Conference Proceeding Open Access
Factors Alleviating Cadmium Toxicity in White Rot Fungus
| Kuber Chandra Bhainsa1,2, Khajamohiddin Syed1 and Jagjit S Yadav1* | |
| 1Environmental Genetics and Molecular Toxicology Division, Department of Environmental Health, University of Cincinnati College of Medicine, Cincinnati, OHIO, USA | |
| 2Nuclear Agriculture and Biotechnology Division, Biomedical Group, Bhabha Atomic Research Centre, Mumbai - 400085, India | |
| Corresponding Author : | Jagjit S Yadav Environmental Genetics and Molecular Toxicology Division Department of Environmental Health University of Cincinnati College of Medicine Cincinnati, OHIO, 45267, USA Tel: 001-513-558-4806 E-mail: yadavjs@ucmail.uc.edu |
| Received August 01, 2013; Accepted December 30, 2013; Published January 05, 2014 | |
| Citation: Bhainsa KC, Syed K, Yadav JS (2014) Factors Alleviating Cadmium Toxicity in White Rot Fungus. J Bioremed Biodeg S18:005. doi:10.4172/2155-6199.S18-005 | |
| Copyright: © 2014 Bhainsa KC, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Co-contamination with heavy metals and polycyclic aromatic hydrocarbon (PAHs) poses a challenge for bioremediation of polluted environments. In order to degrade PAH-metal mixtures, the biodegrading organism needs to be in a metabolically active state to degrade the PAH moiety while being able to tolerate the co-contaminant metal(s). Due to this fact, tolerance studies were carried out on the model white rot fungus, Phanerochaete chrysosporium known to have the ability to degrade PAHs. Toxicity to the organism was investigated using cadmium (Cd) and various PAHs separately as well as in combinations and growth inhibition were measured as an index of the xenobiotic toxicity. Treatment with Cd showed inhibition of fungal growth in a dose-dependent manner. PAHs of varying ring size (3-5 ring) showed inhibitory effect in the 10-25 ppm concentration range and the effect varied with the PAH type. The pattern of initial growth inhibition followed the order pyrene>phenanthrene>benzo(a)pyrene. Both the xenobiotics (metaland PAH)at their respective inhibitory concentrations led to morphological changes in the growth pattern of the organism, observed in terms of variable mycelial pellet morphology. Interestingly, co-exposure with a PAH alleviated the Cd toxicity, indicating a protective effect of the PAHs. Taken together, this is the first report on the metal tolerance properties of this organism in a co-contamination scenarioas well as on demonstration of a protective effect of PAHs in Cd toxicity.
| Keywords |
| Cadmium toxicity; PAH; Tolerance; Bioremediation; Phanerochaete chrysosporium; White rot fungus |
| Introduction |
| Environments are often contaminated with both metals and organic toxicants [1]. In such mixed pollution, metal tolerance plays an important role in growth and metabolic functioningof biodegrading microorganisms, and is a desirable trait in bioremediationapplications. Hence, those organisms that can tolerate and grow in presence of metals while degrading the organic toxicants are the potential bioremediation agents of choice. Filamentous fungi have been investigated for their metal tolerance and utilized in bioremediation due to the ease of growing and handling