Factors Influencing Weight Loss after Bariatric Surgery: A Multivariate Analysis
Received: 10-May-2021 / Accepted Date: 24-May-2021 / Published Date: 31-May-2021 DOI: 10.4172/2165-7904.1000442
Abstract
Introduction: Obesity is a major public health concern due to its association with the development of obesityrelated comorbidities. The aim of this study is to identify the potential relationship between pre-operative patient-related factors with weight loss at 1 and 3 years following Laparoscopic Roux-en-Y gastric bypass (LRYGB), Laparoscopic Sleeve Gastrectomy (LSG) or laparoscopic Adjustable Gastric Banding (LAGB).
Methods: A retrospective cohort study was carried out on 1,210 patients with obesity who underwent primary bariatric surgery at Chelsea and Westminster Hospital NHS Trust between September 1999 and May 2017.
Results: The study sample included 1210 participants. The mean age was 45.3 ± 11.3 years. Females represented 78.3% (n=948) of the sample. The mean BMI at initial assessment was 47.6 ± 7.47 kg/m2. LRYGB was found to be the most effective followed by LSG then LAGB at 1 and 3 years as evidenced by higher number of patients having %EWL>50 (P<0.001). After adjusting for confounders, older age, higher initial BMI, black ethnicity, diabetes, and Obstructive Sleep Apnea (OSA) were associated with suboptimal weight loss. The presence of hypertension, smoking, and gender were not associated with %EWL or TBWL% at any time point.
Conclusion: Patient age, race, type of surgery, pre-operative BMI, and co-morbidity status influence weight loss after bariatric surgery, with older age, black ethnicity, LAGB, higher initial BMI, diabetes, and OSA predicting a lower weight loss in the short and medium term. Hypertension, smoking, and gender did not seem to influence weight loss in our study. The study findings could alert the surgeon to take more aggressive measures towards patients with risk factors, in part, by choosing a more effective bariatric procedure.
Keywords: Bariatric Surgery; BMI; Weight loss; Obesity
Introduction
Obesity is a major public health concern due to its association with the development of obesity-related comorbidities [1,2]. Bariatric surgery is considered the most effective intervention for ensuring significant and sustained weight loss and amelioration of obesityrelated comorbidities [3-6]. Despite reported successful weight loss in the short term following bariatric surgery [7,8], many patients do not reach their weight loss goals, and some are found to regain weight [9-11], which poses a substantial financial burden in the management of obesity and its related co-morbidities [12]. Weight regain has been attributed to several factors; such as Body Mass Index (BMI), advanced age, gender, existing co-morbidities, and consistency and intensity of post-operative follow-up [13-15]. In fact, several studies have suggested socio-demographic, medical, and psychosocial factors to influence weight loss following bariatric surgery [16-18]. The choice of surgery for each patient also plays a role and the type of procedure chosen should be tailored to each patient based on specific factors that include but are not limited to BMI, medical considerations, and the existence of comorbidities [19-22]. The aim of this study is to identify patient-related factors; such as sociodemographic and habitual data as well as co-morbidity status, that are associated with worse weight loss outcomes after bariatric surgery (LRYGB, LSG and LAGB), helping to identify patients in need of more aggressive measures to achieve long-term weight loss outcomes and remission of comorbidities after bariatric surgery.
Methods
Study design
A retrospective cohort study was carried out on 1,210 patients with obesity who underwent primary bariatric surgery following standardized protocols and surgical techniques at the Bariatric and Metabolic Surgery unit, Chelsea and Westminster Hospital NHS Trust between September 1999 and May 2017. Inclusion criteria were patients who entered the bariatric pathway, which involved an initial visit to the advanced bariatric nurse practitioner, bariatric dietitian, anesthesiologist, and bariatric psychologist, followed by weekly discussions in bariatric multi-disciplinary team (MDT) meeting. Patients who were deemed suitable for surgery by the MDT were those who met the criteria for bariatric surgery and had successfully completed at least one year in the bariatric pathway. All eligible patients were referred for assessment and scheduling of surgery (LRYGB, LSG or LAGB) based on the MDT recommendation. All patients were advised to stop smoking for at least six months prior to surgery. Exclusion criteria included patients who had a previous bariatric procedure or those who had a subsequent revision bariatric intervention.
