Research Article Open Access
Extrafine Beclometasone Dipropionate and Formoterol in Single and Separate Inhalers
| Piccinno A1*, Poli G1, Monno R2, Goethals F3, Nollevaux F3 and Acerbi D1 | |
| 1Clinical Pharmacology Unit, Corporate Clinical Development, Chiesi Farmaceutici S.p.A., Parma Italy | |
| 2Scientific Affairs Department, Chiesi Farmaceutici S.p.A., Parma Italy | |
| 3SGS Life Science Services, SGS Belgium S.A., Belgium | |
| Corresponding Author : | Piccinno A Clinical Pharmacology Unit Corporate Clinical Development Chiesi Farmaceutici S.p.A., Parma Italy E-mail: a.piccinno@chiesi.com |
| Received May 25, 2012; Accepted August 09, 2012; Published August 16, 2012 | |
| Citation: Piccinno A, Poli G, Monno R, Goethals F, Nollevaux F, et al. (2012) Extrafine Beclometasone Dipropionate and Formoterol in Single and Separate Inhalers. Clinic Pharmacol Biopharm. 1:102. doi:10.4172/2167-065X.1000102 | |
| Copyright: © 2012 Piccinno A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
The aim of this study was to evaluate the pharmacokinetics and pharmacodynamics of single administration of extrafine beclometasone (BDP)/formoterol hydrofluoroalkane (HFA) (Foster®, Chiesi Farmaceutici S.p.A., Italy) by pressurized metered dose inhaler (pMDI) in healthy volunteers, compared to the free combination of extrafine BDP pMDI (QVAR®, 3 M) and extrafine formoterol pMDI (Atimos®, Chiesi Farmaceutici S.p.A.) delivered via separate inhalers.
Twelve healthy male volunteers completed this open label, randomized, single-dose, placebo-controlled, threeway cross-over study. Volunteers received (i) 4 inhalations of the fixed-combination of BDP/formoterol HFA (100/6 μg), (ii) 4 inhalations of BDP HFA (100 μg) and 2 inhalations of formoterol HFA (12 μg) administered separately or (iii) 4 inhalations of placebo HFA. There was a 7-day washout between treatment periods.
PK parameters of BDP, FF, and of the main BDP metabolite (B17MP) were calculated.
The systemic exposure to B17MP and formoterol after administration as a fixed combination or via separate inhalers was comparable. For B17MP, Cmax (794 ± 316 vs 663 ± 329 pg/mL), AUC0-t (3076 ± 1102 vs 2773 ± 833 pg.h/mL) and AUC0-30min (296 ± 11.7 vs 230 ± 13.3 pg.h/mL) were slightly higher, but not statistically significant, with the fixed than with the free combination.
For formoterol, neither Cmax (27.6 ± 12.2 vs 28.7 ± 16.3 pg/mL) nor AUC0-t (69.3 ± 28.5 vs 67.4 ± 28.7 pg.h/mL) were significantly different between fixed and free combination. There was also no significant difference between the two combinations in serum cortisol levels, or cortisol urinary excretion adjusted for creatinine.
Use of a single inhaler rather than separate inhalers to deliver extrafine drug particles of both BDP and formoterol to the lung does not modify relevant pharmacokinetics and pharmacodynamic parameters in healthy volunteers, therefore it may be considered as a therapeutic option to improve compliance in asthma patients who need chronic treatment.
| Keywords |
| Beclometasone dipropionate; Formoterol; Pharmacokinetics; Pharmacodynamics |
| Introduction |
| The addition of a long-acting β2-agonist (LABA) to a low-tomoderate dose of inhaled corticosteroid (ICS) is recommended for those asthmatics not adequately controlled on ICS alone [1]. This simplified approach to therapy has a strong scientific rationale. Clinically, the complementary effects of LABAs and ICSs in asthma are well established [2-6], as they target different pathophysiological aspects of the disease, the former inducing bronchodilation, and the latter suppressing the chronic inflammatory component and reducing airway hyper-responsiveness [7]. At the molecular level corticosteroids increase the expression of β2-receptors [8], and β2-agonists may potentiate the molecular mechanism of corticosteroid actions by priming inactive glucocorticoid receptors, and via additive (and sometimes synergistic) suppression of inflammatory mediator release [7]. |
| Inhalers delivering fixed combinations of ICSs and LABAs are now available. A hydrofluoroalkane (HFA)-propelled extra-fine fixed combination of the ICS beclometasone dipropionate (BDP) and the LABA formoterol (100/6 μg) delivered by pressurized metered dose inhaler (pMDI) (Foster®, Chiesi Farmaceutici S.p.A., Italy) has recently been developed using Modulite® technology with a Marketing Authorization in more than 28 countries (EU countries) for the treatment of asthma in patients older than 18 years of age when use of a combination of ICS and LABA is appropriate. The rationale behind the development of an extrafine LABA/ICS formulation was due to the accumulating evidence that pathophysiological processes in asthma and chronic obstructive pulmonary disease (COPD) take place in all parts of the airways, including peripheral bronchioles [9-11], and that devices which generate smaller particles produce a more peripheral lung deposition of drug [12]. In addition, both corticosteroid- and β2-adrenergic receptors are distributed along the entire bronchial tree [13,14]. |
