Extranodal Marginal Zone Lymphoma of the Lung: Evolution from an Underlying Reactive Lymphoproliferative Disorder
Received: 22-Dec-2014 / Accepted Date: 28-Jan-2015 / Published Date: 03-Feb-2015 DOI: 10.4172/2161-0681.1000208
Abstract
Extranodal Marginal Zone Lymphoma of mucosa-associated lymphoid tissue (ENMZL) is a problematic and sometimes controversial diagnosis. While commonly seen in the stomach in the setting of chronic Helicobacter pylori infection, other extranodal sites, such as the lung, may also present with disease. ENMZL is clinically and morphologically heterogeneous; however, regardless of presentation, the etiology lies in the accumulation of lymphoid tissue in non-traditional sites. This phenomenon is typically secondary to an underlying inflammatory stimulus such as chronic infection or autoimmune states. The current case report details the clinical history of a patient with Sjögren syndrome over a four year period who eventually developed ENMZL. The patient initially presented with an atypical, but polyclonal, lymphoproliferative process diagnosed as lymphocytic interstitial pneumonia. Over time, the patient showed evolution to a monoclonal process with associated radiologic progression of disease. This evolution manifested as a dense lymphoid infiltrate with prominent plasmacytic differentiation and the development of a lung mass radiologically. This case contributes to the growing body of knowledge that suggests ENMZL lies along a biological spectrum of lymphoproliferative disorders whereby a benign, reactive process may eventually undergo malignant transformation. This evolution likely represents the acquisition of genetic abnormalities that allow autonomous proliferation in the absence of the initial immune stimulus. In practice, determining when this event occurs and, thus, distinguishing between reactive and neoplastic disorders within this spectrum may be difficult as no single clinicopathologic feature may be present to establish the diagnosis. This case further illustrates the importance of correlating the clinical, radiologic and pathologic data to evaluate patients with atypical pulmonary lymphoproliferative disorders and to allow the optimal management of their disease.
Keywords: Extranodal marginal zone lymphoma; MALT lymphoma; Lymphocytic interstitial pneumonia; Atypical lymphoplasmacytic infiltrate; Sjögren syndrome
311935Introduction
Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT) is a B-cell neoplasm and type of indolent, non-Hodgkin lymphoma. Although the clinical presentation and site of involvement may show considerable variation, extranodal marginal zone lymphoma has been defined by the World Health Organization as a tumor composed of a heterogeneous population of small B-cells which resemble the marginal zone B-cells of the Peyer’s patches, lymph node and spleen. Despite the morphologic diversity, a significant proportion of these lymphomas are composed of cells with abundant pale-staining lymphocytes, so-called monocytoid B-cells, and frequently demonstrate plasma cell differentiation [1].
Extranodal marginal zone lymphoma may occur in many sites including the gastrointestinal tract, and less commonly, the salivary glands, thyroid, respiratory tract, skin, ocular adnexa, genitourinary tract and breast. This malignancy is believed to arise in areas of chronic inflammation, where there is proliferation of extranodal lymphoid tissue. An infectious agent, as is the case in most gastric MALT lymphoma, may be the underlying stimulus of chronic inflammation, predisposing the patient to the development of lymphoma [1]. Moreover, autoimmune conditions, such as Sjögren syndrome and Hashimoto thyroiditis, greatly increase the risk of developing lymphoproliferative disorders in general, but especially those of MALT type [2].
The diagnosis of extranodal marginal zone lymphoma, of any site, is problematic due to the extensive overlapping features with reactive lymphoproliferative processes [1]. In fact, extranodal marginal zone lymphoma may represent one extreme of a spectrum of disorders, ranging from polyclonal inflammatory conditions to autonomous proliferations with associated genetic abnormalities [1]. Here we present a case of extranodal marginal zone lymphoma of the lung, developing in a background of lymphocytic interstitial pneumonia and Sjögren syndrome.
