Research Article Open Access
Fabrication of a Dual-Enzyme Sensing System Based on Surface Plasmon Resonance Imager (SPRi) for Simultaneous Determination of Bio-Markers
Yuhei Hida1 and Hiroaki Shinohara1,2*
1Graduate School of Innovative Life Science for Education, University of Toyama, 3190 Gofuku, Toyama, Japan
2Graduate School of Science and Engineering for Research, University of Toyama, 3190 Gofuku, Toyama, Japan
- *Corresponding Author:
- Hiroaki Shinohara
Graduate School of Innovative Life Science for Education
University of Toyama, 3190 Gofuku
Toyama 930-8555, Japan
Tel: +81 076 445 6832
Fax: +81 076 445 6832
E-mail: hshinoha@eng.u-toyama.ac.jp
Received date: August 31, 2015; Accepted date: September 21, 2015; Published date: September 28, 2015
Citation: Hida Y, Shinohara H (2015) Fabrication of a Dual-Enzyme Sensing System Based on Surface Plasmon Resonance Imager (SPRi) for Simultaneous Determination of Bio-Markers. J Anal Bioanal Tech 6:279. doi:10.4172/2155-9872.1000279
Copyright: © 2015 Hida Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Saccharides such as glucose, galactose and amino acids like lysine, phenylalanine are known as bio-markers of metabolic disorders, and several analytical methods have been developed to determine these bio-markers in blood and urine by enzymatic method, HPLC analysis and so on. Multi-biosensing systems for simultaneous determination of these bio-markers are presently expected to be necessary for troublesome diagnosis. We have previously proposed enzyme-sensors based on a two-dimensional surface plasmon resonance imager (2D-SPRi), which can sensitively monitor refractive index change of local region in the evanescent field on an Au chip. L-lysine sensor, L-glutamate sensor and galactose sensor has been developed each by combining oxidase reactions with 2D-SPR imaging of Os polymer-redox state. In this study, we aimed to develop a dual-enzyme sensor capable of the simultaneous detection of lysine and galactose by means of co-immobilizing LysOx/Os-HRP polymer spot and GalOx/Os-HRP polymer spot adjacently on the Au chip, which is divided into two sections.
Keywords
Surface plasmon resonance imager; Bio-markers; Saccharides; HPLC analysis
Introduction
Saccharides and amino acids are essential metabolites, playing an important role in energy production, because ATP, NADH, and FADH2 as energy carriers are made with saccharides and amino acids in cells through glycolysis and TCA cycle. Therefore, various kinds of sensors, which target some saccharides and amino acids have been developed for diagnoses of metabolic diseases such as diabetes, galactosemia, phenylketonuria, maple syrup urine symptom and high lysine blood symptom, etc. [1-11]. Furthermore, recent statistics data of medical treatments demonstrated that abnormality of the amino acids balance in blood was correlated well with some diseases, and a novel method to diagnose by using the data about free-amino acid concentrations with multi-variable analysis has been proposed recently [12-17]. For example, the Fischer ratio defined as the concentration of branchedchain amino acids (BCAA)/the concentration of aromatic amino acids (AAA), shows less than 2.5 for the blood of cirrhosis patients, while that of the blood of healthy person shows 3.5~4.0 [16]. It is also reported that metabolic syndrome causes variation of some amino acids level in the blood [15].
High performance liquid chromatography (HPLC) and the enzyme method are well known as conventional methods for the amino acids measurement [18-21]. HPLC analysis is good at sensitivity, reproducibility and quantitativity, however, it also has the shortcomings such as time-consuming, high costs and need of some complex treatments before the measurement. On the other hand, enzyme method is simple and easy but not good at multi-determination. Therefore, it is required to develop multi-enzyme sensors, which can determine some saccharides and amino acids simultaneously in easy, quick and sensitive way.