| Several clinical studies have demonstrated that adult and elderly patients require an extrafine BDP dose approximately 2.5 times lower than that of BDP chlorofluorocarbon (CFC) to reach the same degree of asthma control [15,16]. BDP extrafine 100 μg has been shown to be as effective as BDP CFC 250 μg in patients with asthma [17,18]. This reduction in nominal dose led to a reduction in systemic exposure compared to concomitant administration of BDP CFC and extrafine formoterol [19]. In fact, with a BDP dose from Foster® that was 2.5 times less than a BDP dose from Becotide® Forte, pulmonary absorption was 86% higher, despite the fact that systemic exposure was 35% lower, resulting in less cortisol suppression for a clinically equivalent BDP dosage [19]. However, a pharmacokinetic (PK)/pharmacodynamic (PD) comparison of Foster® versus both extrafine components (in particular BDP extrafine) administered via separate inhalers has never been performed. The present study investigates for the first time the PK and PD properties of BDP/formoterol HFA fixed combination after single administration compared to BDP HFA pMDI (QVAR® 3 M) and formoterol HFA pMDI (Atimos®, Chiesi Farmaceutici S.p.A., Italy) delivered via separate inhalers in healthy volunteers. |
| Materials and Methods |
| In vitro study design |
| To support the in vivo PK and PD comparisons of BDP/formoterol HFA versus both extrafine components administered separately, the lung deposition of both components (BDP and formoterol) of the fixed combination (100/6 μg) was assessed In vitro [20]. The In vitro deposition of BDP HFA from Foster® was also compared with that of BDP HFA from QVAR® [21]. The mass median aerodynamic diameter (MMAD), geometrical standard deviation (GSD) and fine particle dose (FPD) of BDP were determined using an Andersen Cascade Impactor (ACI; Copley Instruments, Nottingham, UK) operated at 28.3 L/min, with ten doses per determination. FPD was defined as the fraction of particles <4.7 mm. The drug deposition profile was assessed with a fully validated high-performance liquid chromatography-UV method. |
| In vivo study design |
| This was an open label, randomized, single-dose, placebocontrolled, three-way, cross-over study, consisting of one single administration of (i) 4 inhalations of the fixed combination BDP/ formoterol HFA (100/6 μg), (ii) 4 inhalations of BDP HFA (100 μg) and 2 inhalations of formoterol HFA (12 μg) administered separately or (iii) 4 inhalations of placebo HFA. There was a 7-day washout between treatment periods. PK and PD parameters - described later in the Methods section- were assessed for BDP, its main metabolite beclometasone-17-monopropionate (B17MP) and formoterol after active treatments. Safety was assessed by documenting all adverse events which occurred during the study. The study was carried out in accordance with the Declaration of Helsinki, the ICH Harmonized Tripartite Guideline for Good Clinical Practice and with applicable regulatory requirements. The study protocol was approved by an Independent Ethics Committee. |
| Subject selection |
| 12 male healthy volunteers, aged 18-55 with a BMI of 18-28 kg/ m2 were invited to participate to the study. Healthy volunteers, when comparing orally inhaled products represent a more simple and controlled system than the patient population, decreasing the level of complexity [22]. Volunteers were required to be non-smokers or exsmokers (less than 5 pack-years and stopped smoking for more than 1 year), have normal blood pressure and heart rate (supine), normal laboratory tests, have a 12-lead ECG with computerized protocol interpretation considered as normal or without any clinically relevant abnormalities and have the ability to correctly use a pMDI. All subjects gave their written informed consent to participate to the study. |