these organisms and their versatility in biodegradation of normally recalcitrant organopollutants. However, little is known on their ability to tolerate heavy metals in presence of organic toxicants. Heavy metals such as cadmium (Cd) and polycyclic aromatic hydrocarbons (PAHs) such as phenanthrene (Phe) , pyrene (Pyr) and benzo(a)pyrene [B(a)P] are commonly occurring toxicants, that are frequently encountered in various contaminated environmental matrices [1]. Bioremediation of suchcontaminated media requires the organism to tolerate both metal such as Cd and PAHs. White rot fungi are known to possess extraordinary ability to oxidize a broad range of organic toxicants. Limited studies have also shown their ability to tolerate certain metals [2]. Our group has focused on the model white rot fungus Phanerochaete chrysosporium for investigating biodegradation of recalcitrant pollutants including PAHs [3,4]. However, metal tolerance of this organism in presence of PAHsin a mixed pollution scenario is not yet understood. In light of the above background, we investigated Cd tolerance of this PAH-degrading white rot fungus. The study involved monitoring of tolerance at varying concentrations of Cd ion and PAHs of varying ring size (3 to 5 rings) in terms of mycelial growth rate as well as morphological changes observed during the growth. Particularly the study was an attempt to unravel the influence of PAHs on metal tolerance property of the organism in a mixed pollution scenario. |
| Materials and Methods |
| Chemicals |
| All polycyclic aromatic hydrocarbon (PAH) compounds were obtained from Sigma-Aldrich (St. Louis, MO). CdSO4.4H2O was purchased from Fisher Scientific (Pittsburgh, PA). |
| Fungal strain and culture conditions |
| Phanerochaete chrysosporium (BKMF-1767) obtained from ATCCwas maintained on malt extract (ME) agar, as reported earlier [5]. |
| Xenobiotic tolerance experiments |
| Tolerance and/or removal of metal (Cd) and PAHs (Phe, Pyr and BaP) by the model white rot fungus, Phanerochaete chrysosporium was investigated in shaken liquid cultures using the nutrient-rich malt extract broth medium, pH 4.5 containing malt extract (20 g/l), dextrose (20 g/l) and peptone (1 g/l) [5]. Tolerance to Cd was investigated in the 0-2 mM concentration range whereas that to PAHs was tested in the 10- 25 ppm range. For preparation of fungal inoculum, a 50 mlstationary culture grown in a Fernbach flask for 48 hours at 37°C was homogenized aseptically to prepare a uniform suspension as described previously [4]. Liquid cultures (50 ml) were set up in 125 ml Erlenmeyer flasks by inoculating the culture medium (malt extract broth, pH 4.5) at 10% v/v. The cultures were grown for 24 hours by shaking (180 rpm) at 37°C. For assessing the effect of metal and/or PAH on the growth, calculated amount of the test xenobiotic were spiked and the growth continued until 72 hours under the same incubation conditions. Mycelial biomass was harvested by vacuum filtration using Whatman No.1 filter paper (Fisher Scientific, USA) and dried at 80°C for 24 hours or until a constant weight was achieved. |
| Fluorescence microscopy |