Case Report
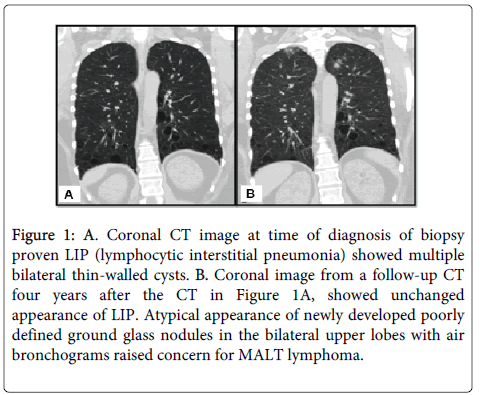
A 47-year-old Caucasian female presented to the pulmonology services at our institution for regular follow-up of a chronic lung condition initially diagnosed four years earlier in 2010 as Lymphocytic Interstitial Pneumonia (LIP). At the time of this diagnosis, she had an extensive evaluation that included a Computed Tomography (CT) scan (Figure 1A) which showed multiple, bilateral thin-walled cysts that proved negative for malignancy. At the time of her follow-up appointment, she had no complaints. The patient was morbidly obese (BMI 46.5 kg/m2) and her past medical history was significant for Sjögren syndrome and allergic rhinosinusitis. For her autoimmune condition, she was on a regimen of chronic immune suppressive therapy including prednisone, mycophenolic acid, and hydroxychloroquine. At this visit, a repeat CT scan of her lungs showed new, bilateral ground glass nodular opacities in the upper lobes with air bronchograms concerning for lymphoma in addition to the unchanged pulmonary parenchymal cysts characteristic of lymphocytic interstitial pneumonia (Figure 1B). A CT-guided core biopsy of this newly apparent right lung nodule was retrieved.
Figure 1: A. Coronal CT image at time of diagnosis of biopsy proven LIP (lymphocytic interstitial pneumonia) showed multiple bilateral thin-walled cysts. B. Coronal image from a follow-up CT four years after the CT in Figure 1A, showed unchanged appearance of LIP. Atypical appearance of newly developed poorly defined ground glass nodules in the bilateral upper lobes with air bronchograms raised concern for MALT lymphoma.
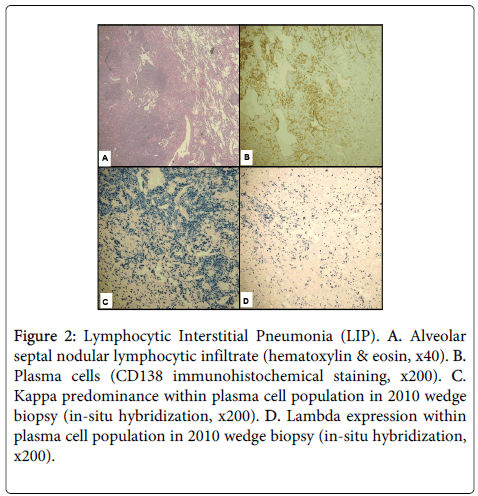
For comparison, the lung wedge resections previously diagnosed as lymphocytic interstitial pneumonia were pathologically reviewed. The specimen showed a predominantly bronchiolocentric nodular proliferation of lymphocytes with occasional germinal center formation. By immunohistochemistry, the lymphoid nodules were composed of a mixture of CD3-positive T-cells and CD20-positive Bcells. Interstitial and alveolar space inflammation was minimal except in the lower lobe of the right lung, where the lymphocytic infiltrate appeared more extensive along the alveolar septae (Figure 2A). This population was composed predominantly of CD4-positive T cells. CD43 highlighted the T-cell population; no CD43-positive B-cells were appreciated. Plasma cells, characterized by expression of CD138 in the absence of CD20, were present and were focally numerous (see Figure 2B). The plasmacytic infiltrate appeared most dense around an eosinophilic, fibrotic nodule that demonstrated a slightly skewed kappa:lambda ratio of approximately 5:1 (see Figure 2C and Figure 2D). Elsewhere, the kappa predominance did not appear as marked. Because of this apparent increase in kappa-expressing plasma cells and concern for lymphoma, PCR for IGH gene rearrangement and FISH for the API2-MALT1 t(11;18)(q21;q21) translocation were performed; however, neither genetic abnormality was detected. These results, coupled with the clinical and radiologic findings, suggested the inflammatory process at that time was most consistent with lymphocytic interstitial pneumonia. The patient’s history of anti-Ro and anti-La antibodies, suggestive of an underlying autoimmune condition, was also noted at this time, further supporting a diagnosis of LIP.