| Subjects were excluded from the study if they had a history or presence of cardiovascular, respiratory, endocrinological, renal, gastrointestinal or neurological disorders capable of altering the absorption, metabolism, distribution or elimination of drugs. Volunteers were also excluded if they had hypersensitivity to the active principles and/or formulations ingredients (or other corticosteroids), or had a history of anaphylaxis to drugs or allergic reactions, if they had recent (<2 months) relevant infectious disease, positive HIV1, HIV2 or hepatitis serology results or lung function measurements outside normal limits. Use of drugs (except paracetamol) in the 14 days before study drug administration, of enzyme-inducing drugs in the previous 2 months, or a history of excessive use of drug, alcohol (>28 units/week) or xanthine-containing substance (>5 cups coffee/tea/cola per day) were also considered as exclusion criteria. |
| Test drug inhalation |
| At screening and at each administration day, volunteers were trained in the correct inhalation technique inhaling from placebo inhalers according to the product leaflets and also using a Vitalograph® aerosol inhalation monitor (AIM) with placebo CFC-free inhalers. The AIM allowed the correct inhalation technique to be evaluated and reinforced by monitoring correct timing of the actuation of the pMDI as well as correct inhalation time, inspiratory flow and breath-holding. Volunteers were trained using placebo inhalers at a required peak inhaled flow rate of 30 L/min. After 3 successful inhalations with the AIM device, volunteers used the same technique and inspiratory flow rate for the test drug inhalation. |
| During each treatment period, the inhalers were removed of the protective cap, vigorously shaken and then primed by actuating once. This process was repeated 4 times before inhalation of drug. Device shaking was requested before each subsequent actuation. Doses were administered under fasting conditions, with time 0 defined as the moment when the first inhalation was administered. Subjects inhaled the medication in an upright position and remained upright (either sitting or standing) for at least 5 minutes post-dose. Additionally, subjects were instructed to breath-hold for 10 seconds following each inhalation, and to wait 30 seconds before taking the next inhalation. |
| Pharmacokinetic measurements |
| Patients were required to fast from the evening (22:00 h) before day 1 of each study period, and remained fasting until 2 h post-dose. Blood samples were taken from patients, who had received active treatments only, by venipuncture at the following timepoints: pre-dose (within 10 min before inhalation), 10, 20, 30, 45 min, 1, 2, 3, 4, 6, 8 12 and 24 h post-dose. Plasma was separated (refrigerated centrifuge @ +4°C at approx. 2500 g for 15 mins) and 1ml aliquots were immediately stored at -80°C for formoterol assay or at -20°C for BDP/B17MP assay. Plasma samples were analyzed for the determination of formoterol, BDP and B17MP using validated LC-MS-MS methods (SGS Life Science Services, Belgium) with limit of quantitation (LOQ) of 2 pg/mL for formoterol and 20 pg/mL for BDP and B17MP. The following PK parameters were calculated from the individual plasma drug concentration versus time profiles: maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), area under the plasma concentration versus time curve observed from 0 to 30 min (AUC0-30min) (as an index of lung absorption), from 0 to the last measured concentration time t (AUC0-t) and from 0 to infinity (AUC0–∞), calculated using the linear trapezoidal rule. The elimination half life (t½) was calculated as 0.693/ λz. The PK calculations were performed using WinNonlin version 4.0 (Pharsight Corp., CA, USA). |
| The allowed time deviations for the pharmacokinetic sampling times were: for measurements before 30 minutes post-dose: +/- 1 minute; 1 hour post-dose: +/- 2 minutes, from 2 to 4 hours post-dose: +/- 5 minutes, from 6 to 12 hours post-dose: +/- 10 minutes and 24 hours post-dose or greater: +/- 15 minutes. Priority, for the different measures, was given to PK samples. |
| Pharmacodynamic measurements |
| Determination of potassium in serum: Blood samples were collected for potassium determination in serum at the following timepoints for both active and placebo treatments: pre-dose, 15, 30 min, and 1, 2, 4, 6, 8, 12, and 24 h post-dose. Blood samples were analyzed by potentiometry (Ektachem) at the Laboratory Algemeen Ziekenhuis Stuivenberg (Antwerp, Belgium). The following PK parameters were derived from the individual potassium concentration time profiles: minimum serum concentration (Cmin), time to minimum serum concentration (tmin) and area under the concentration curve observed from 0 to 24 h (AUC0-24h). |
| Determination of cortisol in serum: Blood samples were collected pre-dose and at 2, 4, 6, 8, 12, 16, 20 and 24 h post-dose for both active and placebo treatments. After coagulation (in the fridge), serum was separated (refrigerated centrifuge (+4°C) at approx. 2500 g for 15 mins) and stored at -80°C. Cortisol in serum was determined at SGS Life Science Services Applied Biology Laboratory (Wavre, Belgium) using a standard radio-immuno assay kit (ZENTECH). The following PK parameters were derived from the individual cortisol concentration profiles: Cmin, tmin and AUC0-24h. |
