Mini Review Open Access
Facial Isotope Differences of Carbon and Global Photosynthesis in the Frames of Global Carbon Cycle
AA Ivlev*
Russian State Agrarian University-Moscow Agricultural Academy of Timiryazev, Timiryazevskaya str.,49 Moscow 127550, Russia
- *Corresponding Author:
- AA Ivlev
Russian State Agrarian University-Moscow
Agricultural Academy of Timiryazev
Timiryazevskaya str., 49 Moscow 127550, Russia
Tel: 74999771455
E-mail: aa.ivlev@list.ru
Received Date: April 10, 2017 Accepted Date: June 15, 2017 Published Date: Jyune 21, 2017
Citation: Ivlev AA (2017) Facial Isotope Differences of Carbon and Global Photosynthesis in the Frames of Global Carbon Cycle. J Earth Sci Clim Change 8: 403. doi: 10.4172/2157-7617.1000403
Copyright: © 2017 Ivlev AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Facial isotopic differences are usually regarded as the features of the carbon isotope composition of sedimentary organic matter, reflecting the conditions of formation and transformation in the location under study. In the present work it is shown that facial differences reflect the conditions of photosynthesis that took place in this location during the relevant period of geological time. These conditions are determined by the ratio of CO2 and O2 in the atmosphere, as well as by a set of other environmental parameters that affect on the ratio of these gases in photosynthesizing cells (illumination, water salinity, aeration, etc). The subsequent processes of organic matter transformation in the sediment do not significantly affect on the carbon isotope composition. The mechanism of formation of carbon isotope composition of photosynthetic part of “living matter” is suggested. The relationship of facial isotopic differences with isotope composition of photosynthetic biomass is traced.
Keywords
Facies; carbon isotope composition; Precambrian atmosphere; Photosynthesis; Assimilation; Photorespiration; Oscillatory mechanism; Facial isotopic differences
Introduction
One of the geochemical characteristics of facies is a carbon isotope composition of sedimentary organic matter (δ13C). Considering that carbon isotope composition of coeval organic matter and carbonates are related and their difference is an analog of 13C discrimination in photosynthesis in modern plants [1], both isotopic characteristics can be used to define facies. Oethler et al. [2] were the first, who having studied the most ancient sedimentary organic matter (Svaziland, 3, 3 Ga), found the jump between carbon isotope composition of organic matter in the overlaying younger strata and in the above strata of amounted to 12% on average (from -14.7/-19.5% to 35/40%). Following the actualism principle, the authors ascribed this jump to photosynthesis origin. This finding correlated well with the results of the studies on photosynthetic CO2 assimilation where it was shown that CO2 fixation, occurring at the stage of enzymatic carboxylation of ribulosebisphosphate (RuBP), causes the isotopic effect of the expected value [3]. The isotopic jump also witnessed the appearance in the course of evolution the key enzyme of photosynthesis mentioned above. The above conclusions were also supported by the subsequent examination of the mechanism of carbon isotope fractionation in photosynthesis [4,5]. Nevertheless, the obtained results were insufficient to define isotopic facial features, since isotopic composition of sedimentary organic carbon depends not only on isotope fractionation at the stage of “living matter”, mainly related to photosynthesis, but also on the stage of organic matter oxidation after burial. First of all, it should be noted that carbon isotope composition of “living matter”, which includes biomass of all living organisms on the Earth, is completely determined by carbon isotope composition of photosynthesizing biomass. It is explained by the fact that carbon isotope fractionation in heterotrophic assimilation is negligible relative to photosynthetic assimilation [6], combined with the notion that photosynthesizing organisms give rise to all trophic chains. One more notion should be done. Carbon isotope fractionation at the stage of “living matter” in non-oxidative conditions of environment is different as compared with isotope fractionation in oxidative atmosphere.