Several types of bio-sensors by means of surface plasmon resonance imager (SPRi) have been already reported. Slight change of refractive index in the evanescent field on the sensor chip enables us to image the surface state. Hence, this technology has been extensively used for observing molecular adsorption / desorption reactions, antigenantibody binding reactions and DNA hybridization reactions [22- 28]. In recent years, Os-HRP complex polymer featuring the ability to change the refractive index in accordance with its redox state was utilized along with glucose oxidase (GOD) to develop a SPR-enzyme sensor for glucose [29-32]. From this basic idea, we started the design and the measurements of multi-enzyme sensor using SPRi.
In this study, we aimed to develop a dual-enzyme sensor capable of the simultaneous detection of L-lysine and galactose by means of co-immobilizing LysOx/Os-HRP polymer spot and GalOx/Os-HRP polymer spot adjacently on the Au chip, which was divided into two sections.
Materials and Methods
Reagents and chemicals
All chemicals as enzyme substrates were purchased from Wako (Japan). L-lysine oxidase (from Trichoderma viride) and galactose oxidase (from Dactylium dendroides) were purchased from Sigma. Os complex polymer containing horseradish peroxidase (Os-HRP poymer) was purchased from BAS (Japan). Glutaraldehyde and the reduced form of nicotinamide adenine dinucleotide (NADH) were purchased from TCI (Japan) and Oriental Yeast Co. (Japan), respectively.
A chip of 50 nm Au thin film-coated high refractive index glass (SF6) (18 × 17 mm) was obtained from BAS (Japan) and flexiPERM® (11 × 7 × 10 mm) was obtained from Greiner Bio One (Germany).
Fabrication of 2D-SPR sensor chip
The multi-sensor chip was prepared as follows. First, the Au thin film of a SPR sensor chip was separated into two sections by cutting the chip surface with a cutter knife. Then Os-HRP polymer solution was dropped on both sides of the cutted Au chip with 2 mm interval and dried at 4°C. Next, mixture of L-lysine oxidase (LysOx) solution or galactose oxidase (GalOx) solution and 1% glutaraldehyde solution was dropped onto each Os polymer spot and dried at 4°C. A silicon well (flexiPERM®) was attached onto the dual-enzyme chip to make measurement chamber, and the chamber was filled with a PB solution (0.1 M, pH 6.8) before measurements. The sensor chip was then set on the 2D-SPR measurement prism.
2D-SPR experimental setup and measurement
All sensing experiments were performed with 2D-SPR sensor (SPR imager 04A, NTT Advanced Technology [NTT-AT], Japan), which was equipped with a collimator lens, P and S-polarizer, four kinds of magnification lenses (× 1, 2, 4 and 7) and a CCD camera. A 770 nm LED was used as an incident light source to arouse SPR after passing through a collimator lens and P-polarizer.
SPR curves were obtained by changing measurement angle to determine the resonanse angle (θr) at each enzyme spot, and optimum angle for time-course measurement of reflection intensity before and after injection of the sample solution containing lysine and galactose. Thereafter, time-course measurement of reflection intensity (RI) at the fixed angle was performed. At this time, several sample solutions were injected into the sensor chamber at 1 minute after record starting. RI change was observed at each spot, and all the data were recorded and analyzed by analysis software.
After these experiments, oxidized-state of Os polymer at each enzyme spot was re-reduced by NADH solution to use this sensor repeatedly.
Results and Discussion
Detection principle of this sensing system for lysine and galactose is described as follows. First, lysine or galactose was oxidized to produce H2O2 at each enzyme spot when sample solutions containing each substrate are injected into the sensor chamber. Os complex polymer was transformed from reduced form (Os2+) to oxidized form (Os2+) by H2O2 with HRP catalysis [29]. Refractive index change caused by the oxidation of Os complex polymer was observed as RI change [30,31], and simultaneous detection of lysine and galactose at each spot was possible.
