Fabrication of Pb(II) Selective Polymeric Membrane Electrode Based on 2,2âÃâ¬Ãâ¢[Propane-1,3-Diylbis(Oxy)]Dibenzaldehyde as an Ionophore.
Received: 01-Mar-2018 / Accepted Date: 14-Mar-2018 / Published Date: 19-Mar-2018 DOI: 10.4172/2155-9872.1000399
Abstract
2,2'-[propane-1,3-diylbis(oxy)]dibenzaldehyde has been explored as an ionophore and fabricated in a polyvinylchloride (PVC)-based membrane. The electrode shows excellent potentiometric response characteristics and displays a linear log [Pb(II)] versus emf response over a wide concentration range of 1.2 × 10-7 M to 1.0 × 10-1 M with detection limit 3.3 × 10-8 M and having nernstian slope of 29.06 mV/decade. Potential response remains almost unchanged at pH range of 3.0-8.0. The electrode shows fast response time of <10 s and a lifetime of four months. It shows good selectivity for Pb(II) ions over the other cations. The complexation 2,2'-[propane-1,3- diylbis(oxy)]dibenzaldehyde and Pb(II) cation was studied spectrophotometrically in 1:1 DMF:H2 O mixed solvents. Spectra shows the strong interaction between Ionophore and Pb(II) ion. The electrode can also be used as an indicator electrode in potentiometric titration of Pb(II) ions with standard chromate solution and its determination in real samples.
Keywords: PVC; Ionophore; Lead ions; Nernstian response
Introduction
In the recent years the contamination of environment by the industrial waste have increased day by day which contains toxic substances or toxic metal ions due to the progressive industrial growth. Among these pollutants or toxic substances lead is one of the major environment pollutant due to its large scale industrial applications. It is used in alloys, paints industries, batteries, electronic gadgets, quality glass and used as radiation shielder. In general lead is considered as non-biodegradable and highly toxic metal ion. It is a carcinogen and effective neurotoxin also causes kidney problems, high blood pressure, stroke and lung disease etc. Plumlee et al. reported instantaneous and severe consequence of Pb toxicity in young children via Pbcontaminated soil, food and water. So, it is essential to determine and sensibly monitoring the amount of Pb ion present in drinking water. Sustained Pb intoxication critically interrupts the biosynthesis of hemoglobin [1]. The reason of the dementia that influence various Roman Emperors was because of lead acetate (called as lead sugar) swallowing as a sweetener for wine by the Roman Empire [2]. The Pb(II) ion in solution exists as PbOH+, Pb3(OH)42+, Pb4(OH)44+, Pb6(OH)84+ and Pb9(OH)126+ and they are easily molded in neutral and slightly alkaline solution. Trace level determination of Pb has increased the research attentiveness due to its harmful effects and accumulation in the biological system and environment. Spectrophotometric methods, atomic absorption spectroscopy-electro thermal atomization (AASETS) [3,4], inductively coupled plasma-atomic emission spectroscopy (ICP-AES), inductively coupled plasma-optical emission spectroscopy (ICP-OES) or the inductively coupled plasma-mass spectroscopy (ICPMS) [5], chromatography [6] and photometry [7-9] are used for the low level determination of lead ions in solution. But these methods generally require sample pre-treatment and infrastructure back up and hence are not convenient for routine analysis. But potentiometric sensors are better suited as they offer advantages such as high selectivity, sensitivity, good precision, simplicity and low cost. Ion selective electrodes are versatile devices with extraordinary analytical ease [10,11]. In ionselective electrodes the ion selective membrane is placed between the sample solution and the internal reference solution and interfacial potentials are developed on both sides of the membrane (Figure 1). This result in a very stable sensor and most of the available potentiometric sensors are based on ion selective electrodes [12]. Several neutral compounds with oxygen, nitrogen and sulphur donor atoms have been used as ionophores as lead(II) selective sensors [13-16]. In continuation with the development of electrochemical sensors [17-20], in this paper, we have reported Pb(II) ion selective electrode based on 2,2’-[propane- 1,3-diylbis(oxy)]dibenzaldehyde(PDOD) as an ionophore that exhibits good selectivity to lead ions over other cations. The reported electrode has good response characteristics such as wide concentration range, fast response time and long life time.
