Fabrication and Characterization of Carbon Black and Phenol Formaldehyde Composites
Received: 27-Feb-2018 / Accepted Date: 28-May-2018 / Published Date: 02-Jun-2018
Abstract
Carbon black and Phenol formaldehyde composites were prepared by hot press molding at different temperature (180°C, 200°C, 220°C); pressure (100 kN, 150 kN, 200 kN, 250 kN); heating time (10 min, 20 min, 30 min, 60 min) and several compositions (50%C, 60%C, 70%C, 80%C, 90%C). The different properties such as physical, mechanical, thermal properties were studied by Universal Testing Machine (UTM), Micro hardness tester, TGA/DTA analyser and X-Ray diffractometer etc. In this work, it was observed that the bulk density was slightly changed for 50% carbon black and Phenol formaldehyde composites. Compressive strength of sample was increased with increasing the pressure, heating temperature and time.
Keywords: Carbon black; Phenol formaldehyde; Micro hardness
Introduction
When two or more material with different properties is combined together they form a composite material [1]. The constituents are combined in such a way that they keep their individual physical phases and are non-soluble in each other or do not form a new chemical compound. That is why a composite is considered to be any multiphase material system that exhibits a combination of properties that makes the composite superior to each of the constituent phases. The primary function of matrix is to hold the fiber to form a certain shape. Besides, the functions of the matrix are also to transfer stress between the reinforcing fibers and to protect them from mechanical and environmental damage. The function of reinforcing phase in matrix is to improve the mechanical properties such as strength, stiffness etc. As per Berghezan [2] the composite material is to be designed in such a way that the individual component retains their characteristic are so incorporated that the composite takes advantage of their superior properties without compromising on the weakness of either. When the matrix is a polymer, the composite is called polymer matrix composite.
Carbon black and phenol formaldehyde composites are primarily developed and designed for high temperature applications which require strength retention [3]. These materials are known for their strength, lightness in weight and thermal properties. Phenolic resins are used as matrix precursors in carbon black properties because they are relatively easy to impregnate fibers in traditional polymer composites processing [4]. To develop such composite, carbon black as a filler and resole- type phenol formaldehyde resin (PF) as a polymer matrix have been used in this study. The most important characteristics of carbon black is its high surface area, since the particles are small in size; therefore, the specific surface area is large. This high surface area facilitates excellent interaction between filler and polymer matrix, which delivers composites with improved properties [5].
Phenolic resins are known for their excellent thermal properties and chemical stability. In the field of advanced composite materials, phenolic based composites are known for their excellent flame resistance and are excessively used in the rocket industry because of their ablative characteristics. Carbon fiber-reinforced phenolic resins are particularly effective in resisting high temperatures, since the resin evaporates and burns at the surface creating a thermal protective layer [6].
Phenolic resins have an excellent affinity for graphite and other forms of carbon. Manufactures often use the resin simply as a binder and adhesive for their carbon materials. At high temperature, phenolic resins form a char of amorphous carbon. This means phenolic bonded carbon materials can be heat treated to yield an all carbon structure. Because of these unique properties, phenolic resins find application in the manufacture of electrodes, carbon carbon composites, carbon seals, and washers. Phenolic resins are the binder of choice for manufacturing the carbon brushes used in electrical motors, starters and the like. Depending on the manufacturing process, powdered or liquid solutions of novolac resin-hexa blends, powdered resol resin and liquid resol binding systems provide the desired binding properties [7,8]. This study aims to investigate effect of carbon black on PF resin on the basis of mechanical and thermal properties.
Materials and Methods
Raw materials
The raw materials for preparing the samples were Carbon black and Phenol formaldehyde.
Carbon black
Carbon black are obtained in different sizes such 1-2 cm lump sizes or in the form of fine powder. For making sample fine powder were used.
Phenol formaldehyde
Phenol formaldehyde is also obtained with a size of 1 mm diameter. Phenol formaldehyde is of two types such as cresol type and resol type. In this case resol type was used.
Preparation of the sample
Drying carbon black and phenol formaldehyde: Collected carbon black and phenol formaldehyde from local market were dried separately at 80°C for 24 hours in a drier under vacuum condition.
Grinding carbon black and phenol formaldehyde: First, carbon black of small lump size was ground to fine powder and then phenol formaldehyde was ground to fine powder and kept in separate container.
Weighing and mixing carbon black and phenol formaldehyde: Carbon black and phenol formaldehyde were weighed in electronic balance to make different sample.
