Research Article Open Access
Fabricationof Murine Ventricular Balloons for the Langendorff Heart Preparation
Andrew Miller and Gary L Wright*Department of Pharmacology, Quillen School of Medicine Johnson City, TN, 37604
- Corresponding Author:
- Gary L Wright
Department of Pharmacology- Quillen COM
PO Box 70577, Johnson city, TN 37614-0577
E-mail: wrightgl@etsu.edu
Received date: February 23, 2011; Accepted date: March 23, 2011; Published date: March 24, 2011
Citation: Miller A, Wright GL (2011) Fabricationof Murine Ventricular Balloons for the Langendorff Heart Preparation. J Biotechnol Biomaterial 1:101. doi:10.4172/2155-952X.1000101
Copyright: © 2011 Miller A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
A method for fabrication of ventricular pressure balloons is presented. Murine ventricular balloons with adequately small size, durability and elastic characteristics were produced using silicone dispersion. The forms, onto which the silicone are coated, are constructed of water soluble materials allowing the release of cured silicone balloons with soaking in H2O.
Keywords
Langendorff; Mouse; Ventricular; Balloon
Introduction
Retrograde aortic perfusion of the heart in the Langendorff mode is used by numerous laboratories to evaluate the effect of various factors on ventricular contractile function and ischemia reperfusion injury [1-3]. In this technique the atrium is removed and a water filled balloon is inserted into the left ventricular compartment to measure the pressure developed during cardiac contraction. The heart displays intrinsic rhythmic contractions and can be maintained for hours when perfused with oxygenated Krebs-Hensleit, or comparable, buffer. The isolated perfused heart model is widely used in academia and industry and was instrumental in the discovery of the phenomena of ischemic preconditioning and in the identification of a multitude of ionotropic and chronotropic factors [1,4].
The mammalian perfused heart preparation [5] was developed based upon work done with frog hearts. While physiologists have traditionally employed rat, the emergence of mouse as the dominant genetic model organism has led many to utilize the mouse heart as a model for perfusion studies. Unfortunately ventricular balloons of the appropriate size and elasticity are not commercially available for the mouse heart. Latex balloons are available for larger animal hearts but the rigidity of latex material, at thicknesses that prevent leaks, is not suitable for small mouse hearts.
The obvious need for a pressure balloon suitable for mouse heart studies led us to explore different materials and methods to fabricate balloons that display reliable performance at sizes small enough for insertion into the mouse ventricle. Here we present the culmination of these attempts: a simple and inexpensive method to make mouse balloons using silicone that results in balloons that display exceptional performance in our hands.
Materials and Methods
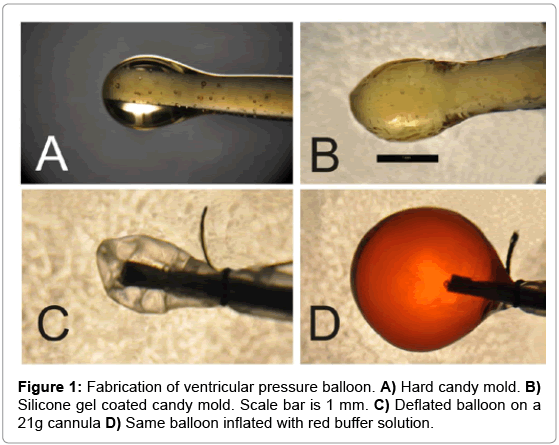
Fabricating the balloon forms: 9.5mL distilled deionized water, 14.2 mL light corn syrup and 33.8g sucrose are mixed in a 100-mL beaker and heated on a hot plate using medium heat. The mixture is stirred once per ~5 minutes avoiding boiling until the sugar is fully dissolved. Once the sugar is dissolved, heat is lowered and heating continues until the solution reaches 154°C. Straight dry pasta about 0.7mm in diameter is broken into thirds and dipped approximately 1cm into molten candy. The noodle is held with candy end down so that a teardrop of candy is formed. The noodle with candy drop is affixed inside a room temperature desiccator chamber so that candy drop faces down. Molds are left overnight to allow hardening of candy.
The hard candy molds are dipped into silicone dispersion gel (NuSil Tech. Carpinteria, CA -Cat # MED 10-6605) about 1 cm past the drop. Each mold is slowly rotated to evenly coat candy. Gel-covered molds are placed in drying oven (37°C) for 2 hours and then stored at RT in a desiccator chamber until the next layer is applied. At least 2 silicone layers are applied to molds. The silicone coated candy forms are simply immersed in water for 2-3 hours for removal of balloons. Balloons detach from candy with gentle pulling. Balloons are stored in a solution of 0.02% sodium azide in water.
Results
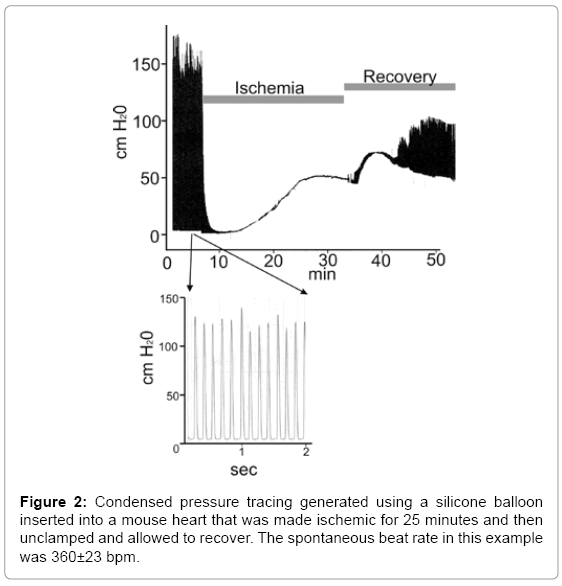
Balloons of sufficiently small size and elasticity were produced (Figure 1). These balloons were successfully situated within the left ventricle of a perfused murine heart and used to obtain pressure recordings (Figure 2).
The general framework of our method where water soluble candy forms are coated with silicone dispersion may prove useful in the fabrication of other small, hollow elastic research devices.
Acknowledgements
This work was supported in part by grant R01HL084302 from NIH.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
References
- Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R (2007) Isolated heart perfusion according to Langendorff---still viable in the new millennium. J Pharmacol Toxicol Methods 55:113-126.
- Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, et al. (2004) Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J 18: 1031-1033.
- Kasiganesan H, Wright GL, Chiacchio MA, Gumina G (2009) Novel l-adenosine analogs as cardioprotective agents. Bioorg Med Chem 17:5347-5352.
- Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124-1136.
- Langendorff O (1898) Untersuchungen am überlebenden. Säugethierherzen p 291-332.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14664
- [From(publication date):
March-2011 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10112
- PDF downloads : 4552