Study variables
Patient information were gathered from medical records. These included sociodemographic data, such as age, gender, marital status (single/married), and ethnic background (black/white/other), and habitual data, such as smoking status (previous/current/never). Preoperative factors included initial BMI and the presence of obesity related co-morbidities, such as type 2 diabetes, hypertension, and obstructive sleep apnea. Initial (preoperative) weight and height were measured using standard protocols by a nurse during patients’ preoperative appointment and BMI was calculated. Percentage Excess Weight Loss (EWL) and Total Body Weight Loss (TBWL)% were calculated postoperatively at each follow up. Co-morbidities were recorded and cross checked with patient hospital medical records.
Post-operative follow-up
Postoperatively patients were regularly followed-up by the bariatric surgeon, dietitian and specialist nurse at 2 weeks, 6 weeks, 3 months, 6 months, 9 months, 12 months and 18 months and annually thereafter. At each follow-up, patients’ weight was measured and BMI and percentage EWL and TBWL were calculated A proton pump inhibitor (PPI) was routinely prescribed for all patients for the first 3 months post-surgery.
Ethical approval
Ethical approval was granted by the Institutional Review Board at Chelsea and Westminster Hospital Trust in London, United Kingdom.
Statistical analysis
Descriptive statistics were calculated for all study variables, using mean, standard deviation for continuous variables and frequencies and percentages for categorical variables. Variables were examined for missing observations prior to the analysis. Variables with 20% or fewer missing observations were included in the analysis. For comparison to investigate the association between surgery type and EWL greater than 50% at 1 and 3 years, Chi squared test was used. Linear regression was used to investigate the association between preoperative factors and surgery type with TBWL% and %EWL at 1 and 3 year follow up. Univariate associations were first analyzed and significant predictor factors were adjusted for in the multivariate analysis. Stepwise linear regression with backward elimination was carried out. All statistical analysis was performed using SPSS v 25 and hypothesis testing was performed at 5% level of significance.
Results
The study sample included 1210 patients. Follow up data was available for 1194 patients (99%) at 1 year, and 935 patients (77%) at 3 years. Response rate at 1 and 3 year of follow-up was 99% (1194 patients) and 77% (935 patients). Descriptive preoperative and postoperative weight loss data of the study population are presented in Table 1. The mean age was 45.3 ± 11.3 years, of which 78.3% were female. The mean BMI at initial assessment was 47.6 ± 7.47 kg/m2. LAGB, LSG and LRYGB was performed in 31.2%, 21.4% and 47.4% of the study population respectively.
| Variables | N=1210 | N |
|---|---|---|
| Patient's Age1 | 45.3 (11.4) | 1210 |
| Gender: 2 | 1210 | |
| Male | 262 (21.7%) | |
| Female | 948 (78.3%) | |
| Ethnic background: 2 | 1210 | |
| White | 697 (57.6%) | |
| Black | 118 (9.75%) | |
| Other | 395 (32.6%) | |
| Type 2 Diabetes: 2 | 1144 | |
| No | 827 (72.3%) | |
| Yes | 317 (27.7%) | |
| Smoking: 2 | 1100 | |
| No | 678 (61.6%) | |
| Yes /ever smoked | 422 (38.4%) | |
| Hypertension: 2 | 1133 | |
| No indication of hypertension; or on no treatment | 698 (61.6%) | |
| Hypertension on treatment | 435 (38.4%) | |
| Sleep Apnea: 2 | 1051 | |
| No diagnosis or indication of sleep apnea | 863 (82.1%) | |
| Sleep apnea on CPAP/BIPAP | 188 (17.9%) | |
| BMI (Initial Assessment) 1 | 47.6 (7.47) | 1203 |
| Weight (Initial Assessment) 1 | 132 (25.3) | 1203 |
| Surgery Type: 2 | 1210 | |
| LAGB | 377 (31.2%) | |
| LSG | 259 (21.4%) | |
| RYGB | 574 (47.4%) | |
| 1Data are presented as means (standard deviations) for continuous variables, 2Data are presented as number (percentage) for categorical variables. |
||
Table 1: Descriptive data of patient characteristics included in the study sample.