| Determination of cortisol and creatinine in urine: Urine samples were collected for 24 h, from 5 min pre-dose until 24 h post-dose for each study period and stored at -20°C. Urinary cortisol was determined using a standard radio-immuno assay kit (ZENTECH) at the SGS Life Science Services Applied Biology Laboratory (Wavre, Belgium). Urinary creatinine was assayed at the Laboratory Algemeen Ziekenhuis Stuivenberg (Antwerp, Belgium). 24 h urinary excretion of cortisol (Ae) and 24 h urinary excretion of cortisol normalized for creatinine excretion (Ae/Aecrea) were derived from the individual cortisol and creatinine concentration profiles. |
| Cardiovascular parameters and peak flow inspiratory flow: Systolic and diastolic blood pressure and heart rate were evaluated (WelchAllyn device) after 5 min in the sitting position pre-dose, 15, 30 min and 1, 2, 4, 6, 8, 12 and 24 h post dose. A 12-lead ECG (CardioMax FX40-10, Fukuda, Denshi) was performed in the supine position at the same time points. Heart rate, pulse rate, QRS and QTc were measured. QTc individual changes relative to baseline measurements were calculated in order to verify the occurrence of changes >30 ms and >60 ms. |
| Peak expiratory flow (PEF) was measured using a Jaeger-Masterlab for both active and placebo treatments during the screening visit, predose (after at least 10 minutes rest, with subjects sitting with the nose clipped) and at 1, 6 and 12 h post-dose. Three technically satisfactory measurements were done for each subject at each time point and the highest value recorded. |
| Data analysis: All statistical calculations were performed using SAS® (version 8.2). Log-transformed values for the continuous PK variables of BDP, B17MP and formoterol (Cmax, AUC0-30min, AUC0-t, AUC0-∞ and t½) and continuous PD variables (cortisol: Cmin, AUC0- 24h, Ae and Ae/Aecrea; potassium: Cmin and AUC0-24h) were analyzed using an ANOVA model adapted to cross-over experimental design with ‘treatment’, ‘period’, ‘sequence’ and ‘subject nested to sequence’ as variables. Tmax (BDP, B17MP and formoterol) was analyzed using a Wilcoxon signed rank test and associated 90% confidence intervals calculated. Values of tmin (cortisol, potassium) were compared using a Friedman’s non parametric ANOVA [23]. A 95% non-parametric interval for each test-reference difference of interest was computed according to the method of Moses [24]. For cardiovascular (QTc, HR, BP) and peak flow measurements, the mean value was calculated as the AUC versus time over time interval (AUC0-12h/12). Safety and tolerability were evaluated using descriptive statistics. |
| In a previous similar study [19], the largest intra-individual coefficient of variation for B17MP and formoterol PK parameters was 29.6% for B17MP Cmax. Given such an intra-individual variability, in the present study it was estimated that 12 subjects were required to reach an 80% power to detect a 1.5 fold variation in B17MP or formoterol systemic exposure at the 0.05 α-level. This 1.5 fold variation corresponds to the confidence limits of 66-150% and was judged able to detect clinically relevant differences between treatments. |
| Results |
| In vitro results |
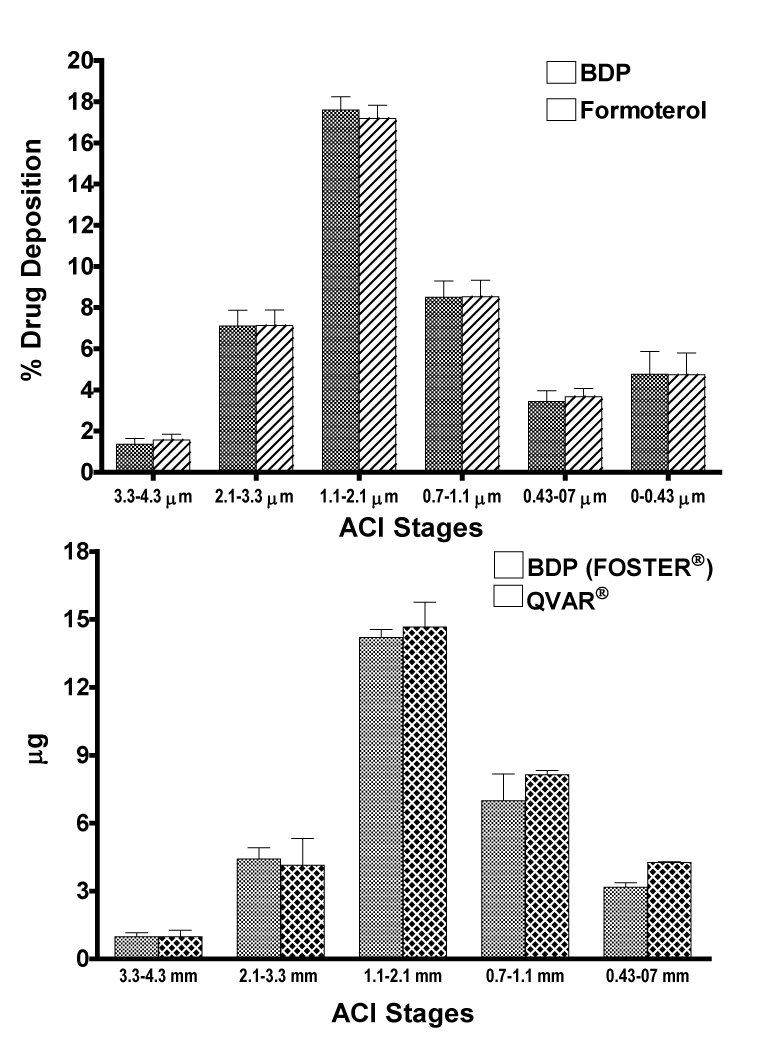
| The In vitro deposition profiles of the two components of Foster® showed that BDP and formoterol had a similar particle size distribution with comparable FPD and MMAD (Figure 1A), suggesting a deposition of both active drugs to the same regions within the lung, including the peripheral airways. The comparison of the In vitro deposition of extrafine BDP from Foster® or QVAR® also revealed comparable deposition profiles in the therapeutically useful range 1.1-4.7 μm (Figure 1B). The FPDs were 36.1 ± 2.3 μg and 43.3 ± 3.3 μg for BDP from Foster® and QVAR® respectively, with almost identical MMADs of 1.3 ± 0.1 μm and 1.1 ± 0.1 μm respectively. These data have been presented previously [20,21]. |
| In vivo results |
| Volunteers: Twenty one subjects were screened and 12 subjects were randomized. All 12 enrolled subjects completed the study as planned. Subjects’ mean age ± standard deviation was 40.7 ± 7.5 years (range 23-49 yrs). Baseline data are presented in Table 1. |
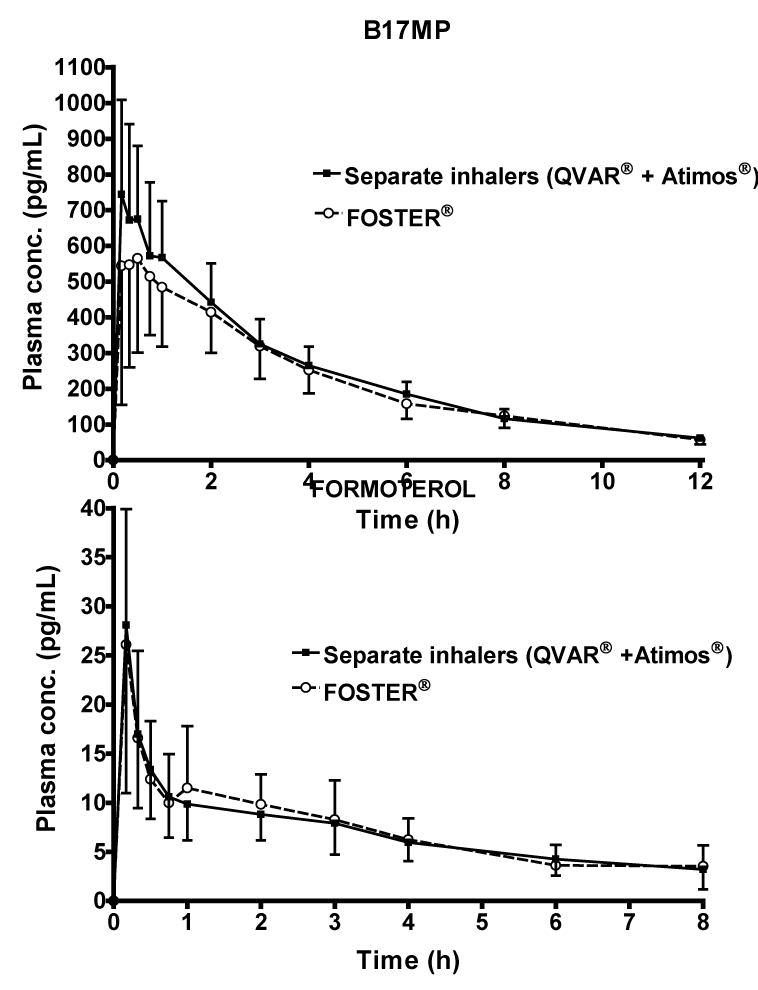