| Superoxide radicals were detected in the fungal mycelium by fluorescence microscopy based on dihydroethedium (DE) staining. Fluorescence microscopy technique was also employed for the live/dead mycelia staining. While staining with 4',6-diamidino-2- phenylindole (DAPI) at 1% revealed all cells (live and dead), the dead cells were selectively detected by propidium iodide (PI) stain (0.5% concentration). |
| Results and Discussion |
| Cadmium tolerance of P. chrysosporium |
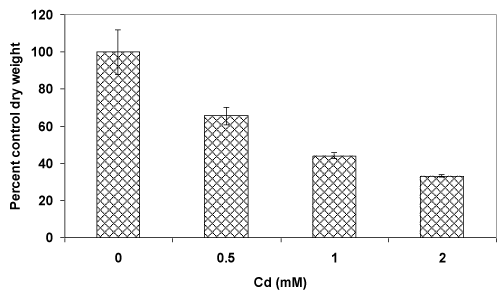
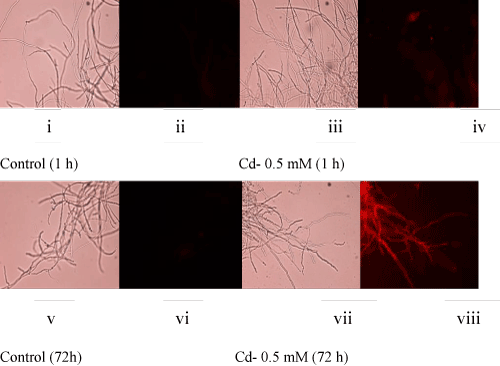
| Atrend of decreasing fungal biomass (in comparison to the control culture) with an increasing concentration of Cd was observed (Figure 1A). The mycelial mass (dry weight-basis) was decreased by 35% and 67% at 0.5 mM and 2 mM concentrations of Cd, respectively. The decrease in biomass could be due to the toxic effect of Cd which is known to generate reactive oxygen species (ROS). In this context, fluorescence staining-based microscopy employed fordetection of super oxide radicals showed increasing number of fungal filaments with a higher red fluorescence (Figure 1B, i-viii) upon metal accumulation [2,6,7]. This implied that with an increasing concentration of Cd, the fungus could experience elevated levels of ROS resulting in oxidative stress-induced toxicity leading to a reduced growth. |
| PAH tolerance of P.chrysosporium |
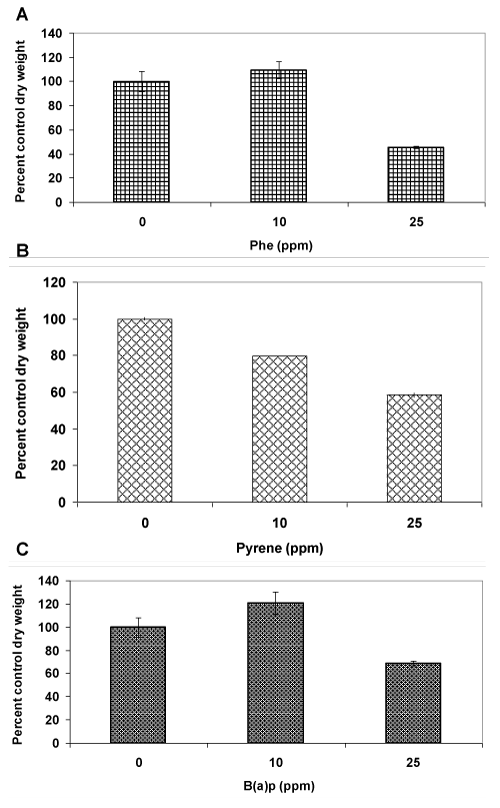
| In order to assess the tolerance to PAHs, three different compounds (containing 3-5 aromatic rings), namely phenanthrene (Phe), pyrene (Pyr) and benzo(a)pyrene (BaP) were tested at 10 and 25 ppm concentrations. Phenanthrene at a 10 ppm concentration had no effect on fungal growth as the mycelia dry weight in the treated cultures was similar to that of the control untreated cultures (Figure 2A). On the other hand, at a 25 ppm concentration, the dry weight reduced by more than 50% indicating toxicity. In contrast, pyrene showed a 20% reduction in mycelia mass even at 10 ppm concentration (Figure 2B), indicating greater sensitivity of the fungus to pyrenein comparison to phenanthrene. A further increase in pyrene concentration to 25 ppm led to a two-fold decrease in biomass level (40% decrease,); this suggestedthat a greater sensitivity to pyrene than phenanthrene at a common low concentration (10 ppm) does not necessarily result in a proportionatelyhigher toxicity at elevated concentrations (such as 25 ppm). A completely different pattern was observed in case of benzo(a) pyrene (Figure 2C) in comparison to phenanthrene and pyrene. At 10 ppm concentration, there was a significant increase (20%) in biomass productionin comparison to the control probably because of the recognized cell proliferation effect of this PAH toward eukaryotic cells [8,9]. At 25 ppm concentration, there was a growth inhibition but the mycelial mass was reduced by only 30%, a figure significantlylower than those observed for phenanthrene (50%) and pyrene (40%). Taken together (10 and 25 ppm), fungal growth inhibition effect exhibited by the PAHs followed the trend pyrene>phenanthrene>benzo(a)pyrene, suggesting that there was no uniform pattern of tolerance across the PAH types. Since pyrene showed the lowest threshold limit of tolerance (10 ppm), it was used as a model PAH for further investigationsin mixture studies with Cd. |