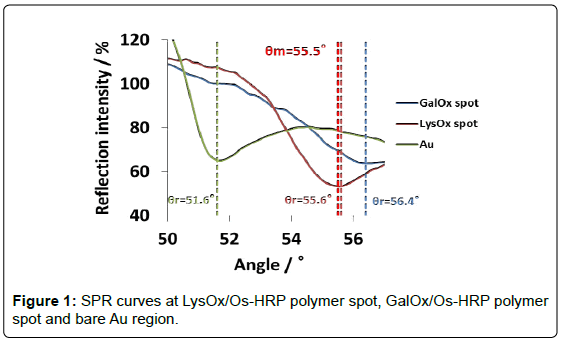
Figure 1 shows SPR curves at LysOx/Os polymer spot, GalOx/Os polymer spot and bare Au region in the angle scan mode. Resonance angles (θr) were 55.6°, 56.4° and 51.6°, respectively. In case of Os complex polymer and enzymes, whose refraction index are higher than that of water on Au chip, θr showed higher, and it indicates that each enzyme/Os polymer spot was certainly immobilized. It was inferred that higher θr of GalOx/Os polymer as compared with that of LysOx/ Os polymer spot may be due to the large extent of cross-linkng between GalOx and glutaraldehyde, and the large mass of GalOx existed on the Os polymer layer.
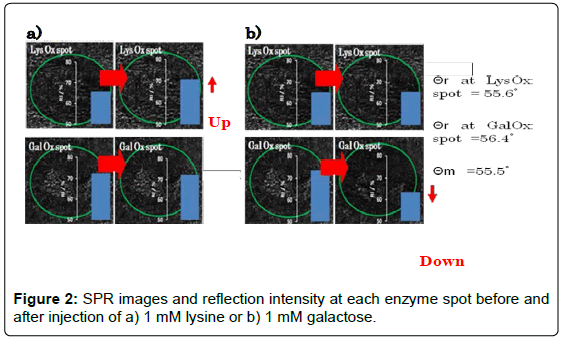
Time-course measurement was performed at a fixed angle (55.5°) determined by the angle scan measurement. We confirmed previously that the enzyme reaction resulted in negative shift of the θr because oxidized form of Os complex polymer has lower refractive index as compared with reduced form. Consequently, it is expected that the RI at LysOx/Os polymer spot should increase and the RI at GalOx/Os poymer spot should decrease at θm = 55.5° by addition of lysine and galactose. Figure 2 shows the RI change at the LysOx/Os polymer spot and the GalOx/Os poymer spot and SPR images of those regions. RI increase was observed at LysOx/Os polymer spot upon lysine injection, whereas no significant change of RI at GalOx/Os poymer spot was observed. In contrast, RI decrease clearly occurred only at GalOx/Os poymer spot after galactose addition as we expected.
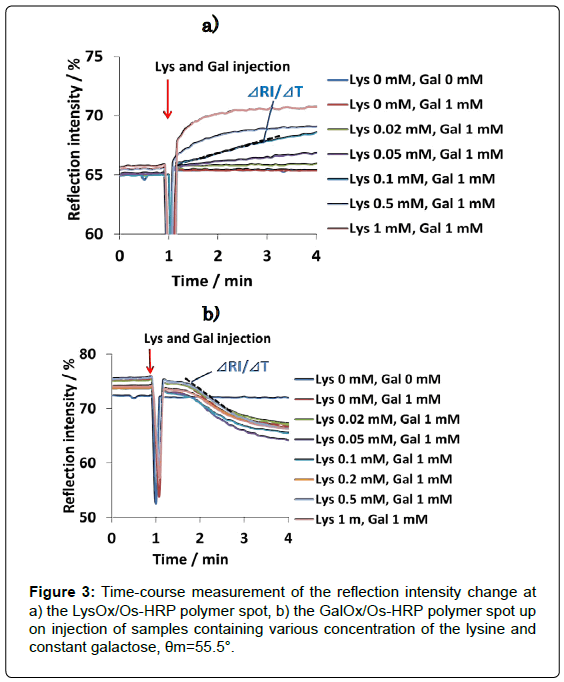
The results of L-lysine measurement performed in a mixed sample solution containing various concentration of lysine and constant concentration of galactose (1 mM) are shown in Figure 3. RI increased in proportion to the lysine concentration at LysOx/Os polymer spot. On the other hand, no change of the RI was observed at GalOx/Os poymer spot. RI decrease according to the galactose concentration was also observed at GalOx/Os poymer spot, whereas RI change at LysOx/ Os polymer spot was nothing (Data not shown).