Experimental
Reagents
High molecular weight PVC, dibutylphthalate (DBP), dioctylsebacate (DOS), dioctylpthalate (DOP), o-nitrophenyloctyl ether (o-NPOE), sodium tetraphenylborate (NaTPB), and potassium carbonate (K2CO3) metal nitrates were received from Sigma Aldrich USA, Analytical reagent grade tetrahydrofuran (THF), hydrochloric acid (HCl), sodium hydroxide (NaOH), dimethylformamide (DMF), dibromopropane, salicyladehyde, ethanol, were obtained from SD-fine Chemical Pvt. Limited India. Solutions of metal nitrates were prepared in deionized water.
Apparatus
All the potentiometric measurements were made with a digital potentiometer model-118. The electrochemical system used was Ag- AgCl|3 M KCl| internal solution, 1.0 × 10-2 M Pb(NO3)2| PVC membrane |test solution |AgCl/Ag.KCl(satd.). An ultrasonic cleaner-2510 (Branson Inc., Danbury, CT 06810 USA) was used to degassed the solution and separate impurities. Elico SL-164 double beam UVVisible spectrophotometer loaded with spectra treatz software was used to record the spectra with quartz cuvettes. All pH measurements were made with an ATC pH meter model 132-E (Electronics, India).
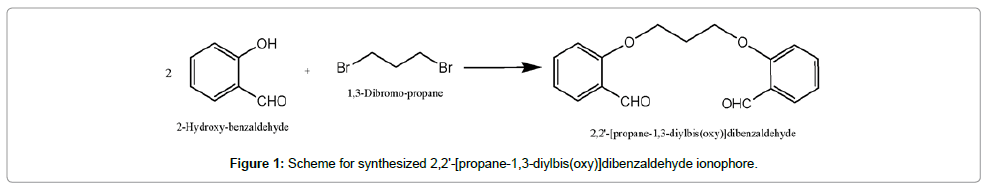
Synthesis of 2,2'-[propane-1,3-diylbis(oxy)]dibenzaldehyde Ionophore (PDOD)
PDOD ionophore was prepared with constant stirring of the mixture of salicyldehyde and K2CO3 in DMF by drop wise addition of dibromoethane for 10 h at 150-155°C and then stirred at room temperature for 5 h. After completion of reaction, 100 mL of distilled water was added; keep this reaction mixture in refrigerator for about 1 h. The precipitate of product was obtained (Figure 2). Crystalline products thus obtained were recrystallized in ethanol white color needle shaped crystals formed [21,22]. The confirmation of the completion of reaction was done with the help of thin layer chromatography (TLC) which gave only a single spot. Further characterization of crystalline products was done with the help of FTIR and 1H NMR (Supplementary Data).
Complexation study
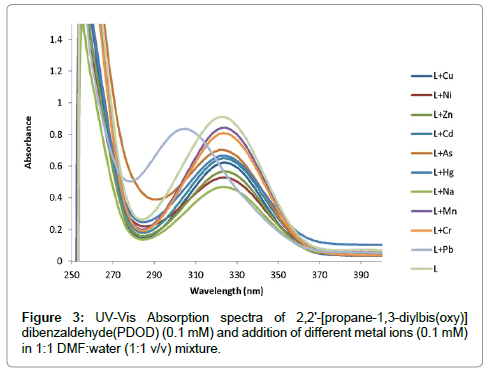
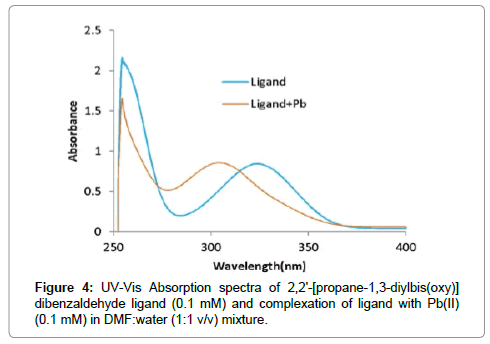
In preliminary studies the complexation of ligand with a different ions was examined spectrophotometrically in 1:1 dimethylformamide(DMF):water (H2O) mixed solvents with concentration(1 × 10-4 M). A UV-visible spectrum of ligand after the addition of different metal ions recorded over 200 to 800 nm spectra is shown in Figure 3. PDOD ionophore shows absorption peaks at 327 nm After the addition of required amounts of these ions separately, spectra were taken. When Pb(II) interacts with ionophore, observed the change in the absorption spectra, absorbance peak at 327 nm shifted to 318 nm (blue shift) and intensity of the band increased. In case of the other metal ions, the peak at 327 nm remains same in the complexation state. This observation confirms that the presence of Pb(II) results in noticeable change absorbption maxima. Hence, this study confirmed selectivity of the ionophore for Pb(II)ions (Figure 4).