| Effect of cadmium and pyrene, singly and in mixture on mycelial pellet morphology |
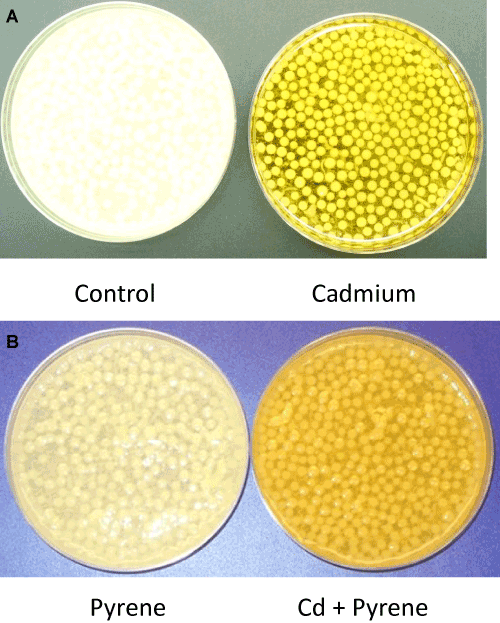
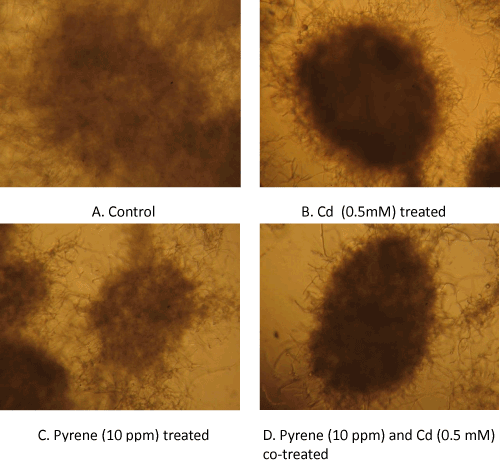
| Visual differences: Morphological changes in the mycelial mass in P. chrysosporium cultures grown in presence of Cd, pyrene, or Cd+pyrene were monitored after 72 hours of growth; equal aliquots from the individual cultures (freshly stirred to generate a uniform suspension) were transferred to the respective petriplates and visual differences in mycelia morphology were recorded using photography. The control (untreated) culture was filled with ‘loose ball-shaped pellets’ (mycelia pellets) in addition to the free suspended filaments in a light clear liquid phase of the medium (Figure 3A-Left panel). In contrast, the Cdcontaining cultures showed light yellow colored, ‘tightly-knit spherical ball-shaped mycelia pellets’ in a colored liquid phase containing only a few suspended filaments (Figure 3A-Right panel). The pyrene culture was similar to the control culture in that the mycelial pellets covered the entire liquid phase (light, clear) albeitata lower density (Figure 3B). In the mixed treatment (Cd+pyrene), the mycelia pellets turned out to be more like those in the pyrene culture (loose ball-shaped pellets surrounded by free suspended filaments) albeit with a darker appearance (Figure 3B). This indicates the overarching influence of pyrene on pellet morphology in the co-contaminated medium. |
| Microscopic differences: A closer look at the culture suspensionusing microscopy provided further insights into the mycelial growth patterns. The culture suspensions were loaded onto the glass slides (without any treatment) and examined under a light microscope (10×magnification). Micrographs of the control (untreated) culture showed loose mycelial masses (pellets) with a fuzzy boundary (Figure 4A), whereas the mycelia growing in presence of Cd alone (Figure 4B) showed tightly packed ball-shaped structures (pellets) with a distinct boundary and hairy small filaments protruding at the surface. On the other hand, the pellet morphology in pyrene cultures were similar to that in the control culture except that it had a more distinct appearance of the otherwise loosely bound smaller mycelia mass (Figure 4C). In theco-treated (Cd+pyrene) cultures (Figure 4D), the mycelial mass appeared as a tightly knit ball-shaped structure with a distinct boundary albeit with a lower density than that inthe Cd-onlycultures. |
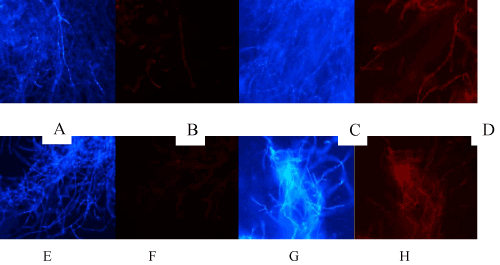
| Toxicity of Cd and Pyrene based on live/dead staining: The results on toxicity of Cd (0.5 mM) and pyrene (10 ppm) measured in terms of cell death based on live/dead staining are presented in (Figure 5A-5H). Red fluorescence due to PI staining of dead cells indicated cell death. While control (untreated) mycelia (Figure 5B) and Pyrene-treated mycelia (Figure 5E and 5F) showed no considerable dead filaments, both Cd-treated (Figure 5C and 5D) and Cd+Pyr-treated (Figure 5G and 5H) showed the dead filament population, indicating that Cd was toxic but presence of Cd along with pyrene (Figure 5G and 5H) increased the toxicity more than Cd alone (Figure 5C and 5D). This filament-specific observation contrasted with the overall protective influence of pyrene on the fungal pellet morphology and biomass yield in the co-contaminated medium. |