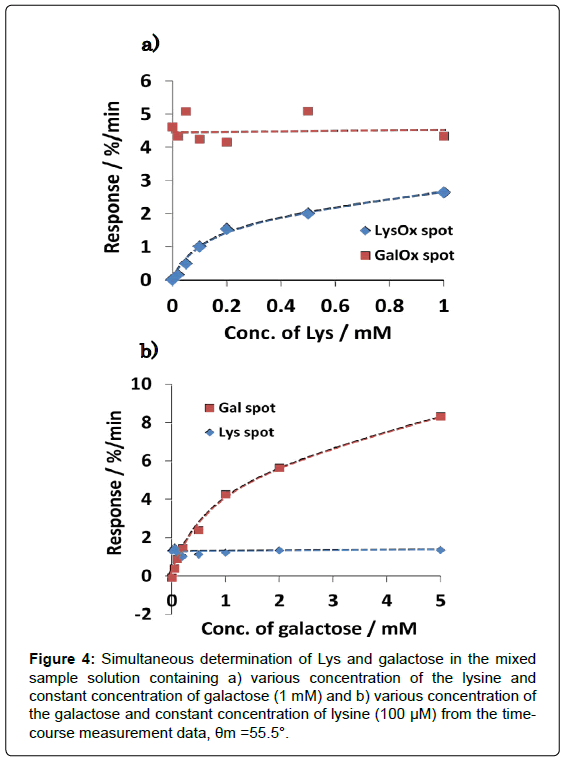
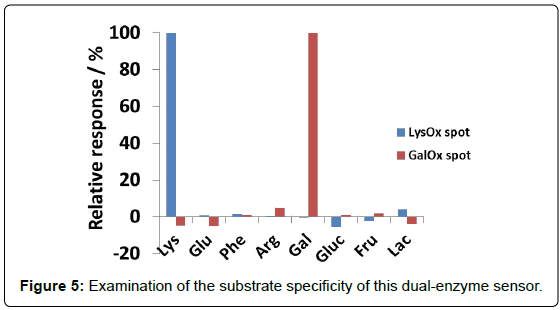
Figure 4 shows the dependence of reaction rate (response) of each enzyme spot on the lysine and galactose concentration. It was demonstrated that lysine can be determined from 20 to 200 μM without influence of galactose, and galactose also can be sensed in the rage from 50 to 1000 μM without influence of lysine. It was confirmed that calibration curves of dual-sensing were almost same as compared with that of each single sensing. The mean and SD values at LysOx and GalOx spot were calculated at 1.26 ± 0.141%/min and 4.55 ± 0.392%/ min in a concentration (100 μM Lys at LysOx spot, 1 mM Galactose at GalOx spot, n=7) on a chip, respectively. And CV values were also calculated at 11.2% and 8.6%, respectively. These results demonstrated that developed dual-enzyme sensing system has capability of measuring these two analytes simultaneously at each enzyme spot without any cross-talk. Substrate specificity was also investigated with several kinds of saccharides and amino acids in Figure 5 (It was tested for separate amino acids and saccharides samples). As we expected, each enzyme spot showed high specificity for lysine and galactose respectively comparing other saccharides and amino acids.