Effectiveness of surgery type
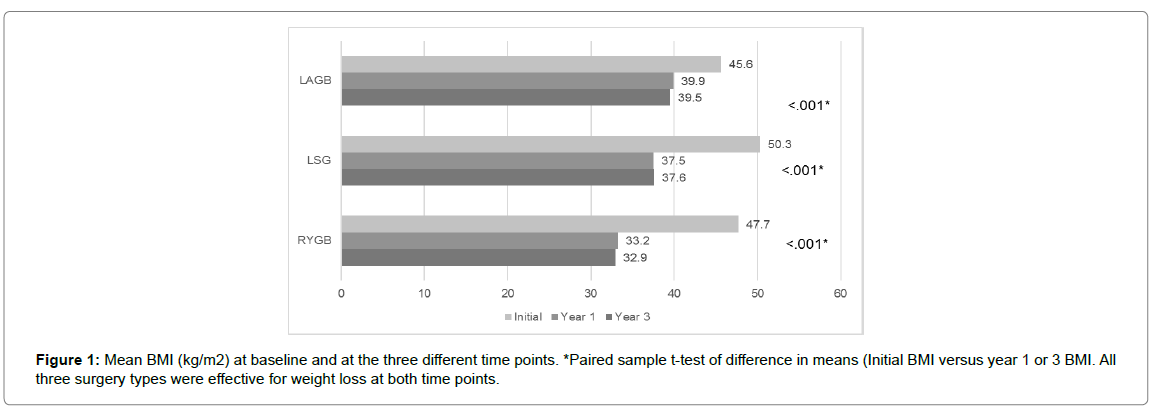
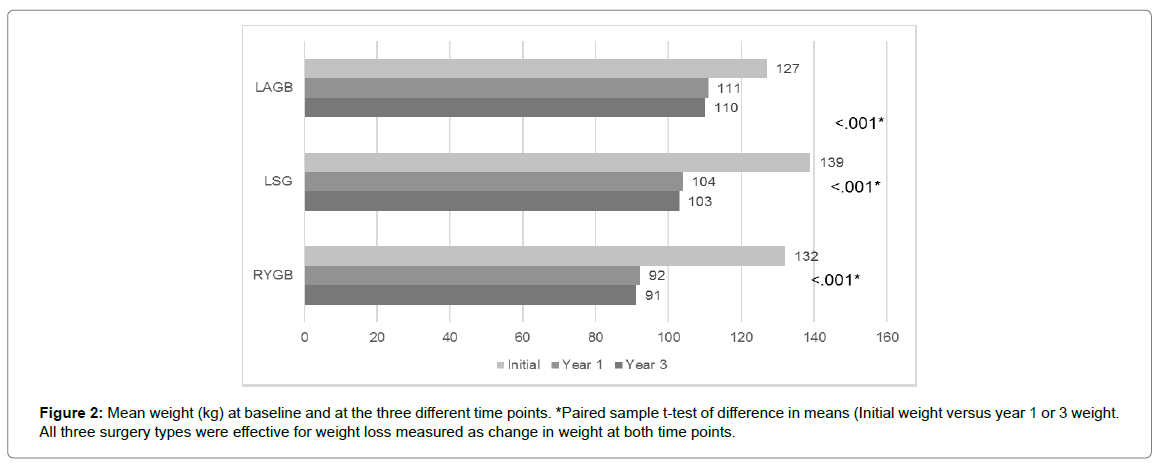
All three surgery types resulted in significant (P<0.001) weight loss presented as a change in weight and BMI from initial assessment at 1 and 3 year of follow-up. Change in BMI and weight at each follow up are presented in Figure 1 and 2. In terms of effectiveness, LRYGB was the most effective bariatric procedure followed by LSG and then LAGB at 1 and 3 years, with the proportion of patients having %EWL >50% at 3 years being 77.5% as compared to 51.2% of those who underwent LSG, and 18.8% of those who underwent LAGB at a significance level of <0.001 (Table 2). This was also illustrated as a change in BMI where the average BMI in kg/m2 before RYGB was 47.7 dropping to 32.9 (- 15.7 kg/m2) while that of LSG was 50.3 dropping to 37.6 (- 12.7 kg/m2) and that of LAGB was 45.6 dropping to 39.5 (- 6.1 kg/m2) at 3 years of follow up. These results are not different than previously published data [3,4,7,8,13,20].