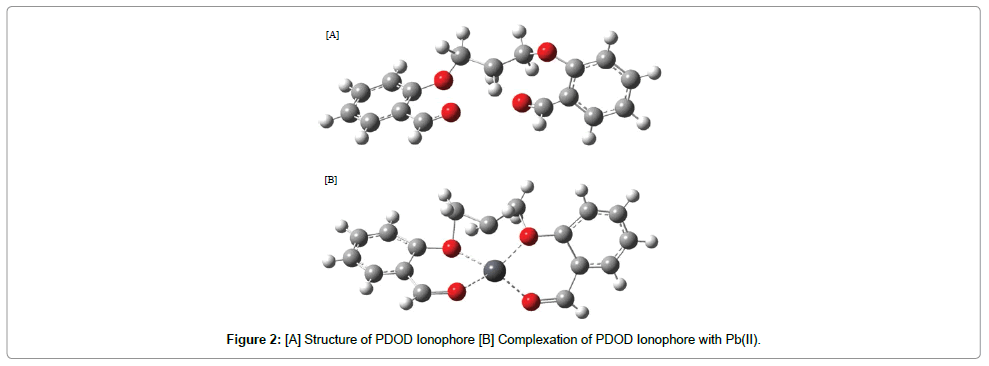
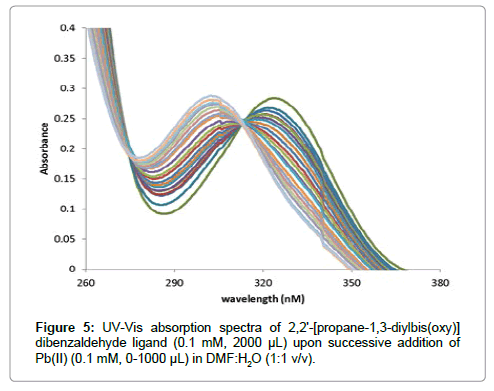
The stoichiometry of formed complexes between L and Pb(II) ion was determined by mole ratio method where the additions of lead ion to ionophore(PDOD) solution result in the formation of 1:1 [PDOD: Pb(II)] (Figure 5). Therefore, the general mechanism for the complexation process can be suggested as follows:
PDOD+ Pb(II) → (PDOD)2-Pb
The metal Pb(II) coordinated in the ligand with two aldehyde oxygen and another two oxy groups present in the ligand via π-back bonding. The results for 1:1 complexes were obtained which indicate the strong reaction between L and Pb(II)cation (Figure 2) [23].
Preparation of PVC membranes
The polymeric membranes were synthesized by mixing varying amounts of the ionophore (L), plasticizers o-NPOE, anionic additive NaTPB, in THF solvent. The mixed components were in weight percentages given in Table 1. all the components were dissolved completely, so that the homogenous mixture was obtained this mixture was placed into the uniform glass rings till the complete evaporation of solvent. Colourless membranes were obtained. The membranes were cut out and glued to one end of the ‘Pyrex’ glass tube. The prepared membrane electrode was equilibrated for 24 hours in Pb(II) (0.01 M) solution. Several membranes of different composition were developed and tested. The membrane which performs reproducible and best characteristics was used for further studies.
| Sensor no. | Composition of membranes(mg) | Working concentration range (M) | Slope (mV/decade) | Response time (s-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDOD | PVC | DOP | DOS | DBP | o-NPOE | NaTBP | ||||
| 1 | 5.0 | 31 | 62 | - | - | - | 2 | 1.4 × 10-6- 1.0 × 10-1 | 23.60 ± 0.3 | 15 |
| 2 | 5.0 | 31 | - | 62 | - | - | 2 | 3.1 × 10-6-1.0 × 10-1 | 30.60 ± 0.2 | 20 |
| 3 | 5.0 | 32 | - | - | 62 | - | 2 | 2.5 × 10-6-1.0 × 10-1 | 28.62 ± 0.3 | 17 |
| 4 | 5.0 | 32 | - | - | - | 60 | 3 | 1.2 × 10-7 -1.0 × 10-1 | 29.06 ± 0.1 | 5 |
| 5 | 3.0 | 32 | - | - | - | 60 | 5 | 2.9 × 10-6-1.0 × 10-1 | 27.83 ± 0.2 | 15 |
| 6 | 5.0 | 33 | - | - | - | 60 | 2 | 3.0 × 10-7-1.0 × 10-1 | 28.80 ± 0.2 | 11 |
| 7 | 2.0 | 33 | - | - | - | 62 | 3 | 1.8 × 10-6-1.0 × 10-1 | 27.82 ± 0.3 | 18 |
| 8 | 5.0 | 31 | - | - | - | 62 | - | 1.3 × 10-6-1.0 × 10-1 | 28.08 ± 0.1 | 22 |
Table 1: Response characteristics of the PVC based membranes having different amounts of ionophore (PDOD).