Figure 4: Simultaneous determination of Lys and galactose in the mixed sample solution containing a) various concentration of the lysine and constant concentration of galactose (1 mM) and b) various concentration of the galactose and constant concentration of lysine (100 μM) from the timecourse measurement data, θm =55.5°.
Conclusion
In this paper, we proposed 2D-SPR based dual-enzyme sensing system by which simultaneous determination of each substrate was feasible without cross-talk by using an Au chip separated into two sections. L-lysine oxidase and galactose oxidase were chosen for sensing oxidase in this time and combined with Os-HRP complex polymer to prepare two adjacent enzyme spots (LysOx/HRP-Os spot and GalOx/ HRP-Os spot) on an Au sensor chip. L-lysine and galactose were determined specifically at each spot from 20 to 200 μM and from 50 to 1000 μM, respectively. This sensor system also has great advantage of simple and quick measurement (within 2 min without pretreatment). Then, we will try to demonstrate accuracy of this method for plasma sample, in which lysine and galactose were determined with other methods like HPLC as future prospects. These results suggested that our sensing strategy based on a 2D-SPRi was capable to measure many kinds of enzyme substrates simultaneously and applicable to medical screening.
References
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448-453.
- Neville S, O'Sullivan S, Sweeney B, Lynch B, Hanrahan D, et al. (2015) Friedreich Ataxia in Classical Galactosaemia. JIMD Rep. pp: 1-5.
- Timson DJ (2015) The molecular basis of galactosemia-Past, present and future. Gene (In Press).
- Pyhtila BM, Shaw KA, Neumann SE, Fridovich-Keil JL (2015) Newborn screening for galactosemia in the United States: looking back, looking around, and looking ahead. JIMD Rep 15: 79-93.
- Seki M, Takizawa T, Suzuki S, Shimizu T, Shibata H, et al. (2015) Adult phenylketonuria presenting with subacute severe neurologic symptoms. J Clin Neurosci 22: 1361-1363.
- Lee JY, Chiong MA, Estrada SC, Cutiongco-De la Paz EM, Silao CLT, et al. (2008) Maple syrup urine disease (MSUD)-Clinical profile of 47 Filipino patients. J Inherit Metab Dis 31: S281-S285.
- Wang XL, Li CJ, Xing Y, Yang YH, Jia JP (2015) Hypervalinemia and hyperleucine-isoleucinemia caused by mutations in the branched-chain-amino-acid aminotransferase gene. J Inherit Metab Dis 38: 855-861.
- Stabler S, Koyama T, Zhao Z, Martinez-Ferrer M, Allen RH, et al. (2011) Serum Methionine Metabolites Are Risk Factors for Metastatic Prostate Cancer Progression. PLoS ONE 6: e22486.
- Saudubray JM, Rabier D (2007) Biomarkers identified in inborn errors for lysine, arginine, and ornithine. J Nutr 137: 1669S-1672S.
- Houten SM, Te Brinke H, Denis S, Ruiter JP, Knegt AC, et al. (2013) Genetic basis of hyperlysinemia. Orphanet J Rare Dis 8: 57.
- Sacksteder KA, Biery BJ, Morrell JC, Goodman BK, Geisbrecht BV, et al. (2000) Identification of the alpha-aminoadipic semialdehyde synthase gene, which is defective in familial hyperlysinemia. Am J Hum Genet 66: 1736-1743.
- Noguchi Y, Zhang QW, Sugimoto T, Furuhata Y, Sakai R, et al. (2006) Network analysis of plasma andtissue amino acids and thegeneration of an amino index for potential diagnostic use. Am J Clin Nutr 83: 513S-519S.
- Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, et al. (2011) Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLOS ONE 6: e24143.
- Fukutake N, Ueno M, Hiraoka N, Shimada K, Shiraishi K, et al. (2015) A Novel Multivariate Index for Pancreatic Cancer Detection Based On the Plasma Free Amino Acid Profile. PLOS ONE 10: e0132223.
- Yamakado M, Nagao K, Imaizumi A, Tani M, Toda A, et al. (2015) Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Scientific Reports 5: 11918.