Figure 2: Lymphocytic Interstitial Pneumonia (LIP). A. Alveolar septal nodular lymphocytic infiltrate (hematoxylin & eosin, x40). B. Plasma cells (CD138 immunohistochemical staining, x200). C. Kappa predominance within plasma cell population in 2010 wedge biopsy (in-situ hybridization, x200). D. Lambda expression within plasma cell population in 2010 wedge biopsy (in-situ hybridization, x200).
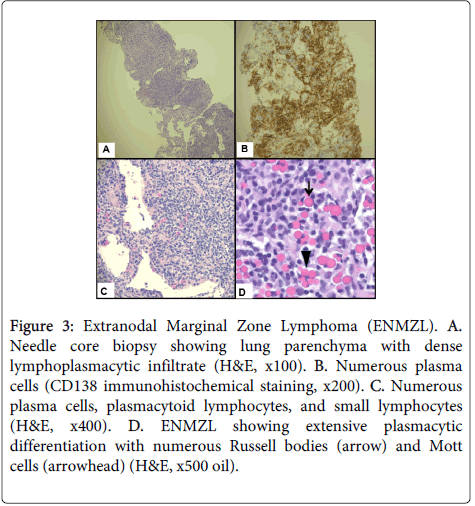
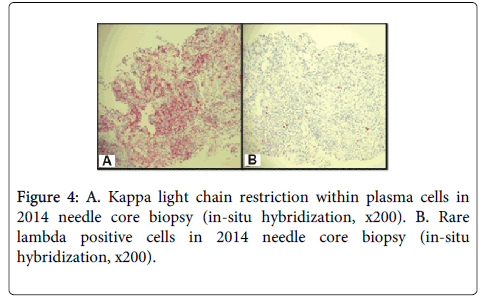
The CT imaging analysis associated with Figure 1B prompted the current core biopsy of the right upper lobe lung nodule, revealing lung parenchyma partially obscured by a dense, interstitial lymphoplasmacytic infiltrate (Figure 3A). Although the infiltrate was somewhat heterogeneous, much of it appeared plasmacytic and was highlighted with CD138 (Figure 3B). Numerous Russell bodies and Mott cells, as well as rare Dutcher bodies, were identified throughout the specimen (Figure 3C and Figure 3D). Of note, these features were absent in the earlier specimen from 2010. Also in contrast to the earlier specimen, immunoglobulin light chain analysis by in-situ hybridization appeared essentially restricted for kappa light chains (Figure 4A and Figure 4B). The kappa:lambda ratio was approximately 50:1 overall in this specimen, and the areas with numerous Russell bodies and rare Dutcher bodies showed the greatest degree of kappa skew. Small aggregates of B-cells were also associated with the plasma cell infiltrate, which lacked co-expression of CD5 and CD10. In conjunction with the clinical history, the overall morphologic and immunophenotypic features of this case were that of an atypical lymphoplasmacytic infiltrate consistent with extranodal marginal zone lymphoma of the lung. The overall features were low-grade with no increase in large cells and no significant mitotic activity.
Figure 3: Extranodal Marginal Zone Lymphoma (ENMZL). A. Needle core biopsy showing lung parenchyma with dense lymphoplasmacytic infiltrate (H&E, x100). B. Numerous plasma cells (CD138 immunohistochemical staining, x200). C. Numerous plasma cells, plasmacytoid lymphocytes, and small lymphocytes (H&E, x400). D. ENMZL showing extensive plasmacytic differentiation with numerous Russell bodies (arrow) and Mott cells (arrowhead) (H&E, x500 oil).