S-1 to S-11 contain 50% mixture of carbon black and phenol formaldehyde i.e., 50 g carbon black and 50 g phenol fromaldehyde.
• S-3(1) contain 60% carbon black and 40% phenol fomaldehyde. • S-3(2) contain 70% carbon black and 30% phenol fomaldehyde. • S-3(3) contain 80% carbon black and 20% phenol fomaldehyde. • S-3(4) contain 90% carbon black and 10% phenol fomaldehyde. • S-3(00) contain 100% carbon black.
After weighing, fine powder of carbon black and phenol formaldehyde were mixed according to sample and again ground to mix uniformly for 30 minutes.
Replacing into molds: Weighed and uniform mixture of carbon black and phenol formaldehyde were put into a rectangular mold and was ready for further operation by fitting the mold with nut and bolt.
Pressing and heating
Then the mold was placed in a Weber-Press Hydraulic Press Machine and then specific condition for sample preparation was set. For example, for the preparation of sample S-1, first temperature was set at 200°C then the mold was kept under 100 kN pressure. With the rise of temperature up to 200°C, pressure was maintained at 100 kN. Then the mold was allowed to heat for 10 minutes at 200°C and 100 kN pressure. After 10 minutes, water was circulated in order to reach at room temperature. Then the composite sample was released from the mold (Table 1).
| Variables | Constant variables | Sample Name | Specific condition |
|---|---|---|---|
| Pressure | Temperature 200°C, Time 10 minutes |
S-1 | 100 kN |
| S-2 | 150 kN | ||
| S-3 | 200 kN | ||
| S-4 | 250 kN | ||
| Temperature | Pressure 250 kN, Time 10 minutes |
S-5 | 180°C |
| S-6 | 200°C | ||
| S-7 | 220°C | ||
| Time | Temperature 200°C Pressure 200 kN |
S-8 | 10 min |
| S-9 | 20 min | ||
| S-10 | 30 min | ||
| S-11 | 60 min | ||
| Composition | Temperature 200°C Pressure 200 kN Time 10 minutes |
S-3 | 50%C |
| S-3(1) | 60%C | ||
| S-3(2) | 70%C | ||
| S-3(3) | 80%C | ||
| S-3(4) | 90%C | ||
| S-3(0) | 100%C |
Table 1: Specific condition for sample preparation.
Specific conditions are pressure, temperature and time which are given in the following table.
Methods for measuring physical properties
Bulk density specimen was prepared according to the ASTM C135-76.
D=WS/V;
Where,
D=Density of the specimen (g/Cm3);
WS=Weight of the specimen (g);
V=Volume of the specimen (Cm3).
Methods for measuring mechanical properties
Micro hardness: Microhardness of the samples was measured by using following formula:
HV=1.854 F/d2 (approximately);
Where,
F=Load in Kgf;
d=Arithmetic mean of the two diagonals, d1 and d2 in mm;
H=Vickers hardness.
Universal testing machine (compression test)
Compressive strength of the composites was determined by using Universal testing machine ((UTM) (FS – 300 kN, Testometric, England)). There is fixed jaw and a movable jaw. The sample is inserted within this jaw and then pressure is applied by an input system.
Results
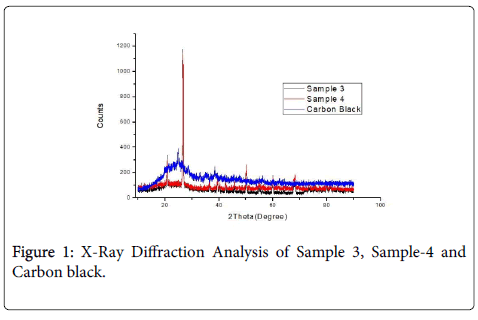
X-Ray diffraction analysis
Figure 1 shows the X-Ray Diffraction pattern of Carbon black and Phenol formaldehyde composites. The pattern was identified with Graphite for S-3 and S-4. Carbon black X-RD pattern was identified with Carbon by the sharp peak at about 24.7°C two theta values (002 plane) [9].
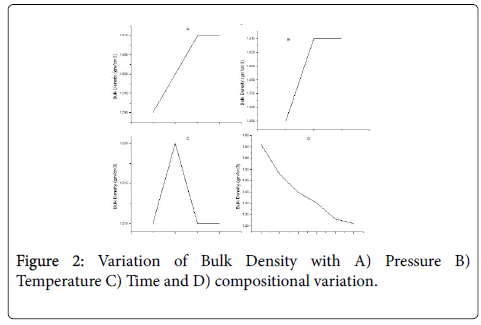
Bulk density
For 50% mixture of carbon black and phenol formaldehyde, bulk density variation for temperture, pressure and pressure and temperature holding time is very slight. With the change of composition of carbon black and phenol formaldehyde, bulk density variation is observed. As the percentage of carbon black increases, bulk density decreases gradually as shown in Figure 2.