Potential measurements
All the potentiometric measurements were made with a Digital Potentiometer Model-118. The electrochemical system was as follows:
Ag-AgCl|3 M KCl |internal solution, 1.0 × 10-2 M PbNO3| PVC membrane |test solution |AgCl/Ag.KCl (satd.)
Results and Discussion
Optimization of membrane composition
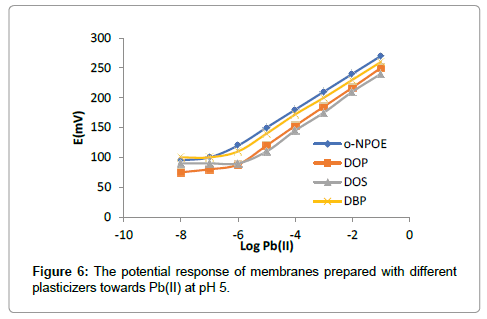
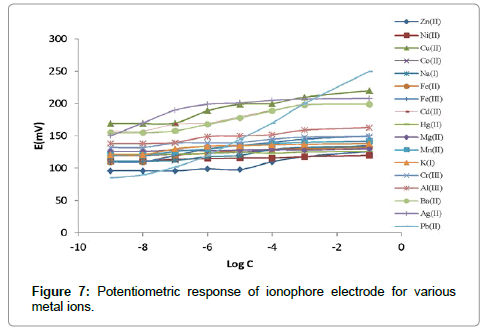
The composition of immobilized membrane performing a major role in the selectivity and sensitivity of an ionophore. The ISE-membranes were optimized by varying the ratio of the PVC, ionophore, stabilizers, additive and the results are summarized in Table 1. PDOD used, as an ionophore, plays significant role in ISE-membranes towards Pb(II) ion. The membrane no. 4 mention in Table 1, having a composition ration of 5:32:60:3 mg (PDOD:PVC:o-NOPE:NaTBP) shows the significant results with a linear working concentration range from 1.2 × 10-7 M-1.0 × 10-1 M, and nernstian slope of -29.06 ± 0.10 mV per decade. The amount and nature of plasticizers provide mobility to the ionophore within the membranes and make channels for target species. The change in potential of the ISE towards the Pb(II) ion were depend upon L as an ionophore, and various plasticizers with different polarities (DBP, DOS, DOP and oNPOE) in the PVC matrix. In this study among all these plasticizer, o-NPOE was determined to be the most appropriate material, as it improves the selectivity and nernstian slope of composite membrane illustrated in Figure 6. The most favorable PVC/o-NPOE ratio was determined to be about 1:2. Even though the fact that plasticizers lack any role in related to the change in selectivity of ionophore-based ISE, it is sensible to consider that various plasticizers having different dielectric constants affect the partition coefficient of cations in the form of cation-ionophore complex formation constants. Also mention in Table 1, the presence of lipophilic anionic additives improves the selectivity of the membrane sensor and the electrode resistance was lowered, and the long-term stability improved. The effects of internal solution on the response of the fabricated ISEs were determined on four measurements from 10-4 M to 10-1 M. working electrode concentration range and slope were obtained with an internal solution of activity 1.0 × 10-2 M shows best results. Therefore, an internal solution of Pb(II) ion activity 1 × 10-2 M was used throughout studies (Figure 7).
Working concentration range and slope
The potential response of the PDOD based proposed membrane towards Pb(II) ion is shown in Figure 6. The electrode shows a linear response to lead ion in a wide working concentration range (1.2 × 10-7-1.0 × 10-1 M), with a detection limit of 3.3 × 10-8 M and a Nernstian slope of -29.06 ± 0.10 mV per decade of concentration. The optimized membrane sensor provides a good selectivity toward the lead ion over other ions.