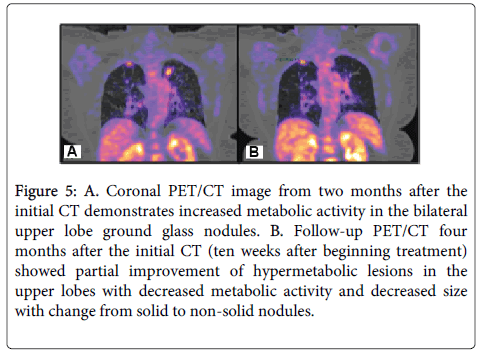
Figure 5: A. Coronal PET/CT image from two months after the initial CT demonstrates increased metabolic activity in the bilateral upper lobe ground glass nodules. B. Follow-up PET/CT four months after the initial CT (ten weeks after beginning treatment) showed partial improvement of hypermetabolic lesions in the upper lobes with decreased metabolic activity and decreased size with change from solid to non-solid nodules.
Two months after the CT scan in which malignancy was suspected, a Positive Emission Tomography-Computed Tomography scan (PET/CT) was performed for staging purposes (Figure 5A) and showed the expected findings of increased metabolic activity in the areas of lymphoma in both upper lung lobes. Due to the largely CD20- negative, plasmacytic nature of the neoplasm, rituximab therapy was not used and, instead, the patient was started on weekly bortezomib. Ten weeks after the first PET/CT scan, a second scan was performed to assess treatment response (Figure 5B). This scan showed less intense metabolic activity and decrease in size and density of the lesions. The patient’s oncologist also advocated for a tapering of her systemic immune suppression during this time. At the time of this manuscript’s submission, the patient has completed six months of chemotherapy with marked improvement of her lymphoma, achieving near complete resolution of the previously seen nodules within the upper lobes bilaterally.
Discussion
The diagnosis extranodal marginal zone lymphoma of pulmonary Mucosa-Associated Lymphoid Tissue (MALT) is difficult and, at times, may be controversial. Pulmonary MALT lymphoma must be differentiated from other malignancies, including a number of other low-grade B-cell lymphomas, as well as from a variety of benign lymphoproliferative disorders. Distinction from this latter group of conditions is especially problematic due to the morphologic heterogeneity encountered in this neoplasm and the lack of a specific immunohistochemical marker for marginal zone lymphoma. Despite these limitations in interpreting the pathologic features alone, correlation with the clinical, laboratory and CT findings frequently allows the diagnosis of extranodal marginal zone lymphoma to be made with confidence.
The lymphoma cells of extranodal marginal zone lymphoma are typically composed of marginal zone (centrocyte-like) cells that have abundant, pale cytoplasm with small nuclei containing irregular, moderately dispersed chromatin and inconspicuous nucleoli [1]. The cell morphology can vary, producing cells with monocytoid, small lymphocytic, or plasmacytic appearances [1]. Scattered immunoblasts and centroblast-like cells are also present [1]. These cells infiltrate the marginal zone of a follicle, located externally to the mantle zone and germinal center and eventually form confluent reactive follicles [3,4]. This mixture of cells tends to infiltrate the epithelia causing characteristic Lymphoepithelial Lesions (LEL). These lesions, which are characteristic of extranodal marginal zone lymphomas of MALT type, are defined as three or more marginal zone cells forming an aggregate that disrupts the epithelial architecture and often causes epithelial degeneration [1]. Dutcher bodies (intranuclear inclusions), Russell bodies (cytoplasmic vesicles containing immunoglobulin), and Mott cells (plasma cells that contain numerous Russell bodies) are also common findings in MALT lymphoma [5,6]. MALT lymphomas are usually composed of a heterogenous mixture of the cells; however, between one-fourth and one-third of MALT lymphomas display prominent plasmacytic differentiation [7,8] as was seen in this case. This variant is not associated with a difference in clinical presentation or prognosis [7], although MALT lymphomas arising outside the gastrointestinal tract are more likely to demonstrate this morphology [9]. Of note, patients treated with rituximab therapy may eventually show a shift in differentiation toward the plasmacytic variant due to selective proliferation of the CD20-negative cells [9,10].