| Pharmacokinetics: Maximum plasma concentrations of BDP were significantly lower with the fixed combination (390 ± 270 pg/mL) compared with the separate inhalers (781 ± 444 pg/mL) the. However, in most subjects BDP plasma concentrations were quantifiable only up to 45 minutes after administration, due to the fast lung conversion to its metabolite B17MP. For B17MP, both Cmax (633 ± 329 vs 794 ± 316 pg/mL; p=0.174) and AUC0-t (2773 ± 833 vs 3076 ± 1102 pg.h/ mL; p=0.274) were slightly lower after administration of BDP and formoterol as fixed combination (Table 2, Figure 2A). However, this difference was not statistically significant, and the 90% confidence intervals were entirely within the usual 80-125% bioequivalence limits for AUC0-t (90% CIs: 81.64; 104.61), and only partially outside for Cmax (90% CIs: 66.83; 104.54) (Table 2). The median time to peak B17MP plasma concentration was 30 min and 20 min for the fixed and free combinations respectively (Table 2, Figure 2A). The AUC0-30min of B17MP was also partially outside the confidence intervals (90% CI: 54.03; 102.99) between the fixed combination (230 ± 13.3 pg.h/mL) and separate inhalers (296 ± 11.7 pg.h/mL). The elimination half-life of B17MP was approximately 4 h in both active groups (Table 2). |
| Formoterol systemic exposure was comparable after the two treatments (Table 2, Figure 2B). Neither Cmax (27.6 ± 12.2 vs 28.7 ± 16.3 pg/mL) nor AUC0-t (69.3 ± 28.5 vs 67.4 ± 28.7 pg.h/mL) was significantly different after administration of BDP/formoterol as a fixed or free combination. The median time to peak formoterol plasma concentration was 10 minutes with both active treatments (Table 2). |
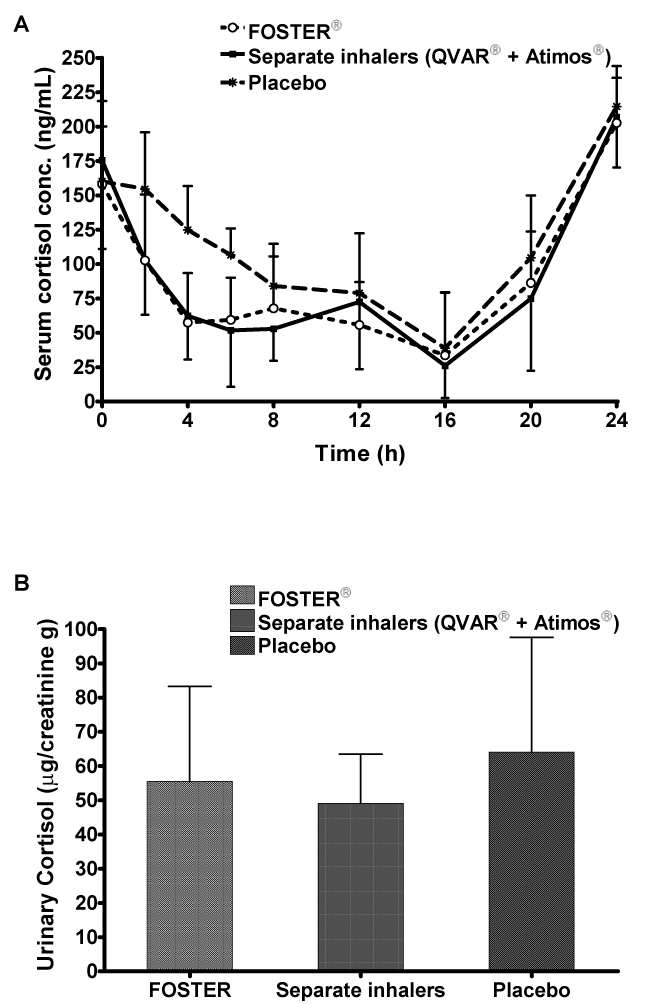
| Pharmacodynamics: Mean cortisol serum concentrations were lower after administration of both active treatments compared to placebo as expected, (Table 3, Figure 3A). The fixed combination decreased minimum serum cortisol concentration by 41% and the free combination by 42% compared with placebo, but these reductions were not statistically significant (Table 3). Differences between active and placebo treatments were significant for serum cortisol AUC0- 24h values. The fixed combination significantly (95% CI 61.88; 88.20) decreased serum cortisol AUC0-24h by 26% and the free combination by 30% (p=0.001; 95% CI 58.72; 83.69) compared to placebo (Table 3). Differences in Cmin (22.9 ± 16.8 vs 22.8 ± 18.3 ng/mL) and AUC0- 24h (1.91 ± 0.56 vs 1.86 ± 0.65 μg.h/mL) between fixed dose and free combination were not significant. All treatments showed a median tmin of 16.00 h, suggesting that treatments did not alter the normal circadian variation in cortisol secretion. |
| The urinary creatinine-normalized excretion of cortisol decreased by 16% with the fixed combination (95% CI 67.10; 105.74) and by 21% with the free combination (95% CI 63.08; 99.40) compared to placebo (Table 3, Figure 3B). There was no significant difference (95% CI 84.74; 133.53) between BDP/formoterol administered as a fixed combination (55.5 ± 27.8 μg/g) or as two separate inhalers (49.1 ± 14.4 μg/g). These findings are in agreement with the similar systemic exposure to B17MP observed in both the fixed and free combination groups. |
| The minimum serum potassium concentration was lower in the fixed combination group (3.6 ± 0.2 mmol/L) compared to either the free combination group (3.8 ± 0.2 mmol/L) or placebo (3.8 ± 0.1 mmol/L) (Table 3). The time to achieve the minimum serum potassium concentration was 4 h in each group. The plasma potassium AUC0-24h did not significantly (95% CI 96.36; 100.79 for fixed- vs freecombination) differ between active treatments, but it was significantly (95% CI 94.86; 99.22) reduced in the fixed combination group (94.5 ± 4.0 mmol.h/L) compared with placebo (97.4 ± 4.4 mmol.h/L) (Table 3). |
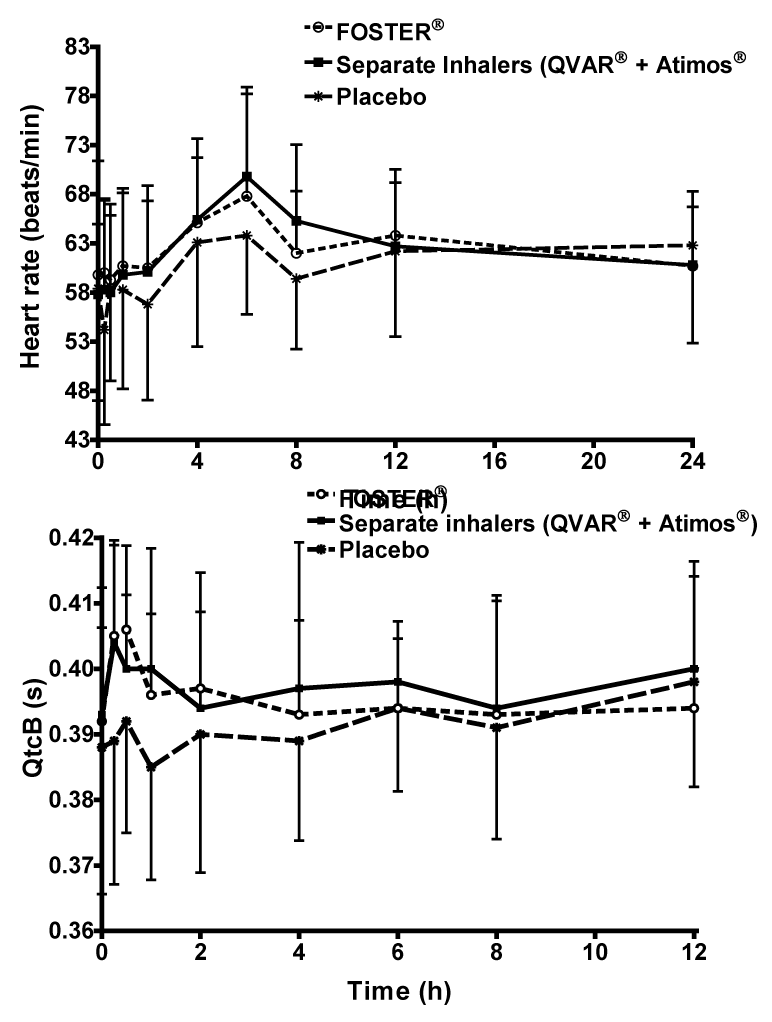
| Over 12h recording period of time, the administration of the fixed combination increased HR by 4.5% compared to placebo (95% CI 100.53; 108.74), while the increase in HR with the free combination was approximately 6.2% (95% CI 102.13; 110.48) (Figure 4A). The difference between the fixed (63.4 ± 7.5 bpm) and free combinations (64.2 ± 6.0 bpm) was not statistically significant (95% CI 94.64; 102.37). No changes were observed for systolic and diastolic blood pressure. |