| LAGB | LSG | RYGB | P | |
|---|---|---|---|---|
| N=371 | N=253 | N=570 | ||
| EWL > 50% at year 1: | 46 (12.4%) a | 141 (55.7%) b | 444 (77.9%) c | <0.001 |
| EWL > 50% at year 3: | 63 (18.8%) a | 88 (51.2%) b | 331 (77.5%) c | <0.001 |
Table 2: Excess Weight Loss greater than 50% according to surgery type at years 1 and 3 years.
Factors associated with TBWL% and %EWL
Linear regression was used to identify independent preoperative factors associated with %TBWL and %EWL at 1 and 3 years of follow up.
Following the adjustment for confounding factors in multivariate analysis, patient age, type 2 diabetes, sleep apnea, ethnicity, surgery type, and initial BMI were found to be significantly associated with TBWL% at 1 year (Table 3). Age and initial BMI were inversely associated with TBWL% (p=0.01). Significant less TBWL% was observed in patients with type 2 diabetes (-1.74%, p=0.007) and OSA (-1.77%, p=0.026). In addition, patients of black ethnic background were found to have a lower TBWL% when compared to white patients (-2.2%, p<0.001). However, TBWL% was greater among patients who underwent RYGB (5.86%) or LSG (5.89%) as compared to LAGB (p<0.001).
| β | SE | Standardize β | P | |
|---|---|---|---|---|
| TBWL (%) at 1 year | ||||
| Patient's Age | -.062 | .03 | -.058 | .01 |
| Type 2 Diabetes | -1.74 | .64 | -.062 | .007 |
| Sleep Apnea | -1.77 | -.79 | -.054 | .026 |
| BMI Initial Assessment | .253 | .04 | .150 | < 0.001 |
| Surgery Type LAGB |
Ref | |||
| RYGB | 5.68 | .22 | .676 | < 0.001 |
| LSG | 5.89 | .40 | .383 | < 0.001 |
| Ethnicity White race |
Ref | |||
| Black race | -2.2 | .49 | -.104 | < 0.001 |
| TBWL (%) at 3 year | ||||
| Patient Age | -.064 | .03 | -.056 | .03 |
| Type 2 Diabetes | -1.70 | .76 | -.059 | .026 |
| Initial BMI | .285 | .05 | .165 | < 0.001 |
| Surgery Type LAGB |
Ref | |||
| RYGB | 5.52 | .25 | .633 | < 0.001 |
| LSG | 4.98 | .49 | .296 | < 0.001 |
| Ethnicity White race |
Ref | |||
| Black race | -2.39 | .54 | -.113 | < 0.001 |
Table 3: Linear regression analysis for total body weight loss % at 1 and 3 years.