As noted above, there are no definitive immunophenotypic markers for the diagnosis of marginal zone lymphoma in most cases [1]; however, such assays may be used to exclude other lymphomas. Flow cytometry and/or immunohistochemistry are useful in detecting not only light chain restriction, but also aberrant cell surface marker expression [5]. The cells of extranodal marginal zone lymphoma are typically CD20+, CD79a+, CD21+, and CD35+ with CD21 and CD35 acting as non-specific marginal-zone cell associated antigens [1]. Importantly, MALT lymphoma cells are also typically negative for CD5, CD10, and CD23, which may be useful in distinguishing it from other types of B-cell lymphoma. Of note, IRTA1 is a promising immunohistochemical marker which appears to be strongly associated with lymphomas of marginal zone differentiation and may aid in classifying B-cell malignancies [11]. Unfortunately, IRTA1 has not been shown to be useful in distinguishing reactive from neoplastic marginal zone proliferations [12,13].
The diagnosis of extranodal marginal lymphoma may be further complicated by the phenomenon of clonal expansions that are known to occur in chronic inflammatory and autoimmune conditions, but do not necessarily represent the presence of overt malignancy. Genetic analyses may also be performed to detect Immunoglobulin Heavy chain (IGH) gene rearrangements to provide relatively objective evidence for determining clonality and to further assist in establishing the diagnosis of lymphoma [14,15]. A rearrangement of the IGH gene in a clonal pattern would support malignancy in this setting. Furthermore, MALT lymphoma is frequently associated with the translocation t(11;18)(q21;q21) that produces the fusion gene API2- MALT1 in pulmonary and gastric sites in 40% and 30% of cases, respectively [1,16]. Other genetic aberrations including a number of translocations and trisomies may also be seen in these lymphomas with varying frequency depending on site of involvement. These abnormalities can be detected using fluorescence in-situ hybridization (FISH) and would be another means of confirming malignancy [1].
Extranodal marginal zone lymphoma is thought to arise in response to prolonged antigenic stimulation, which leads to a focused immune response and continual turnover of B-cells, facilitating the acquisition of mutations [17]. As such, chronic inflammatory disorders have been implicated in their pathogenesis [1]. Infectious agents known to cause chronic inflammatory states with such focused immune responses have been identified and are associated with the development of MALT lymphomas. For example, Helicobacter pylori infection is known to play a role in the development of gastric MALT lymphoma [18], Chlamydia psittaci in ocular adnexa MALT lymphoma [19], Campylobacter jejuni in Immunoproliferative Small Intestinal Disease (IPSID) [20], and Borrelia burgdorferi in cutaneous MALT lymphoma [21]. In addition to infectious stimuli, autoimmune diseases may cause the accumulation of mucosa-associated lymphoid tissue and chronic inflammation in general, serving as a substrate for lymphoma development [22]. For example, Sjögren syndrome is known to precede salivary gland MALT lymphoma, increasing the risk for its development by 44 times above the general population [23,24]. Sjögren syndrome has also been shown to stimulate the lung to form Bronchus-Associated Lymphoid tissue (BALT) [25], a specific type of MALT, resulting in a spectrum of lymphoproliferative conditions to occur in the lungs [26]. This appears to be a major driving force for the development of lymphoid neoplasms in patients with Sjögren syndrome, as MALT lymphomas represent approximately 85% of lymphomas in this population [1].
Radiologic imaging is critical in characterizing lymphoid lesions of the lung. Patients often undergo imaging studies as part of their initial evaluation, allowing correlation with the pathologic material to establish a diagnosis and, importantly, providing baseline data that can be used for comparison purposes as these typically indolent processes evolve. In this case, suspicion of malignancy was initially raised by the CT scan findings, which revealed the development of ground glass nodules with air bronchograms, not typical of LIP. Radiologic features that typically favor lymphoma in this setting include pleural effusions, consolidations and large nodular opacities or masses [27].