| Both active treatments had similar QTcB interval profiles with an increase from 392 ms to 406 ms 30 min after administration of BDP/ formoterol fixed combination and from 393 ms to 404 ms 15 min after administration of the free combination , followed by a decrease to values close to baseline values 24 h post-dose (Figure 4B). The increase in QTcB with the active treatments was associated with a significant increase in heart rate, supporting the concept that the QTcB method of correction is not fully adequate to control for the effect on HR. Placebo administration resulted in a decrease of QTcB 1 h post-dose (from 388 to 385 ms), followed by an increase to 398 ms 12 h post-dose and a return to baseline value (388 ms) (Figure 4B). The QTc intervals AUC0- 12h/12 values for the fixed, free and placebo groups were 393 ± 13.4, 397 ± 13.1 and 392 ± 14.6 ms respectively, with no significant difference between groups. No subject had a QTcB increase versus baseline greater than 60 ms. |
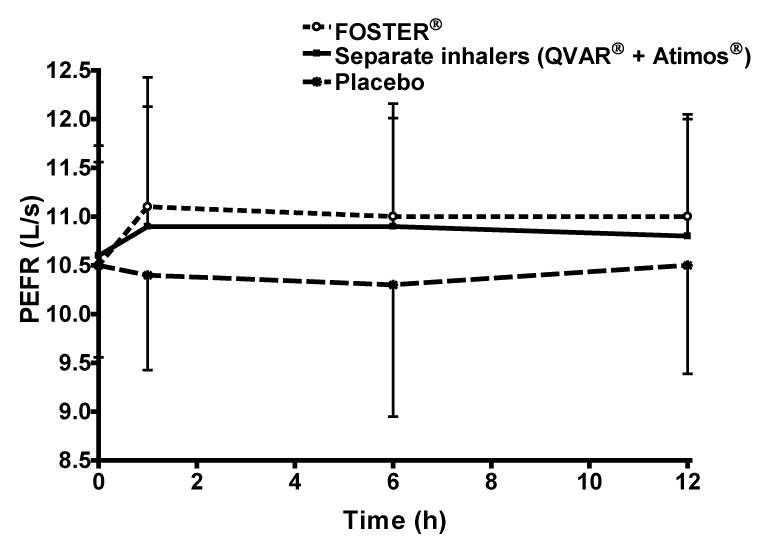
| Both active treatments produced similar PEF profiles, which peaked 1 h post-dose (666 L/min fixed combination; 654 L/min free combination) significantly greater compared with placebo (624 L/min placebo) (Figure 5). |
| Safety and tolerability: A total of 4 adverse events (AEs), all mild in intensity and none of these related to study drugs, were reported by four subjects. No AEs occurred after administration of the fixed combination, 1 AE (pharyngolaryngeal pain) occurred in one subject after administration of formoterol HFA in the free combination group and 3 AEs (1 arthralgia, 1 cough and 1dizziness) occurred in 3 subjects after placebo treatment. All subjects had recovered from their AEs at the end of the study. No serious adverse events were reported. |
| Discussion |
| The addition of a long-acting inhaled β2-adrenergic receptor agonist (LABA) to the recommended first-line ICS therapy is preferred in patients whose asthma is not controlled with either low or high doses of an ICS and provides better control of asthma symptoms and lung function than increasing the dose of the ICS [2-6]. In this context, fixed combinations of ICS and LABA have been developed (Symbicort®, Seretide®). With a new fixed combination, it is necessary to investigate possible interactions between the combination components and if the systemic exposure to the single components given as a fixed combination is comparable to the systemic exposure of the components given separately. |
| In the present study the PK and PD properties of extrafine BDP/ formoterol administered as pMDI fixed combination versus the individual extrafine components administered separately already available on the market (QVAR® and Atimos®) are compared. |
| QVAR® (BDP HFA extrafine 100 μg) was the first extrafine corticosteroid formulation available on the market. Using HFA as a propellant, drug solutions can be manipulated to vary particle size distribution, permitting precise control of delivered dose and optimization of drug delivery. Particle size is a major determinant of the proportion of drug that reaches the lung, with small drug particle size being associated with increased and more peripheral lung deposition [25,26,12]. |
| The benefits of HFA drug solutions in producing extrafine drug particles capable of homogeneously distributing throughout the bronchial tree, whilst keeping the systemic exposure low may result in some clinical advantages over non extrafine formulations. This has been observed for the extrafine BDP/formoterol fixed combination, where treatment of patients with moderate-to-severe asthma was associated with significantly higher percentages of controlled days and nights, and a better asthma control score versus separate administration of BDP CFC plus formoterol dry powder inhaler or BDP alone [27]. |
| In vitro studies showed similar particle size distribution with comparable FPD and MMAD for both BDP and formoterol within the same formulation suggesting a deposition of both active drugs to the same regions within the lung, including the peripheral airways, and for the HFA BDP of the combination product and BDP HFA delivered separately (QVAR®). These results support the findings observed in-vivo. |
| A significantly higher maximum plasma concentration of BDP with the free versus the fixed combination was observed. Considering that BDP is rapidly metabolized to its metabolite B17MP, and has 30 times lower affinity for the corticosteroid receptor compared to B17MP [28-30], the increase in the unchanged BDP systemic exposure observed with the free combination has a very limited impact on the overall systemic activity. |
| Our results confirmed the same pulmonary absorption (AUC0-30min) [31,32] and total systemic exposure (Cmax and AUC0-t) of the active metabolite B17MP delivered as a free and fixed combination and are in agreement with Singh et al. [33] who reported remarkably similar AUC0-30min, Cmax and AUC0-t values for B17MP delivered in a fixed combination with formoterol. |