We detected some differences in the results when the regression model was carried out with %EWL as the dependent variable (Table 4). For example, patient age (p<0.02), ethnicity (p<0.001), initial BMI (p<0.001), and surgery type (p<0.001) were still significantly associated with %EWL but type 2 diabetes and sleep apnea were not. We attribute that to the greater variability of %EWL as a measure of weight loss [23]. Age and initial BMI were negative predictors of %EWL. In contrast to patients who underwent LAGB, those who underwent RYGB or LSG have a %EWL that is higher by 12.8% and 13.6%, respectively. Patients with black ethnic background have a lower %EWL by 4.4% when compared to those of white ethnic background.
| β | SE | Standardize β | P | |
|---|---|---|---|---|
| EWL at 1 year (%) | ||||
| Patient's Age | -.13 | .06 | -.053 | .022 |
| BMI Initial Assessment | -.56 | .09 | -.15 | < 0.001 |
| Surgery type LAGB |
Ref | |||
| RYGB | 12.8 | .49 | .688 | < 0.001 |
| LSG | 13.6 | .93 | .401 | < 0.001 |
| Ethnicity White race |
Ref | |||
| Black race | -4.4 | 1.1 | -.094 | < 0.001 |
| EWL at 3 years (%) | ||||
| Initial BMI | -.43 | 0.11 | -0.113 | < 0.001 |
| Surgery type LAGB |
Ref | |||
| RYGB | 12.3 | .58 | .637 | < 0.001 |
| LSG | 11.3 | 1.1 | .303 | < 0.001 |
| Ethnicity White race |
Ref | |||
| Black race | -4.4 | 1.3 | -.094 | < 0.001 |
Table 4: Linear regression analysis for % Excess Weight loss at 1 and 3 years.
The factors that were significantly associated with TBWL% at 1 years were also associated with TBWL% at 3 years, except for OSA, as presented in Table 4. At 3 years after bariatric surgery a patient with diabetes is expected to have a TBWL% that is less by 1.7% when compared to a patient without diabetes. Those who undergo RYGB or LSG are expected to have a higher TBWL% by 5.52% and 4.98%, respectively, as compared to someone who underwent LAGB. In contrast to patients of white ethnic background, those with black ethnic background are predicted to have a lower TBWL% by 2.2%.
Gender, smoking, and hypertension were not found to be significant predictors of TBWL% or %EWL at 1 or 3 years of follow-up (p>0.1).
Discussion
In this study of 1210 patients undergoing primary bariatric surgery we report our result investigating the association of socio-demographic (age, gender, and ethnicity), pre-operative factors (hypertension, diabetes, OSA, smoking, and initial BMI), and surgery type (LAGB, LSG, RYGB) with TBWL% and % EWL at different 1 and 3 years following bariatric surgery using multivariate analysis.
The negative association of initial BMI with TBWL% and %EWL is extensively studied and reported by many studies in the literature [14,24]. For example, Wood et al. have reported their results studying the association between preoperative clinical factors and long term weight loss after RYGB with a sample size of 726 and a median follow-up of 9.3 years and found that preoperative hyperlipidemia, older age, and high BMI were associated with a poorer TBWL % of -2.8%, -8.8%, and -4.1%, respectively [18]. The present study replicates the negative association between higher initial BMI with weight loss. This association could be explained by the fact that heavier patients may be less active, have genetic determinants of a depressed energy expenditure or thermogenesis, or simply have a lot of excess weight to lose.
Similar to the present study, a large cohort study by Coleman et. al with a sample size of 20,296 found that non-Hispanic white patients who underwent LRYGB had significantly higher %EWL (at 3 years followup) than non-Hispanic black and Hispanic patients [25]. Likewise, Lufti et al. [15] found an association between race and EWL, where Blacks Americans were found to have a less favorable %EWL compared to White Americans (60% for Blacks versus 82.9% for White Americans). However, they failed to find significance (p=0.06) and reported it could be due to the small number of African American patients in their sample. Other older studies that examined the effect of race showed less weight loss achieved in patient with black ethnicity after different bariatric interventions [26,27]. The inconclusive evidence on race could be the result of genetic differences or lifestyle and socioeconomic factors. Designing bariatric surgery programs should take into consideration racial difference in outcomes and target certain groups with higher intensity follow up and support programs.