Preceding the diagnosis of lymphoma for several years, the patient showed lung pathology characteristic of Lymphocytic Interstitial Pneumonia (LIP). LIP falls in the category of non-neoplastic pulmonary lymphoid proliferations and is frequently associated with autoimmune phenomena and may be considered a precursor to lymphoma in a subset of cases. The group also includes follicular bronchiolitis and Nodular Lymphoid Hyperplasia (NLH). Follicular bronchiolitis, the least severe of these pulmonary lymphoid proliferations, presents with proliferating lymphoid nodules that are restricted to airways, blood vessels, and interlobular septa [28]. Nodular lymphoid hyperplasia is localized reactive lymphoid proliferations forming one or more nodules or pulmonary infiltrates [29]. LIP diffusely affects the lung [5] with prominent interstitial lymphoid infiltrates [30] that invade the alveolar septa and is composed of small lymphocytes and variable numbers of plasma cells [31]. The infiltrate is comprised of a mixture of T- and B-cells, the latter of which typically has polyclonal surface immunoglobulin light chain expression [29,32,33]. However, in some reported cases, minor clones of lymphoid cells were found with high homology to the hypervariable regions of antibodies produced by autoreactive lymphocytes [34]. The detection of these minor populations of monoclonal lymphoid cells, within the background of reactive LIP, support the concept that lymphoma may evolve from LIP, especially in association with Sjögren syndrome [35]. Findings from radiologic images of LIP are relatively nonspecific, including parenchymal consolidation and bilateral reticular, nodular, and ground glass opacities (Figure 1A) [36]. As was demonstrated in this case, however, the baseline radiologic findings were critical in terms of allowing meaningful comparison as the patient’s pulmonary disease progressed.
This case report demonstrates the difficulty in distinguishing LIP and extranodal marginal zone lymphoma due to the morphologic overlap that frequently exists between these entities [34]. Extranodal marginal zone lymphoma of the lung can present with interstitial and/or bronchiolocentric pattern and have reactive lymphoid follicles, both features typical of LIP [35]. Due to the similarity of these diseases on both clinical and pathologic levels, it has been proposed these types of lymphoproliferative lesions of the lung lie along a biological spectrum, ranging from benign/reactive conditions to atypical proliferations to lymphoma [29,35,37]. The patient in this case report had a history of Sjögren syndrome and was originally diagnosed with LIP. She then developed extranodal marginal zone lymphoma, representing a progression along this theoretical spectrum to its malignant endpoint, presumably due to a long-standing inflammatory process affecting the lungs.
Sjögren syndrome is an autoimmune disease that targets the body’s epithelial cells for destruction using TH1-type cytokine expression [38]. A normal, polyclonal immune response is initially produced, but the focused immune stimulus of the autoimmune disorder causes chronic replication and response of a narrowing group of cells that share increasingly higher levels of homology in their immunoglobulin gene rearrangements [34]. This group of cells replicates at a relatively faster rate, causing oligoclonal populations to emerge. The continued inflammatory responses surrounding LIP and Sjögren syndrome create a situation which allows a dominant clone to arise from this oligoclonal population, thereby producing a monoclonal lymphoid lesion that remains under immune regulation [35,39]. However, this chronic inflammatory state in the setting of a monoclonal response provides an opportunity for malignant transformation to occur as each replication is prone to the acquisition of genetic mutations. In the prototypical case, these mutations eventually affect the regulatory control of the cell, resulting in replication in the absence of an immune stimulus (i.e., autonomy) and malignant transformation of the monoclonal population [40].
The diagnosis of extranodal marginal zone lymphoma may remain problematic despite correlating all the clinicopathologic data, due to a lack of consensus regarding the feature or features that define an appropriate “cut-off” for malignancy. This controversy is well illustrated by the observation of spontaneous regression of a clonal lymphoproliferation following the removal of the inciting inflammatory stimulus [35]. This is seen most dramatically in gastric MALT lymphomas in which antibiotic treatment and eradication of H. pylori may cause the spontaneous remission of the gastric MALT lymphoma [35]. Furthermore, as previously discussed, atypical precursor lesions such as LIP of the lung may demonstrate monoclonality, but, in conjunction with other available clinicopathologic features, do not meet criteria for the diagnosis of lymphoma. These situations suggest that while monoclonality is typically used to diagnose malignancy for most lymphomas, it may not be a sufficient or an appropriate criterion alone to diagnose extranodal marginal lymphoma.