| Formoterol peak concentrations were reached 10 min postdose and were similar with both treatments (around 28 pg/mL). PK parameters of formoterol did not significantly differ between treatments. |
| Similar results with comparable ICS plasma concentrations following free (budesonide pMDI [8×160 μg] and formoterol DPI [8×4.5 μg]) versus fixed dosing (one pMDI inhaler (8×160/4.5 μg)) of budesonide and formoterol have been reported for the Budesonide/ Formoterol fixed combination [34]. |
| In contrast with the present study, the systemic exposure to formoterol when delivered via the fixed combination product was about 20% lower compared to administration via separate inhalers (515.7 pmol.h/l versus 627.1 pmol.h/l). |
| The effects on serum and urinary cortisol excretion were similar for the BDP/formoterol extrafine fixed dose formulation versus both extrafine formulations administered via separate pMDIs, in agreement with the results of total systemic exposure. As expected, both fixed and free combinations of extrafine BDP/formoterol reduced serum cortisol concentrations and urinary cortisol excretion (relative to creatinine), compared to placebo. Interestingly, the serum and urinary cortisol results for the BDP/formoterol HFA fixed combination (4 inhalations of 100/6 μg) were remarkably similar between the present study and that published by Bousquet and colleagues [19]: serum cortisol Cmin: 22.9 vs 25.0 ng/mL; AUC0-24h: 1.91 vs 2.26 μg.h/mL; urinary cortisol relative to creatinine: 55.5 vs 48.8. |
| At the same time, in a clinical study with 24 weeks treatment period in patients with moderate-to-severe asthma, adrenal function, measured through morning plasma cortisol levels, increased significantly versus baseline with BDP/formoterol HFA fixed-dose combination (400/24 μg/day), but remained substantially unchanged when BDP (1000 μg day) and formoterol (24 μg/day) were co-administered via CFC pMDI and DPI respectively [27]. The lack of cortisol suppression observed with beclomethasone dipropionate/ formoterol suggest a favourable safety profile compared with non-extra-fine formulations. |
| The average minimum serum potassium concentration was lower in the fixed combination group compared to either the free combination group or placebo; however it was above the lower limit of the normal range (3.5-5.1 mmol/L). No clinically relevant abnormality was observed for the individual potassium values. The plasma potassium AUC0-24h did not significantly differ between active treatments. Systemic effects of higher doses of inhaled β2-agonists have previously been reported including tremor, tachycardia, hypokaliemia and associated electrocardiographic signs [35,36]. However, a recently published study with high cumulative doses of BDP/formoterol showed tolerability generally similar to formoterol alone when administered to stable asthmatic patients [37]. Formoterol caused a significantly (p<0.05) greater decrease in serum potassium than BDP/formoterol or placebo. QTc, plasma lactate and vital signs did not significantly differ between the combination product and formoterol alone [37]. |
| In the present study there was no significant difference in heart rate between active treatment groups. Heart rate was significantly elevated in both fixed and free combination groups compared to placebo, although the increase was small and considered not clinically relevant (4.5% fixed; 6.5% free). Furthermore, all groups were associated with similar QTcB interval profiles, with no significant intra-group differences. |
| One of the limitations of this study is the fact that it was carried out in healthy volunteers rather than in a patient population. However, deposition of BDP and formoterol has been shown to be independent of pathophysiological condition, showing high and homogeneous deposition in the airways of healthy subjects as well as asthmatic and COPD patients with the average lung deposition (relative to nominal dose) reported as 34% in healthy subjects, 31% in asthmatics and 33% in COPD patients [25]. |
| The present study was a single-dose design, and so cumulative and long term effects were not assessed. However, the systemic exposure to BDP and Formoterol of the fixed combination in the present study are comparable to the study with the same fixed combination in asthmatic patients after one week of treatment [37]. |