Age has been previously reported to be negatively correlated with weight loss after bariatric surgery [18,24]. Chang et al. [16] investigated factors associated with poor weight loss after LSG and RYGB on 247 patients and found that age and type of surgery were associated with %EWL at 1,3, and 5 years follow up, with younger age at time of surgery and undergoing LRYGB having a more favorable %EWL. Our study found a negative association of age with TBWL% at 1 and 3 years and with %EWL at the first year of follow-up.
The relationship between the presence of comorbidities and weight loss has contradictory results in the literature. For example, Chang et al. reported that although hypertension and diabetes seemed to affect weight loss on univariate analysis, their impact was negligible on multivariate analysis after adjusting for other factors [16]. However, Melton et al, Still et al. and Ma et al. [14,24,28] reported observing suboptimal weight loss for patients with diabetes. In addition, Sillen et al. found both type 2 diabetes and hypertension to be significant predictors of weight loss at longer but not short term of follow up [17]. Ma et al, reported hypertension and diabetes to show an inverse association with weight loss in bivariate analyses but the association with hypertension was lost after adjusting for age, and gender among others. De Raaf et al. have previously studied the association between having OSA and weight loss after bariatric surgery on 816 patients and concluded that after adjustment, the difference in weight loss between patients with OSA and those without OSA was not significant [29]. We found OSA to be a predictor of a lower TBWL% at 1 year of follow up with the decrease in TBWL% being 1.77%. We also report type 2 diabetes to be associated with suboptimal weight loss after bariatric surgery but not hypertension. Although some studies report diabetes to be a negative predictor of weight loss after bariatric surgery, this disease tends to resolve or improve after surgery with a resolution rate of 83.7% [4] and the improved glycemic control is hypothesized to be unrelated to the effect of weight loss but due to the alteration in the entero-insulin axis [14] thus bariatric surgery remains advisable for such patients when indicated.
The influence of gender and history of smoking on weight loss after bariatric surgery is not yet clear. Melton et al. [14] reported males to have a less favorable outcome following a bariatric intervention while Ma et al. [28] reported less favorable weight loss for females. Few studies investigated the effect of smoking on post-operative weight loss. Still Ma et al. [28] found history smoking to favor more %EWL. The study found no effect for gender or history of smoking in multivariate analysis.
Strengths and Limitations
The present study has several strengths and limitations. Strengths include the large number of patients enrolled and the follow up period for 3 years. Additionally, the study investigated the influence of several preoperative factors on weight loss using both TBWL% and %EWL among patients undergoing three types of bariatric surgery and thus findings can help tailor procedures to patients with specific risk factors to optimize weight loss. However, we did not investigate the effect of other psychosocial factors on weight less such as marital or socioeconomic status. Another limitation is the single center nature of the study, making generalization of findings difficult. Also, grouping of ethnicity into black, white, or other limits the utility of this variable as other ethnicities were not taken into account.
Conclusion
In conclusion, the present study found that different bariatric surgeries have different effectiveness in TBWL% and %EWL with LRYGB being a superior bariatric procedure over LSG and LAGB in short and medium term of follow-up. In addition, we are able to conclude that patient age, race, type of surgery, pre-operative BMI, and co-morbidity status influence weight loss after bariatric surgery, with older age, black ethnicity, LAGB, higher initial BMI, diabetes, and OSA predicting a lower weight loss in the short and medium term. The study findings could alert the surgeon to take more aggressive measures towards patients with risk factors, in part, by choosing a more effective bariatric procedure and more intense postoperative follow up. Hypertension, smoking, and gender did not seem to influence weight loss in our study.
References
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384: 766-781.
- Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev 2014: Cd003641.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. (2004) Bariatric surgery: A systematic review and meta-analysis. JAMA 292: 1724-1737.
- Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, et al. (2005) Meta-analysis: Surgical treatment of obesity. Ann Intern Med 142: 547-559.