- Yanagisawa R, Kataoka M, Inami T, Momose Y, Kawakami T, et al. (2015) Usefulness of circulating amino acid profile and Fischer ratio to predict severity of pulmonary hypertension. Am J Cardiol 115: 831-836.
- Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, et al. (2015) Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 47: 603-615.
- Cheng F, Wang Z, Huang Y, Duan Y, Wang X (2015) Investigation of salivary free amino acid profile for early diagnosis of breast cancer with ultra performance liquid chromatography-mass spectrometry. Clin Chim Acta 447: 23-31.
- Shimbo K, Oonuki T, Yahashi A, Hirayama K, Miyano H (2009) Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 23: 1483-1492.
- Waterval WA, Scheijen JL, Ortmans-Ploemen MM, Habets-van der Poel CD, Bierau J (2009) Quantitative UPLC-MS/MS analysis of underivatised amino acids in body fluids is a reliable tool for the diagnosis and follow-up of patients with inborn errors of metabolism. Clin Chim Acta 407: 36-42.
- Yoshida H, Kondo K, Yamamoto H, Kageyama N, Ozawa S, et al. (2015) Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 998-999: 88-96.
- Uludag Y, Tothill IE (2012) Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal Chem 84: 5898-5904.
- Wegner GJ, Lee H, Marriott G, Corn RM (2003) Fabrication of Histidine-Tagged Fusion Protein Arrays for Surface Plasmon Resonance Imaging Studies of Protein-Protein and Protein-DNA Interactions. Anal Chem 75: 4740-4746.
- Sakai T, Shinahara K, Torimaru A, Tanaka H, Shoyama Y, et al. (2004) Sensitive detection of glycyrrhizin and evaluation of the affinity constants by a surface Plasmon resonance-based immunosensor. Anal Sciences 20: 279-283.
- Altintas Z, Uludaga Y, Gurbuzb Y, Tothill IE (2011) Surface Plasmon resonance based immunosensor for the detection of the cancer biomarker carcinoembryonic antigen. Talanta 86: 377-383.
- Hemmi A, Mizumura R, Kawanishi R, Nakajima H, Zeng H, et al. (2013) Development of a novel two dimensional surface plasmon resonance sensor using multiplied beam splitting optics. Sensors (Basel) 13: 801-812.
- Wegner GJ, Wark AW, Lee HJ, Codner E, Saeki T, et al. (2004) Real-Time Surface Plasmon Resonance Imaging Measurements for the Multiplexed Determination of Protein Adsorption/Desorption Kinetics and Surface Enzymatic Reactions on Peptide Microarrays. Anal Chem 76: 5677-5684.
- Nelson BP, Grimsrud TE, Liles MR, Goodman RM, Corn RM (2001) Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal Chem 73: 1-7.
- Vreeke M, Maidan R, Heller A (1992) Hydrogen Peroxide and beta-Nicotinamide Adenine Dinucleotide Sensing Amperometric Electrodes Based on Electrical Connection of Horseradish Peroxidase Redox Centers to Electrodes through a Three-Dimensional Electron Relaying Polymer Network. Anal Chem 64: 3084-3090.
- Koide S, Iwasaki Y, Horiuchi T, Niwa O, Tamiya E, et al. (2000) A novel biosensor using electrochemical surface plasmon resonance measurements. Chem Commun 2000: 741-742.
- Iwasaki Y, Horiuchi T, Niwa O (2001) Detection of electrochemical enzymatic reactions by surface plasmon resonance measurement. Anal Chem 73: 1595-1598.
- Iwasaki Y, Tobita T, Kurihara K, Horiuchi T, Suzuki K, et al. (2002) Imaging of electrochemical enzyme sensor on gold electrode using surface plasmon resonance. Biosens Bioelectron 17: 783-788.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13685
- [From(publication date):
December-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9325
- PDF downloads : 4360