Facial carbon isotope differences in non-oxidative Precambrian atmosphere
It was established that Proterozoic carbonates in the period 2.3/1.9 Ga were abnormally enriched in 13C (from 10% up to 20%) [7]. In the period under review the atmosphere was anoxic, because molecular oxygen, produced in photosynthesis hasn’t been collected, but completely was spent for oxidation of igneous rocks and other reduced forms. The atmosphere predominantly consisted of carbon dioxide and methane. The methanogens were the prevalent life form. It allows concluding that the cause of the abnormally 13C-enriched carbonates could be bound to the microbial conversion of carbon dioxide into methane. This process is known to be followed by carbon isotope fractionation [7,8]. Protobionts, living in Precambrian, needed oxygen to supply their energy metabolism. However because of anoxic atmosphere in Precambrian they were enforced to take it from oxygencontaining compounds. Carbon dioxide was the most appropriate form in the primitive “atmosphere-hydrosphere” system to provide oxygen. It contributed to the occurrences of methanogens that produced methane [7]. The Raleigh effect, which commonly accompanies the isotope fractionation in natural conditions, strengthens isotopic discrepancies by multiplying one-off isotope effects of the reaction. Anaerobic photosynthesis continued until the ocean has not ceased to absorb oxygen and it appeared in the atmosphere in a molecular form. A toxic release of oxygen has happened in 2.5 to 2.3Ga interval [9]. Two billion years ago due to photosynthesis of cyanobacteria the atmosphere has become oxidative. It was the crucial point of evolution.
Facial carbon isotope differences in oxidative Precambrian atmosphere
In Late Proterozoic (0.8-0.6 Ga) the abnormally “heavy” carbonates were also found. In oxidative atmosphere of that time the reason for the appearance of unusually 13C enriched sedimentary carbonates was quite different as compared with previous case. The 13C enrichment was due to CO2 consumption in photosynthesis followed by carbon isotope effect. It resulted in a depletion of CO2 in “atmosphere-hydrosphere” system during orogenic cycle and was accompanied with Raleigh effect. The extent of depletion was abnormally high, since initial filling of the system with CO2 in orogenic period of the cycle wasn’t great because the amount of sedimentary organic matter, the source of CO2, accumulated in the first orogenic cycles, was much less as compared with the subsequent periods of Phanerozoic [10]. The emergence of free oxygen in the atmosphere resulted in dramatic events in biosphere. Cyanobacteria got photorespiration. It means they adapted to oxygen and have learned to use it. The enzyme RuBP carboxylase has acquired a new oxygenase function and together with it the oscillatory mechanism of photosynthesis [5]. Notably, that assimilatory part of the photosynthetic mechanism was associated with carbon isotope fractionation related to RuBP carboxylation and resulted in 12C enrichment of the assimilated carbon. The photorespiratory part of the mechanism was also associated with carbon isotope fractionation but with the effect of opposite sign relative. The effect was bound to glycine decarboxylase reaction in photorespiratory chain of cell metabolism. Both CO2 assimilation and photorespiration, contributed to carbon isotope composition of total biomass. The summary isotope composition of biomass depended on CO2/O2 concentration ratio in the environment. The increase of oxygen concentration intensified photorespiration and strengthened 13С the enrichment of biomass. The growth of carbon dioxide stimulated assimilation and 12С accumulation in biomass. Organisms with such an organization of photosynthesis are usually attributed to the C-3 type.
