chaos? An evaluation of the regulation of protein kinase CK2. Biochem Cell Biol 82: 681-693.
- Yim H, Lee YH, Lee CH, Lee SK (1999) Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Med 65: 9-13.
- Battistutta R, Sarno S, De Moliner E, Papinutto E, Zanotti G, et al. (2000) The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. J Biol Chem 275: 29618-29622.
- Sarno S, Moro S, Meggio F, Zagotto G, Dal Ben D, et al. (2002) Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther 93: 159-168.
- Fredenhagen A,Mett H, Meyer T, Buchdunger E, Regenass U, et al. (1995) Protein tyrosine kinase and protein kinase C inhibition by fungal anthraquinones related to emodin. J Antibiot (Tokyo) 48: 1355-1358
- Reddy KB, Nabha SM, Atanaskova N (2003) Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev 22: 395-403
- Wang R, Wan Q, Zhang Y, Huang F, Yu K, et al.(2007) Emodin suppresses interleukin-1beta induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK. Life Sci 80: 2481-2488.
- Hahn WC, Weinberg RA (2002) Rules for making human tumor cells. N Engl J Med 347: 1593-1603.
- Serrero G, Lu R (2001) Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid Redox Signal 3: 969-979.
- De Ledinghen V, Monvoisin A, Neaud V, Krisa S, Payrastre B,et al. (2001) Trans-resveratrol, a grapevine-derived polyphenol, blocks hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells. Int J Oncol 19: 83-88.
- Kaneuchi M, Sasaki M, Tanaka Y, Yamamoto R, Sakuragi N, et al. (2003) Resveratrol suppresses growth of Ishikawa cells through down-regulation of EGF. Int J Oncol 23: 1167-1172.
- Huang N, Lu H, Chang L, Zhang H, Zhang H, et al. (2010) Resveratrol inhibits EGF-induced invasion of human lung adenocarcinoma A549 cells. Zhongguo Fei Ai Za Zhi 13: 287-291.
- Azios NG, Dharmawardhane SF (2005) Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia 7: 128-140
- Heo SK, Yun HJ, Noh EK, Park SD (2010) Emodin and rhein inhibit LIGHT-induced monocytes migration by blocking of ROS production. Vascul Pharmacol 53: 28-37.
- Muto A, Hori M, Sasaki Y, Saitoh A, Yasuda I,et al. (2007) Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol Cancer Ther 6: 987-994.
- Kwak HJ, Park MJ, Park CM, Moon SI, Yoo DH, et al. (2006) Emodin inhibits vascular endothelial growth factor-A-induced angiogenesis by blocking receptor-2 (KDR/Flk-1) phosphorylation. Int J Cancer 118: 2711-2720.
- Huang Q, Shen HM, Ong CN (2005) Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol Life Sci 62: 1167-1175.
- Bråkenhielm E, Cao R, Cao Y (2001) Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J 15: 1798-1800
- Woo JH, Lim JH, Kim YH, Suh SI, Min DS, et al. (2004) Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene 23: 1845-1853
- Szende B, Tyihák E, Király-Véghely Z (2000) Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med 32: 88-92.
- Igura K, Ohta T, Kuroda Y, Kaji K (2001) Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett 171: 11-16
- Bruder JL, Hsieh T, Lerea KM, Olson SC, Wu JM (2001) Induced cytoskeletal changes in bovine pulmonary artery endothelial cells by resveratrol and the accompanying modified responses to arterial shear stress. BMC Cell Biol 2: 1
- Lin MT, Yen ML, Lin CY, Kuo ML (2003) Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 64: 1029-1036
- Abou-Agag LH, Aikens ML, Tabengwa EM, Benza RL, Shows SR, et al. (2001) Polyphyenolics increase t-PA and u-PA gene transcription in cultured human endothelial cells. Alcohol Clin Exp Res 25: 155-162
- Bertelli AA, Baccalini R, Battaglia E, Falchi M, Ferrero ME (2001) Resveratrol inhibits TNF alpha-induced endothelial cell activation. Therapie 56: 613-616.
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, et al. (2006) Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol 291: H1694-H1699.
- Li HF, Chen SA, Wu SN (2000) Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc Res 45: 1035-1045.
- Fulgenzi A, Bertelli AA, Magni E, Ferrero E, Ferrero ME (2001) In vivo inhibition of TNFalpha-induced vascular permeability by resveratrol. Transplant Proc 33: 2341-2343.
- Rakheja D1, Kapur P, Hoang MP, Roy LC, Bennett MJ (2005) Increased ratio of saturated to unsaturated C18 fatty acids in colonic adenocarcinoma: implications for cryotherapy and lipid raft function. Med Hypotheses 65: 1120-1123.
- Huang Q, Shen HM, Shui G, Wenk MR, Ong CN. (2006) Emodin inhibits tumor cell adhesion through disruption of the membrane lipid Raft-associated integrin signaling pathway. Cancer Res66: 5807-15.
- Wang X, Zhen Y (2001) Inhibitory effect of emodin on metastasis-associated properties of PG cells from a human highly metastatic giant lung carcinoma cell line. Chinese J Cancer 20: 789
- Zhu F, Liu XG, Liang NC (2003) Effect of emodin and apigenin on invasion of human ovarian carcinoma HO-8910PM cells in vitro. Ai Zheng 22: 358-362.
- Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H, et al. (2005) Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int J Oncol 27: 839-846.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 16692
- [From(publication date):
November-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 15795
- PDF downloads : 897