| Role of pyrene in alleviation of Cd toxicity |
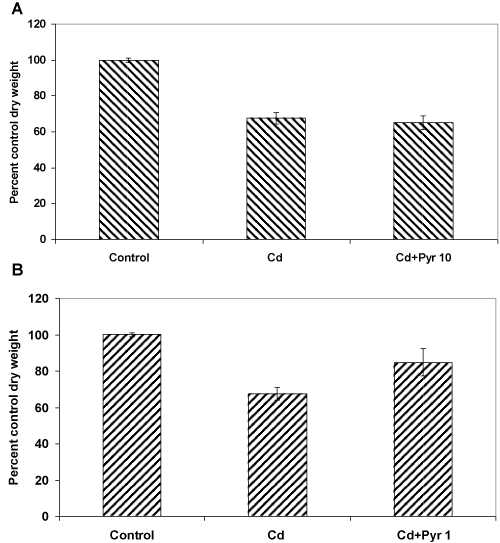
| These co-exposure experiments were carried out in presence of 0.5 mM Cd and 10 ppm pyrene, considering the preceding observations on the independent toxicity of these test concentrations (as evident in terms of their effect on both fungal biomass yield and morphology). Interestingly, the overall toxicity outcome in the co-exposure treatment did not increase beyond what was seen with Cd alone indicating some kind of protective effect of pyrene. To further prove this point, the experiments were carried out at a much safer nontoxic concentration (1 ppm) of pyrene. Interestingly, it improved the biomass growth, meaning that presence of even low amount of pyrene in the medium containing Cd interacts in a manner that protects the fungus from metal toxicity. |
| In specific terms, when Cd (0.5 mM) and pyrene (10 ppm) were added alone at their respective toxic concentrations, the fungal growth was reduced by 35% and 20%,respectively (Figures 1 and 2A ). Interestingly, in a co-exposure at these toxic concentrations, the growth was reduced by 35% (Figure 6A), same as that for the Cd only cultures. This means, the two toxicants do not exert an additive effect. Instead, pyrene seems to exert a protective effect. We then asked the question, if this assumption was true even co-exposure to a lower non-toxic amount of pyrene should protect the fungus from the toxic effect of Cd. For this, cultures were treated with 0.5 mM Cd and 1ppm pyrene (a 10 times lower concentration of pyrene) and the results (Figure 6B) showed merely a 15% reduction in fungal growth. This meant an overall improvement in biomass yield, 20% over the Cd onlycultures and 5% over the pyrene only cultures. This significant enhancement of growth in presence of 1 ppm pyrene in a 0.5 mM Cd containing medium therefore confirmed the role of pyrene in alleviation of Cd toxicity. This protective effect could be due to metabolic changes [10] caused by pyrene as corroborated by the observations on cellular and structural changes in mycelia morphology (Figure 3A, 3B and Figure 4A-4D). Additionally, pyrene being hydrophobic in nature may interact favorably with the fungal cell wall and cell membrane that presumably dictate the toxic effects of Cd [11]. |
| Overall, the results of this study provide the first evidence of alleviation of Cd toxicity by a PAH compound in a co-contamination scenario. Further studies are underway to understand the mechanism(s) underlying this protective effect that may have useful implications in bioremediation of mixed pollution. |
| Acknowledgements |
| The study was supported in part by the University of Cincinnati funds. Kuber Chandra Bhainsa acknowledges the financial support awarded to him in the form of DBT-CREST Fellowship (2011-12) by the Department of Biotechnology (DBT), Government of India. |
References
|
Figures at a glance
 |
 |
 |
 |
| Figure 1a | Figure 1b | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13373
- [From(publication date):
specialissue-2014 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 9022
- PDF downloads : 4351