How does CO2/O2 concentration ratio in the environment determines the carbon isotope composition of “living matter”
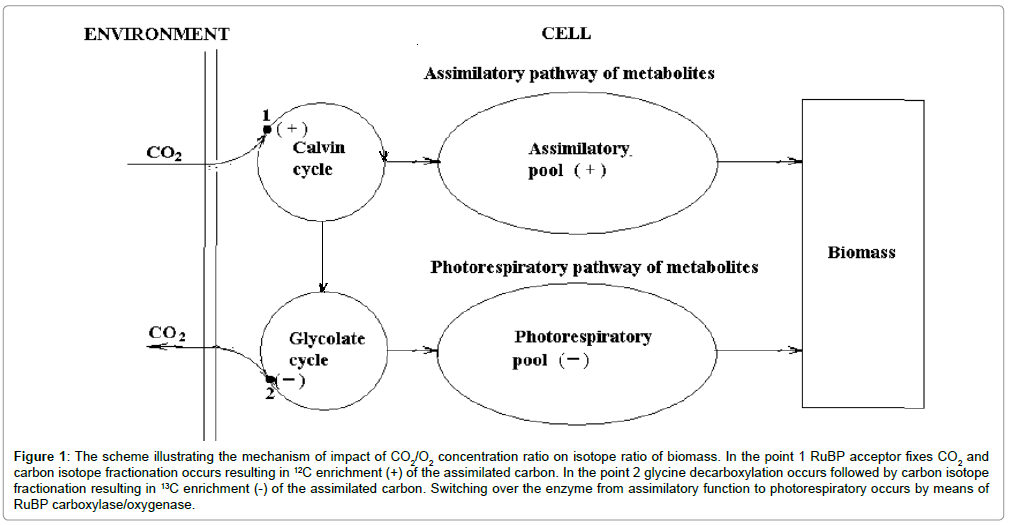
The “living matter” consists of two parts – photosynthetic and heterotrophic. Since photosynthesizing organisms give rise to all trophic chains carbon isotope composition of them determines isotope composition of whole “living matter”. It means to understand carbon isotope composition of “living matter”, it is necessary to find out how carbon isotope composition of photosynthesizing biomass is formed. Basing on previous studies [4], let’s see, how it is formed carbon isotope composition of biomass of a generalized cell of C-3 organisms. (Figure 1) illustrates the mechanism of formation of carbon isotope composition of biomass of C-3 organisms. Carbon dioxide enters the cell and, fixed by RuBP acceptor, is involved into Calvin cycle. The fixation is followed by carbon isotope effect resulting in 12C enrichment of the assimilated carbon. Then a part of the pool of assimilated carbon is used to provide glycolate cycle with substrates, where photorespiration occurs. In glycine dehydrogenase reaction of the glycolate cycle a part of the carbon flow is subjected to oxidative decarboxylation and is evolved from the cell. Due to isotope effect of decarboxylation the photorespiratory flow of the cycle is enriched in 13C. Both flows of assimilatory and photorespiratory carbon are separated in time due to oscillatory mechanism and to a strict temporal cell organization. Thus, carbon of assimilatory pool enriched in 12C and carbon of photorespiratory pool enriched in 13C, make the total biomass. The contribution of each pool to biomass determines its carbon isotope composition and depends on CO2/O2 concentration ratio in the environment. In the accordance with the suggested oscillatory mechanism of photosynthesis [5], the alternation of assimilation and photorespiration is controlled by the switching of enzyme RuBP carboxylase/oxygenase from carboxylase to oxygenase function and back depending on the CO2/O2 ratio in the cell. The latter in turn depends on the ratio in the environment. Thus carbon isotope composition of total biomass is a function of CO2/O2 ratio. Basing on actualism principle, we believe that oscillatory mechanism of photosynthesis is typical to photosynthesis of C-3 type organisms at present and in the past. Passing from the photosynthesis of the individual organism to the concept of global photosynthesis, which characterizes the photosynthesis in the global carbon cycle, it should be underlined that the latter has all the characteristics of photosynthesis of an individual organism, excepting ontogenesis, i.e. it doesn’t depend on time [1].
Figure 1: The scheme illustrating the mechanism of impact of CO2/O2 concentration ratio on isotope ratio of biomass. In the point 1 RuBP acceptor fixes CO2 and carbon isotope fractionation occurs resulting in 13C enrichment (+) of the assimilated carbon. In the point 2 glycine decarboxylation occurs followed by carbon isotope fractionation resulting in 13C enrichment (-) of the assimilated carbon. Switching over the enzyme from assimilatory function to photorespiratory occurs by means of RuBP carboxylase/oxygenase.
What are the facial isotopic differences?