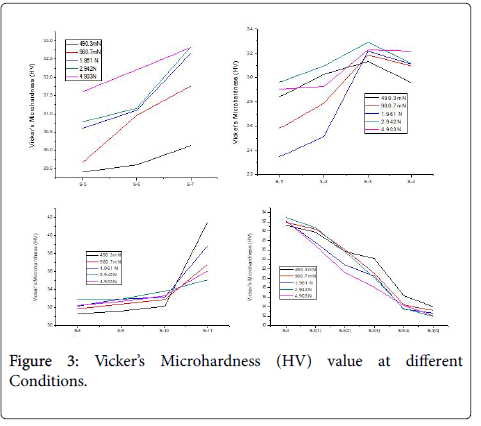
Vicker’s microhardness (HV)
Vicker’s microhardness test was done at different load such as 490.3 mN, 980.7 mN, 1.961 N, 2.942 N and 4.903 N. The sample 1-3, those containing 1: 1 carbon black and formaldehyde resin, shows an increase in hardness value up to a pressure of 200 kN. Sample 4, which is prepared at pressure of 250 kN shows a declination in hardness value, however the maximum hardness value is observed for sample 3 at 2.942 N load. The hardness value increases with the both the heating time and temperature as well. Figure 3 shows that Vicker’s Microhardness (HV) value decreases with increasing the percentage of Carbon black in composite.
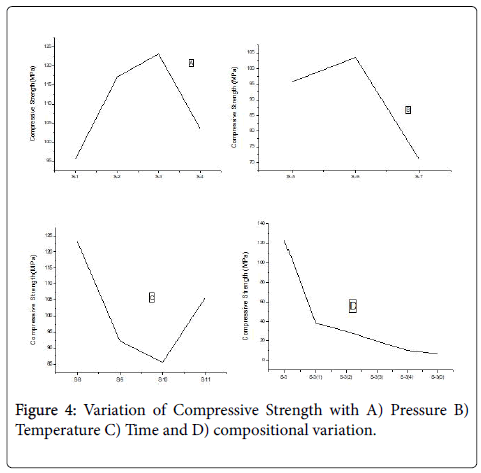
Compressive strength
Compressive strength found to increase with only the mold pressure that is from sample 1-sample 3, but in case sample 4 it shows a declination due to increasing of temperature with pressure. The maximum Compressive strength is observed for 50-50 composition which is around 125 MPa (Figure 4).
Thermal analysis
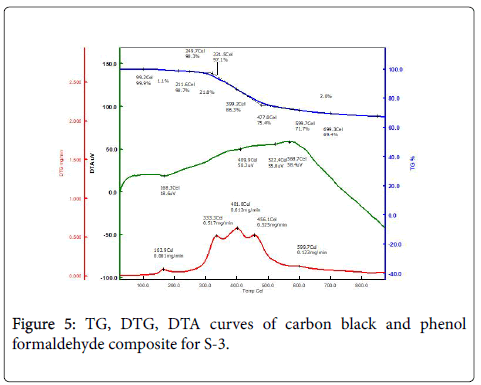
The following table shows the thermal degradation of prepared composite based on the TG-DTA analysis. From this study it is clear that the thermal stability increases with the inclusion of Formaldehyde resin along with temperature and pressure increases. Table 2 demonstrated the effect of introducing the carbon structures had on the thermal stability of PF. Figure 5 shows that the weight loss of composite under nitrogen atmosphere took place in three steps. Below 300°C, (first region) evolution of water, cross-linked byproducts and unreacted oligomers, is the main reaction. The decomposition of composite started at 300°C and the significant degradation occurred between 300-600°C resulting in the generation of large number of gaseous volatile, including CO, H2O, small molecule hydrocarbons and other compounds [10,11]. Between 500-800°C, weight loss curve became relatively flatter which is attributed to the breakage and redistribution reactions of the C–C bonds. Due to the increased carbon black incorporation the char yield increased from 50.20% to 60.04%, which is an indication of improved thermal stability and the bonding strength of the adhesive at high temperature. From the DTG values as shown in Table 2, it can be clearly seen that the decomposition peak temperature (Td, max) of composite was higher and the decomposition rate was lower, suggesting that carbon black effectively protect the matrix from degradation.