Response and life time
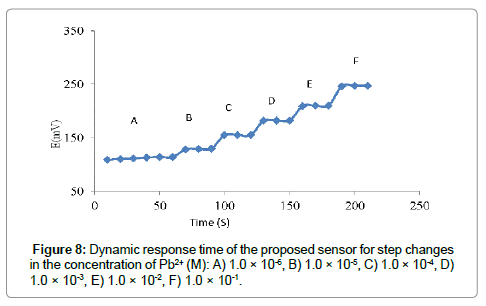
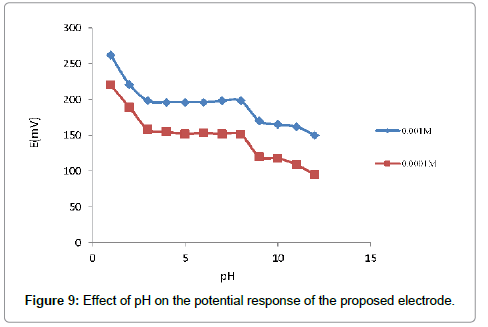
It is necessary to that the developed sensor generates not only stable but also reproducible potentials at short response time over a period of time. The dynamic responses of the prepared polymeric membrane electrode with stepwise increase in the concentration of the Pb(II) ion from 1.2 × 10-7 to 1.0 × 10-1 is shown in Figure 8. The time required to reach 95% the steady potential response was less than 10 s. Moreover, the stability and life time of the developed ISE was evaluated by using the proposed sensor over a period of 6 month without any significant changes in the Nernstian slope (Table 2). The limitation of life time in the prepared sensor might be due to the loss of membrane ingredients by leaching into the sample [24] (Figure 9).
| No. | Period | Working concentration range (M) | Detection limit (M) | Slope (mV/decade) |
|---|---|---|---|---|
| 1 | 1 Initial | 1.0 × 10-6 -1.0 × 10-1 | 2.1 × 10-8 | 29.06 ± 0.1 |
| 2 | 2 weeks | 1.0 × 10-6 -1.0 × 10-1 | 4.0 × 10-8 | 29.1 ± 0.1 |
| 3 | 4 weeks | 1.0 × 10-6 -1.0 × 10-1 | 9.0 × 10-8 | 29.3 ± 0.2 |
| 4 | 8 weeks | 1.0 × 10-6 -1.0 × 10-1 | 1.0 × 10-7 | 28.9 ± 0.2 |
| 5 | 14 weeks | 1.0 × 10-6 -1.0 × 10-1 | 1.6 × 10-7 | 28.1 ± 0.3 |
| 6 | 26 weeks | 1.0 × 10-6 -1.0 × 10-1 | 2.5 × 10-7 | 27.8 ± 0.2 |
Table 2: The life time behavior of the lead-selective electrode based on L.
Effect of pH
The potential responds of the proposed electrode for the two-test solution of Pb2+ ion with concentration 1.0 × 10-3 M and 1.0 × 10-4 M was studied by change the pH range between 1.0 to 13.0. The pH was adjusted with hydrochloric acid or sodium hydroxide. From Figure 10, it is cleared that the response potential is independent of pH 3.0-8.0, and the same can be taken as the working pH range of the proposed electrode. Above pH~8.0 gradual reduction in the potential was observed may be due to the formation of some hydroxyl complexes of Pb(II) in the solution. Meanwhile at lower pH, the potential increased, indicating that the electrode responded to hydrogen ion.
Potentiometric selectivity
The selectivity is the one of the most important feature of an electrode which tells the utility of the sensor. The study of interfering ions on the response of electrodes is usually termed as selectivity coefficient. To examine the membrane sensor response selectivity its potentials changes was observed in the presence of other interfering foreign cations. The methods like mixed solution method and fixed interference method are the generally used for the determination of the selectivity coefficient. However, these methods suffer some limitations if ions of different charges are involved. Thus, in our work we followed the match potential method (MPM), which is totally independent of the Nicolsky-Eisenman equation and also the varying charge of interfering ions. According the MPM, primary ions (A) in a specified activity, are added to a reference solution containing fixed concentration of primary ions and the potentials change is recorded. In a separate experiment, interfering ions (B) are successively mixed to an identical reference solution, until the measured potential matched the one obtained before by adding primary ions. A value of selectivity coefficient equal to 1.0 specify that the sensor responds equally to the primary as well as an interfering ion and in such a case the sensor is said to be selective to the primary ion over interfering ions. Further the selectivity coefficient, higher is the selectivity order [25-28]. The MPM selectivity coefficient, is given by activity (concentration) ratio of the resulting primary ion to interfering ion:
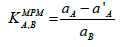
The value of αA (Pb(II) ref) and α'A (Pb(II)) were taken to be 1.0 × 10-2 and 3.0 × 10-3 M, whereas the value of aB (interfering ion) was experimentally determined. The experimental conditions applied, and the resulting values are given (Table 3). It is clear from the obtained data, that selectivity coefficient values are data indicate that logK values are in the order 10-3 for monovalent, divalent and trivalent metal ions (except Cd2+) (Table 4). Selectivity coefficient Therefore, the electrode can be used for the determination of Pb(II) ions in the presence of certain interfering ions. Electrodes already reported in literature generally suffer from strong interference by mercury ions, copper ions, silver ion, etc. but the proposed electrode system shows good selectivity for lead ion even in presence of these ions. The interfering ion does not affect the sensor response unless present at very high concentration. Selectivity coefficient values for Pb(II) selective electrode are shown in Table 5. The selectivity coefficient pattern clearly indicates that the electrodes are efficiently selective to Pb2+ ions.