To proceed to the definition of facial isotopic differences it is necessary to estimate isotopic shifts in the transformation of “living matter” into sedimentary organic matter. We assumed that no carbon isotope fractionation occurs in microbial oxidation of biogenic molecules since at present there are no data evidencing to the contrary. The isotopic shifts might appear as a result of partial oxidation of biochemical fractions because they slightly differ in carbon isotope composition due to intracellular isotope fractionation. But this fractionation isn’t great. Maximal isotopic differences in the range of 5% characterize lipid fraction. It is known that lipid fraction at the same time is most resistant to oxidation. So the maximal isotopic shifts due to partial oxidation can’t exceed 5%. But in real situation it should be much less because full oxidation of other fractions is a low probable case. Indeed, many researchers, who studied carbon isotope composition of organic matter in sedimentation and diagenesis, observed slight 12C enrichment about 2%-3% with transformation depth of organic matter [11,12]. It evidenced in favor of increase of lipid fraction in the composition of the remaining organic matter. If to compare the isotopic shifts for the partial oxidation of organic matter with the shifts due to photosynthesis in different environmental conditions, which exceeds 20%, it becomes clear that the effects of photosynthesis should play a dominant role. As said, oxidation doesn’t contribute to carbon isotope composition of sedimentary organic matter. It is left to find out how chemical destruction of organic matter can change its carbon isotope composition. Obviously, carbon isotope composition could change, if the parts of organic matter, mainly carbon products of its transformation with the other isotopic composition, leave organic matter. It might be petroleum, as a whole, and gas (methane). The 12C enrichment of petroleum is a result of their formation, which is bound to lipid fraction. As to methane, its massive removal occurs at high degree of catagenetic transformation.
Conclusion
From balance consideration it is clear, that even in the cases isotopic shifts should be small. Thus one can conclude that at all stages of organic matter transformation its carbon isotope composition reflects isotope fractionation emerged in photosynthesis. By the other words, facial isotopic differences are inherited from the “living matter” and reflect the environmental conditions of photosynthesis (illumination, water salinity, water availability, temperature, mixing, etc.). A set of the environmental parameters forms isotopic peculiarities of the locality, i.e. facial isotopic differences.
Available data confirm these assertions. It is well established fact that the organic matter from marine, fresh water, salt marsh and terrigeneous sediments distinctively different in carbon isotope ratio. The cause is in that all these localities are characterized by different CO2/O2 ratios determining the photosynthesis conditions. The more this ratio is the more organic matter is enriched in 12C.
References
- Ivlev AA (2016) Comments on the role of photosynthesis in the global redox carbon cycle. J Ecosystems and Ecography 6: 4.
- Oehler DZ, Schopf JW, Kvenvolden KA (1972) Carbon isotopic studies of organic matter in Precambrian rocks. Science 175: 1246-1248.
- Park R, Epstein S (1960) Carbon isotope fractionation during photosynthesis. Geochim et Cosmochim. Acta 21: 110-119.
- O’Leary MH (1993) Biochemical basis of carbon isotope fractionation. In: Stable isotopes and plant carbon - water relations/ Ehleringer JR, Hall AE, Farquhar GD (ed), Academic Press, Inc.San Diego, Boston, New York, London, Sydney, Tokyo, Toronto. pp: 19-27.
- Ivlev AA (2012) Oscillatory nature of metabolism and carbon isotope distribution in photosynthesizing cells Oscillatory nature of metabolism and carbon isotope distribution in photosynthesizing cells. In: Photosynthesis – fundamental aspects, Najafpour MM, (ed), Intec Publishers. Croatia. pp. 341-366.
- Ivlev AA (2013) Chemical evolution vs Biological volution: Coupling effect and Consequences. Transworld Research Network. Kerala. India.
- Vasconcelos C, Bovler C, Bahnluk A, Anderson MB, McKenzie JA (2016) Unusual very positive enrichment of 13C in carbonate sediments deposited in modern hypersaline environment, Brazil: Indicator of salinity controlled. 35th Int. Geol. Congress. Cape Town. South Africa. Paper 2853
- Valentine DL, Chidthaisong A, Rice A, Reeburgh WS, Tyler SC (2004) Carbon and hydrogen isotope fractionation by moderately thermophylic methanogens Geochim. Cosmochim. Acta 68: 1571-1590.
- Farquhar GD, Zerkle AL, Bekker A (2011) Geological constraints on the origin of oxygenic photosynthesis. Photosynthesis Res 107: 11-36.
- Ivlev AA (2015) Global redox cycle of biospheric carbon: interaction of photosynthesis and earth crust processes. BioSystems 137: 1-11.
- Galimov EM (1968) Geochemistry of stable carbon isotopes. Moscow. “Nedra”.
- Meyers PH (1994) Preservation of elemental and isotopic identification of sedimentary organic matter. Chem. Geology 114: 289-302.
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 2421
- [From(publication date):
June-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 1625
- PDF downloads : 796