Conclusion
In conclusion, extranodal marginal zone lymphomas of mucosa associated lymphoid tissue likely represent the malignant transformation of a chronic inflammatory process. While not observed in all cases, an evolution from a reactive to an autonomous lymphoid lesion occurs, often with the acquisition of genetic abnormalities that allow proliferation in the absence of an immune stimulus. In our patient, Sjögren syndrome was the likely underlying condition that resulted in an accumulation of bronchus-associated lymphoid tissue and eventually caused a diffuse lymphocytic interstitial pneumonia that prompted her to seek medical assistance. The four-year follow-up data demonstrated the natural progression of this atypical lymphoid lesion to lymphoma. This case further illustrates the necessity of correlating the clinical, radiologic and pathologic findings to establish the diagnosis of extranodal marginal zone lymphoma and to allow the appropriate management of this patient.
Acknowledgements
The authors would like to thank James E Coad, MD for manuscript guidance.
References
- Isaacson PG, Chott A, Nakamura S, Muller-Hermelink HK, Harris NL, et al. (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. (4th Edn), IARC Press, Lyon, France.
- Papiris SA, Kalomenidis I, Malagari K, Kapotsis GE, Harhalakis N, et al. (2007) Extranodal marginal zone B-cell lymphoma of the lung in Sjögren's syndrome patients: reappraisal of clinical, radiological, and pathology findings. Respir Med 101: 84-92.
- Isaacson PG, Spencer J (1987) Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology 11: 445-462.
- Isaacson PG, Wotherspoon AC, Diss T, Pan LX (1991) Follicular colonization in B-cell lymphoma of mucosa-associated lymphoid tissue. Am J SurgPathol 15: 819-828.
- Yi E, Aubry MC (2010) Pulmonary pseudoneoplasms. Arch Pathol Lab Med 134: 417-426.
- Troch M, Kiesewetter B, Dolak W, Jaeger U, Püspök A, et al. (2012) Plasmacytic differentiation in MALT lymphomas following treatment with rituximab. Ann Hematol 91: 723-728.
- Molina T, Lin P, Swerdlow SH , Cook JR (2011) Marginal zone lymphomas with plasmacytic differentiation and related disorders. Am J ClinPathol 136: 211-211.
- Wöhrer S, Troch M, Streubel B, Hoffmann M, Müllauer L, et al. (2007) Pathology and clinical course of MALT lymphoma with plasmacytic differentiation. Ann Oncol 18: 2020-2024.
- Woehrer S, Streubel B, Chott A, Hoffmann M, Raderer M (2005) Transformation of MALT lymphoma to pure plasma cell histology following treatment with the anti-CD20 antibody rituximab. Leuk Lymphoma 46: 1645-1649.
- Falini B, Agostinelli C, Bigerna B, Pucciarini A, Pacini R, et al. (2012) IRTA1 is selectively expressed in nodal and extranodal marginal zone lymphomas. Histopathology 61: 930-941.
- Attygalle AD, Liu H , Shirali S (2004) Atypical marginal zone hyperplasia of mucosa-associated lymphoid tissue: a reactive condition of childhood showing immunoglobulin lambda light-chain restriction. Blood 104: 3343-3343.
- Kojima M, Morita Y, Shimizu K, Yoshida T, Yamada I, et al. (2007) Immunohistological findings of suppurative granulomas of Yersinia enterocolitica appendicitis: a report of two cases. Pathol Res Pract 203: 115-119.
- Nicholson AG, Wotherspoon AC, Diss TC, Hansell DM, Du Bois R, et al. (1995) Reactive pulmonary lymphoid disorders. Histopathology 26: 405-412.