| Interfering ion | Selectivity coefficient | Interfering ion | Selectivity coefficient |
|---|---|---|---|
| Na+ | 1.2 × 10-3 | Cd2+ | 2.0 × 10-2 |
| K+ | 2.2 × 10-3 | Fe2+ | 7.5 × 10-3 |
| Ag+ | 4.2 × 10-3 | Fe2+ | 8.2 × 10-3 |
| Ca2+ | 4.8 × 10-3 | Cu2+ | 8.9 × 10-3 |
| Ni2+ | 4.9 × 10-3 | Mg2+ | 9.0 × 10-3 |
| CO2+ | 5.6 × 10-3 | Mn2+ | 5.8 × 10-3 |
| Hg2+ | 7.2 × 10-3 | Al2+ | 6.2 × 10-3 |
Table 3: Selectivity coefficient values of Pb2+ ion-selective electrode based on ligand for several interference ions.
| Samples | Method [Pb2+ content (mg/L)] | |||
|---|---|---|---|---|
| Membrane electrode (mg/L)a | % recovery | AAS (mg/L) | % recovery | |
| Sample 1 | 7.15 (± 0.1) | 105 | 7.13 (± 0.2) | 104 |
| Sample 2 | 7.90 (± 0.1) | 106 | 7.83 (± 0.2) | 105 |
| Sample 3 (WS)* | 1.62 (± 0.1) | - | 1.60 (± 0.3) | - |
Where, aAverage of three measurements, (WS)*- Without Spiked.
Table 4: Comparison of results for the determination of the Pb(II) in waste water samples by the proposed method and atomic adsorption spectroscopy (AAS) method.
| Ref. | Working concentration range (M) | Detection limit (M) | Slope (mV/decade) | Response time (s) | pH range |
|---|---|---|---|---|---|
| 1,2-bis(salicylidin aminooxy)ethane [27] | 1.00 × 10-6 to 1.00 × 10-1 | 7.60 × 10-7 | 26.49 | 10< | 4-7 |
| Semi-conducting poly (m phenylenediamine) microparticles [28] | 3.16 × 10-6 to 3.16 × 10-2 | 6.31 × 10-7 | 29.8 mV decade | 14 s | 3.0-5.0 |
| 1,3,7,9-tetraaza-2,8-dithia-4,10-dimethyl-6,12-diphenylcyclododeca-4,6,10,12-tetraene (TDDDCT) [29] | 5.0 9 × 10-7 –1.0 9 × 10-1 |
2.59 × 10-7 | 29.5 (± 0.5) mV decade-1 |
10 s | 2.8-7.0 |
| N,N'-bis-thiophene-2-ylmethylene-ethane-1,2-diamine [30] | 1.00 × 10-5 to 1.00 × 10-1 | 2.04 × 10-6 | 29.79 mV | <5 | 5-7 |
| 3,15,21-triaza-4,5;13,14-dibenzo-6,9,12-trioxabicycloheneicosa-1,17,19-triene-2,16-dione [29] | 1.3 × 10-2 to 3.6 × 10-6 | 2.0 × 10-6 | 29.7 | 16 s | 3.7-6.5 |
| Present paper based on 2,2'-[propane-1,3-diylbis(oxy)]dibenzaldehyde | 1.2 × 10-7 M to 1.0 × 10-1 | 3.3 × 10-8 | 29.06 | <10 | 3.0-8.0. |
Table 5: Comparison of response characteristic of Pb(II) ion-selective electrodes with previous reported electrodes.