| Sample | Conditions | Initial degradation Temperature (°C) |
Maximum Degradation temperature (°C) |
Maximum Degradation rate(mg/min) | Final Residue at 875°C (%) |
|---|---|---|---|---|---|
| 2 | 150 kN, 200°C, 10 minutes | 287.0°C | 333.1°C | 0.294 | 50.2 |
| 5 | 200 kN, 180°C, 10 minutes | 311.1°C | 396.8°C | 0.204 | 58.4 |
| 3 | 200 kN, 200°C, 10 minutes | 321.5°C | 399.2°C | 0.612 | 60.4 |
Table 2: TG and DTA values of the prepared composite at various conditions.
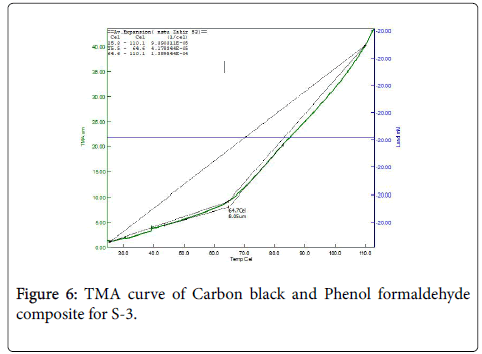
Figure 6 shows the thermo Mechanical Analysis (TMA) curve of Carbon black and phenol formaldehyde composite for S-3. TMA curve shows that the onset of expansion occurs in the range of 25.5°C to 64.7°C, the value of which is 8.05 μm.
Conclusion
Density variation is very slight for 50% carbon black and phenol formaldehyde composites. The highest density is found for Sample S-8 and S-9. Vicker’s Microhardness (HV) value increases with increasing the heating temperature and heating time. The highest compressive strength is found for sample S-3 is 123.13 MPa. Thermal analysis (TG, DTG and DTA) shows that S-3 is more thermally stable than the other samples. X-Ray Diffraction Analysis shows that, the pattern shown by composite S-3, S-4 is identified with Graphite. The result of the present study reveals that composites with good strength could be successfully developed by 50% carbon black and phenol formaldehyde (S-3) for 10 minutes heating at 200°C under 200 kN molding load. From characterization of all samples, S-3 gives the maximum compressive strength than other sample.
References
- Herakovich CT (1998) Mechanics of fibrous composites. New York: Wiley, pp: 1-27.
- Berghezan AN (1966) Nucleus an Editeur,1, rhe, Chalgrin, Paris. 8: 16(e).
- Fitzer E (1987) The future of carbon-carbon composites. Carbon 25: 163-190.
- Weisshaus H, Kening S, Siegmann A (1991) Effect of materials and processing on the mechanical properties of C/C composites. Carbon 29: 1203-1220.
- Knop A, Scheib W (1979) Chemistry and application of phenolic resins, Springer-Verlag, Berlin, p: 53.
- Bryner F (1967) Phenolic resin adhesive extended with carbon and causticized amylaceous materials.
- Gautam RK, Kar KK (2015) The effects of nano-sized carbon black content and particle sizes on the properties of carbon/ phenolic composite bipolar plates. Journal of Multidisciplinary Engineering Science and Technology 2: 191-200.
- Gill RM (1972) Carbon fibers in composite materials, Iliffe Books, London, p: 42.
- Ungár T, Gubicza J, Ribárik G, Pantea C, Zerda TW (2002) Microstructure of carbon blacksdetermined by X-ray diffraction profile analysis. Carbon 40: 929-937.
- Wang F, Huang Z, Zhang G, Li Y (2016) Preparation and thermal stability of heat-resistant phenolic resin system constructed by multiple heat-resistant compositions containing boron and silicon, High Performance Polymers. High Performance Polymers 29: 493-498.
- Norkhairunnisa M, Azizan A, Mariatti M, Ismail H, Sim LC (2012) Thermal stability and electrical behavior of polydimethylsiloxane nanocomposites with carbon nanotubes and carbon black fillers. Journal of Composite Materials 46: 903-910.
Citation: Hossain J, Alam MS, Paul SC, Islam S (2018) Fabrication and Characterization of Carbon Black and Phenol Formaldehyde Composites. Ind Chem 4: 127. DOI:
Copyright: © 2018 Hossain J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5973
- [From(publication date): 0-2018 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 5199
- PDF downloads: 774