- Hatoum IJ, Blackstone R, Hunter TD, Francis DM, Steinbuch M, et al. (2016) Clinical factors associated with remission of obesity-related comorbidities after bariatric surgery. JAMA Surg 151: 130-137.
- Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, et al. (2018) Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg 153: 427-434.
- Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, et al. (2014) Long-term follow-up after bariatric surgery: a systematic review. JAMA 312: 934-942.
- Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, et al. (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683-2693.
- Lauti M, Kularatna M, Hill AG, MacCormick AD (2016) Weight regain following sleeve gastrectomy-a systematic review. Obes Surg 26: 1326-1334.
- Velapati SR, Shah M, Kuchkuntla AR, Abu-Dayyeh B, Grothe K, et al. (2018) Weight regain after bariatric surgery: Prevalence, etiology, and treatment. Curr Nutr Rep 7: 329-334.
- McPherson K, Marsh, T., Brown, M (2007) Foresight report on obesity. The Lancet 370: 1755.
- Puzziferri N, Nakonezny PA, Livingston EH, Carmody TJ, Provost DA, et al. (2008) Variations of weight loss following gastric bypass and gastric band. Ann Surg 248: 233-242.
- Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH (2008) Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg 12: 250-255.
- Lutfi R, Torquati A, Sekhar N, Richards WO (2006) Predictors of success after laparoscopic gastric bypass: A multivariate analysis of socioeconomic factors. Surg Endosc 20: 864-867.
- Chang WW, Hawkins DN, Brockmeyer JR, Faler BJ, Hoppe SW, et al. (2019) Factors influencing long-term weight loss after bariatric surgery. Surg Obes Relat Dis 15: 456-461.
- Sillen L, Andersson E (2017) Patient factors predicting weight loss after roux-en-y gastric bypass. J Obes 3278751.
- Wood GC, Benotti PN, Lee CJ, Mirshahi T, Still CD, et al. (2016) Evaluation of the association between preoperative clinical factors and long-term weight loss after roux-en-y gastric bypass. JAMA Surg 151: 1056-1062.
- Aminian A, Brethauer SA, Andalib A, Nowacki AS, Jimenez A, et al. (2017) Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg 266: 650-657.
- Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, et al. (2011) First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 254: 410-420.
- Nelson LG, Gonzalez R, Haines K, Gallagher SF, Murr MM (2005) Amelioration of gastroesophageal reflux symptoms following Roux-en-Y gastric bypass for clinically significant obesity. Am Surg 71: 950-953.
- Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, et al. (2019) Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth ifso global registry report 2018. Obes Surg 29: 782-795.
- van de Laar A (2012) Bariatric Outcomes Longitudinal Database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obes Surg 22: 1843-1847
- Still CD, Wood GC, Chu X, Manney C, Strodel W, et al. (2014) Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity 22: 888-894.
- Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, et al. (2014) Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis 10: 396-403.
- Capella RF, Capella JF (1993) Ethnicity, type of obesity surgery and weight loss. Obes Surg 3: 375-380.
- Sugerman HJ, Londrey GL, Kellum JM, Wolf L, Liszka T, et al. (1989) Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg 157: 93-102.
- Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, et al. (2006) Predictors of weight status following laparoscopic gastric bypass. Obes Surg 16: 1227-1231.
- de Raaff CA, Coblijn UK, de Vries N, Heymans MW, van den Berg BT, et al. (2016) Predictive factors for insufficient weight loss after bariatric surgery: Does obstructive sleep apnea influence weight loss? Obes Surg 26: 1048-1056.
Citation: ElAbd R, Al-Tarrah D, Almazeedi S, Alyaqout K, Efthimiou E, et al. (2021) Factors Influencing Weight Loss after Bariatric Surgery: A Multivariate Analysis. J Obes Weight Loss Ther 11: 442. DOI: 10.4172/2165-7904.1000442
Copyright: © 2021 ElAbd R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2132
- [From(publication date): 0-2021 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 1540
- PDF downloads: 592