Potential applications
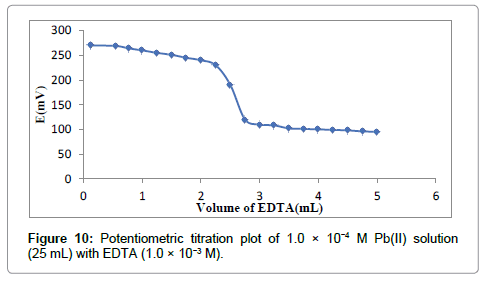
The developed Pb(II)-selective membrane electrode was successfully used under laboratory conditions. It was significantly working as an indicator electrode for the potentiometric titration of 25 mL Pb2+ solution (1.00 × 10-4 M) with EDTA (1.00 × 10-3 M), shown in Figure 10. The obtained plot indicates that the developed sensor is significantly selective towards the Pb2+ ion. Additionally, the end point corresponds to stoichiometry 1:1 of EDTA-Pb2+ complex.
Analysis of Pb (II): The detection of Pb(II) ions in waste water were carried out using potentiometry via spike and standard addition methods. Collected waste water (sample 1 and sample 2) were spiked with standard solutions of Pb(II) ion (1.0 × 10-4, 1.2 × 10-4) and sample 3 of waste water was taken as such without spiking. The validity of Pb(II) sample analysis by developed sensors via potentiometric method was performed. For potentiometric analysis of Pb(II) in test solution the following equation was used.
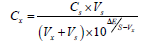
Where Cx and CS are the concentrations of Pb(II) ion in waste water and standard solution. Vx and VS are the volumes of test and added standard solutions. ΔE (EX-ES) is the difference in the potentials before and after standard addition. S is the slope of electrode response calculated from calibration curve respectively [14,19,29,30]. The reported data are the mean values of three measurement for each experiment with maximum RSD less than 2%. For the comparison purpose the Pb(II) content of these samples was also determined using AAS. The results are in satisfactory agreement (approximately 2% variation) with those obtained by atomic absorption spectroscopy (AAS). These results are summarized in Table 4.
Comparison of previous works with the current study
The performance characteristic of L ionophore based Pb(II) ion selective electrode was compared with the reported Pb2+ selective electrodes. As it can be observed from Table 5, the developed electrode in this study demonstrated a very good dynamic working range with low detection limit and fast response time compared to previously reported electrodes. Moreover, the potential responses of the developed electrodes are pH independent over a wide range. It can be concluded that the developed electrodes can be applied successfully for accurate and precise determination of lead(II) concentration in real samples.
Conclusion
The sensor proposed in this paper can be used to determine the concentration of Pb(II) ions in real water sample. The properties of the sensor are characterized by its strong selectivity, good reproducibility and rapid response time. The sensor can be used in a wide range of pH from 3 to 8. It gives a good Nernstian slope of 29.06 mV/decade for concentrations of lead(II) cations between 1.2 × 10-7-1.0 × 10-1 M. The performance of the sensor constructed using PDOD ionophore is favorable compared to previously developed Pb(II) ion sensors. This paper demonstrates that the synthesized ionophore can be successfully used in Pb(II)-ISEs.
Acknowledgments
The author is thankful to the University Grants Commission of India (UGC), New Delhi for the fellowship to complete this work.
References
- Lin C, Jie W, Huangxian J (2015) Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron 63: 276-286.
- Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, et al. (2011) Electrochemical analysis of some toxic metals by ion–selective electrodes. Critical Reviews in Analytical Chemistry 41: 282-313.
- Danielle KW, Gabriel LB, Mika Meschievitz E, David EC (2012) Electrochemical Sensors and Biosensors. Anal Chem 84: 685-707.
- Gregory M, Tuan DN, Benoit P (2015) Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 5: 241-275.
- Manju BG, Swaminathan S, Uma MK, John BBR (2015) A review on detection of heavy metal ions in water – An electrochemical approach. Sens Actuators B Chem 213: 515-533.
- Shailendra KP, Priti S, Jyoti S, Sadhana S, Sameer S, et al. (2016) Nanocarbon-based Electrochemical Detection of Heavy Metals. Electroanalysis 28: 2472-2488.
- Ming L, Honglei G, Al-Ogaidi I, Nianqiang W (2013) Nanostructured Sensors for Detection of Heavy Metals: A Review. Chem Eng 1: 713-723.
- Hangjia S, Danfeng Q, Yuzhen L, Shouzhu L, Chi Y (2016) In situ magnesiothermal synthesis of mesoporous MgO/OMC composite for sensitive detection of lead ions. Electroanalysis 28: 1-9.
- Rajesh S, Woo-Jin C, Sundaram G (2015) Highly Sensitive Detection and Removal of Lead Ions in Water Using Cysteine-Functionalized Graphene Oxide/Polypyrrole Nanocomposite Film Electrode. Appl Mater Interfaces 7:Â 15935-15943.