- Elenitoba-Johnson K, Medeiros LJ, Khorsand J, King TC (1995) Lymphoma of the mucosa-associated lymphoid tissue of the lung. A multifocal case of common clonal origin. Am J ClinPathol 103: 341-345.
- Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, et al. (1999) The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 93: 3601-3601.
- Frizzera G (1994) Immunosuppression, autoimmunity, and lymphoproliferative disorders. Hum Pathol 25: 627-629.
- Hussell T, Isaacson PG, Crabtree JE , Spencer J (1993) The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 342: 571-571.
- Chanudet E, Zhou Y, Bacon CM, Wotherspoon AC, Müller-Hermelink HK, et al. (2006) Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol 209: 344-351.
- Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, et al. (2004) Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 350: 239-248.
- Cerroni L, Zöchling N, Pütz B, Kerl H (1997) Infection by Borreliaburgdorferi and cutaneous B-cell lymphoma. J CutanPathol 24: 457-461.
- Isaacson PG (1994) Gastrointestinal lymphoma. Hum Pathol 25: 1020-1029.
- Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, et al. (1978) Increased risk of lymphoma in sicca syndrome. Ann Intern Med 89: 888-892.
- Talal N, Sokoloff L, Barth WF (1967) Extrasalivary lymphoid abnormalities in Sjögren's syndrome (reticulum cell sarcoma, "pseudolymphoma," macroglobulinemia). Am J Med 43: 50-65.
- Pabst R (1992) Is BALT a major component of the human lung immune system? Immunol Today 13: 119-122.
- Ioannidis JP, Vassiliou VA, Moutsopoulos HM (2002) Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren's syndrome. Arthritis Rheum 46: 741-747.
- Johkoh T, Müller NL, Pickford HA, Hartman TE, Ichikado K, et al. (1999) Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 212: 567-572.
- Joshi VV, Kauffman S, Oleske JM, Fikrig S, Denny T, et al. (1987) Polyclonal polymorphic B-cell lymphoproliferative disorder with prominent pulmonary involvement in children with acquired immune deficiency syndrome. Cancer 59: 1455-1455.
- Kradin RL, Mark EJ (1983) Benign lymphoid disorders of the lung, with a theory regarding their development. Hum Pathol 14: 857-867.
- Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E (1999) Histological Typing of Lung and Pleural Tumours.
- Liebow AA, Carrington CB (1973) Diffuse pulmonary lymphoreticular infiltrations associated with dysproteinemia. Med Clin North Am 57: 809-843.
- Koss MN, Hochholzer L, Langloss JM, Wehunt WD , Lazarus AA (1987) Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 cases. Pathology 19: 178-178.
- Banerjee D, Ahmad D (1982) Malignant lymphoma complicating lymphocytic interstitial pneumonia: A monoclonal B-cell neoplasm arising in a polyclonal lymphoproliferative disorder. Hum Pathol 13: 780-780.
- Kurosu K, Yumoto N, Furukawa M, Kuriyama T , Mikata A (1997) Third complementarity-determining-region sequence analysis of lymphocytic interstitial pneumonia: most cases demonstrate a minor monoclonal population hidden among normal lymphocyte clones. Am J RespirCrit Care Med 155: 1453-1453.
- Koss MN (2004) Malignant and benign lymphoid lesions of the lung. Ann DiagnPathol 8: 167-187.
- Fraser RS, Muller NL, Colman N, Pare PD (1999) Fraser and Pare's Diagnosis of Diseases of the Chest. (4th Edn), Saunders, Philadelphia, PA.
- Nicholson AG (2000) Pulmonary lymphoproliferative disorders. Current Diagnostic Pathology 6: 130-130.
Citation: Rubenstein NJ, Beatty C, Kinkade Z, Bryan C, Paul Hogg J, et al. (2015) Extranodal Marginal Zone Lymphoma of the Lung: Evolution from an Underlying Reactive Lymphoproliferative Disorder. J Clin Exp Pathol 5:208. DOI: 10.4172/2161-0681.1000208
Copyright: © 2015 Rubenstein JN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16394
- [From(publication date): 2-2015 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 11777
- PDF downloads: 4617