- Shunyang Y, Qun Y, Fuhai L, Yongming L (2012) Improved potentiometric response of all-solid-state Pb2+-selective electrode. Talanta 101: 546.
- Tarley CRT, Andrade FN, De Santana H, Zaia DAM, Beijo LA, et al. (2012) Ion-imprinted polyvinylimidazole-silica hybrid copolymer for selective extraction of Pb (II): Characterization and metal adsorption kinetic and thermodynamic studies. Reactive and Functional Polymers 72: 83-91.
- Kumar P, Ashok Kumar SK, Mittal SK (2014) N', N'', N'''-tris (2-pyridyloxymethyl) ethane as ionophore in potentiometric sensor for Pb (II) ions. Journal of Chemical Sciences 126: 33-40.
- Guzinski M, Lisak G, Kupis J, Jasinski A, Bochenska M (2013) Lead (II)-selective ionophores for ion-selective electrodes: A review. Analytica chimica acta 791: 1-12.
- Kulesza J, Bochenska M (2011) Calixthioamides as Ionophores for Transition-and Heavy-Metal Cations. European Journal of Inorganic Chemistry 6: 777-783.
- Wilson D, de los Ãngeles Arada M, Alegret S, del Valle M (2010) Lead (II) ion selective electrodes with PVC membranes based on two bis-thioureas as ionophores: 1, 3-bis (N'-benzoylthioureido) benzene and 1, 3-bis (N'-furoylthioureido) benzene. Journal of hazardous materials 181: 140-146.
- Singh S, Rani G, Singh G, Agarwal H (2013) Comparative study of lead (II) selective poly (vinyl chloride) membrane electrodes based on podand derivatives as ionophores. Electroanalysis 25: 475-485.
- Huang MR, Ding YB, Li XG (2013) Lead-ion potentiometric sensor based on electrically conducting microparticles of sulfonic phenylenediamine copolymer. Analyst 138: 3820-3829.
- Inamuddin NM, Rangreez TA, Alothman ZA (2012) Ion-selective potentiometric determination of Pb(II) ions using PVC-based carboxymethyl cellulose Sn(IV) phosphate composite membrane electrode. Desalination and Water Treatment 24: 806-813.
- Pazik A, Skwierawska A (2012) Chromogenic derivatives of new bis(phenylhydrazono-1 H-tetrazol-5-yl-acetonitriles)–synthesis and properties. Supramolecular Chemistry 24: 726-737.
- Kumar P, Joseph A, Ramamurthy PC, Subramanian S (2012) Lead ion sensor with electrodes modified by imidazole-functionalized polyaniline. Microchimica Acta 177: 317-323.
- Sayed YK, Mojtaba S, Hashem S (2009) Leadselective poly(vinyl chloride) electrodes based on some synthesized benzo-substituted macrocyclic diamides. J Hazard Mater 172: 68-73.
- Khan AA, Baig U (2012) Electrically conductive membrane of polyaniline–titanium (IV) phosphate cation exchange nanocomposite: Applicable for detection of Pb (II) using its ion-selective electrode. Journal of Industrial and Engineering Chemistry 18: 1937-1944.
- Umezawa Y, Umezawa K, Sato H (1995) Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KA, Bpot values. Pur & Appl Chem 67: 518.
- Deyhimi F (1999) A method for the determination of potentiometric selectivity coefficients of ion selective electrodes in the presence of several interfering ions. Talanta 50: 1129-1134.
- Ali H, Farzad M, Zahra A (2013) Lead (II)-Selective Polymeric Electrode Using PVC Membrane Based on a Schiff Base Complex of 1,2-Bis(Salicylidin Aminooxy) Ethane As an Ionophore. Energy and Environmental Engineering 1: 99.
- Sobhana M, Leena R, Laina AL, Theresa J, Krishnapillai GK (2014) J Incl Phenom Macrocycl Chem 78: 171.
- Taejun J, Dae-Cheol J, Hyo KL, Seungwon J (2005) Bull Korean Chem Soc 26: 1219.
Citation: Kaur K, Aulakh JS (2018) Fabrication of Pb(II) Selective Polymeric Membrane Electrode Based on 2,2’[Propane-1,3-Diylbis(Oxy)]Dibenzaldehyde as an Ionophore. J Anal Bioanal Tech 9: 399. DOI: 10.4172/2155-9872.1000399
Copyright: © 2018 Kaur K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3909
- [From(publication date): 0-2018 - Jul 17, 2024]
- Breakdown by view type
- HTML page views: 3206
- PDF downloads: 703