| In conclusion, the systemic exposure to extrafine B17MP and formoterol after administration as a fixed combination or via separate inhalers was comparable. There was no clinically relevant difference in systemic effects between active treatments. The fixed and free combinations of BDP/formoterol were well-tolerated by all subjects. In asthmatic patients, Long-Acting Beta-Agonist (LABA) should not be used without inhaled steroids because alone, as LABA inhalers cause an increase in serious adverse events. LABA does not increase adverse events in COPD patients or in asthma patients who use LABA in conjunction with an inhaled steroid [38-40].Use of a single inhaler rather than separate inhalers to deliver extrafine drug particles of both BDP and formoterol simultaneously thus may be considered as a therapeutic option to improve convenience (adherence to treatment) and potentially compliance in asthma patients who need chronic treatment. |
| Acknowledgements |
| This study was sponsored by Chiesi Farmaceutici S.p.A. Medical writing assistance was provided by Dr Ruth Murray, MedScript Communications, Ireland. Thank you to [other non-authors] for their critical appraisal of this article. |
| References |
style="line-height: 25px; padding-left: 25px" class="ref_width">
References
- Global Initiative for Asthma (GINA).
- Bateman ED, Bantje TA, João Gomes M, Toumbis MG, Huber RM, et al. (2003) Combination therapy with single inhaler budesonide/formoterol compared with high dose of fluticasone propionate alone in patients with moderate persistent asthma. Am J Respir Med 2: 275-281.
- Lalloo UG, Malolepszy J, Kozma D, Krofta K, Ankerst J, et al. (2003) Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest 123: 1480-1487.
- Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, et al. (1997) Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med 337: 1405-1411.
- Ringdal N, Chuchalin A, Chovan L, Tudoric N, Maggi E, et al. (2002) Evaluation of different inhaled combination therapies (EDICT): a randomised, double-blind comparison of Seretide 50/250 microg bd Diskus vs. Formoterol (12 microg bd ) and budesonide (800 microg bd) given concurrently (both via Turbuhaler) in patients with moderate-to-severe asthma. Respir Med 96: 851-861.
- Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, et al. (2002) Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatr Pulmonol 34: 324-350.
- Barnes PJ (2002) Scientific rationale for inhaled combination therapy with long-acting ß2-agonists and corticosteroids. Eur Respir J 19: 182-191.
- Mak JC, Nishikawa M, Barnes PJ (1995) Glucocorticosteroids increase ß2-adrenergic receptor transcription in human lung. Am J Physiol 268: L41-L46.
- Tulic MK, Christodoulopoulos P, Hamid Q (2001) Small airway inflammation in asthma. Respir Res 2: 333-339.
- Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, et al. (1997) Inflammation of small airways in asthma. J Allergy Clin Immunol 100: 44-51.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, et al. (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645-2653.
- Usmani OS, Biddiscombe MF, Barnes PJ (2005) Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med 172: 1497-1504.
- Adcock IM, Gilbey T, Gelder CM, Chung KF, Barnes PJ (1996) Glucocorticoid receptor localization in normal and asthmatic lung. Am J Respir Crit Care Med 154: 771-782.
- Spina D, Rigby PJ, Paterson JW, Goldie RG (1989) Autoradiographic localization of beta-adrenoceptors in asthmatic human lung. Am Rev Respir Dis 140: 1410-1415.
- Magnussen H (2000) Equivalent asthma control after dose reduction with HFA-134a beclomethasone solution aerosol. Comparative Inhaled Steroid Investigation Group (CISIG). Respir Med 94: 549-555.
- Vanden Burgt JA, Busse WW, Martin RJ, Szefler SJ, Donnell D (2000) Efficacy and safety overview of a new inhaled corticosteroid, QVAR (hydrofluoroalkane-beclomethasone extrafine inhalation aerosol), in asthma. J Allergy Clin Immunol 106: 1209-1226.
- Busse WW, Brazinsky S, Jacobson K, Stricker W, Schmitt K, et al. (1999) Efficacy response of inhaled beclomethasone dipropionate in asthma is proportional to dose and is improved by formulation with a new propellant. J Allergy Clin Immunol 104: 1215-22.
- Rigamonti E, Kottakis I, Pelc M, Grzelewska Rzymowska I, Feschenko Y (2006) Comparison of a new extrafine beclometasone dipropionate HFA 134a-formulated pMDI with a standard BDP CFC pMDI in adults with moderate persistent asthma. Eur Respir J 28: 206-207.
- Bousquet J, Poli G, Acerbi D, Monno R, Ramael S, et al. (2009) Systemic exposure and implications for lung deposition with an extrafine Hudrofluroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet 48: 347-358.
- Paggiaro P, Nicolini G, Papi A (2008) Extrafine beclomethasone dipropionate/formoterol hydrofluoroalkane-propelled inhaler in asthma. Expert Rev Resp Med 2: 161-166.
- Piccinno A, Poli G, Acerbi D, Burton I, Druart X (2007) Pharmacokinetics and pharmacodynamics of a new beclomethasone dipropionate and formoterol CFC-free fixed combination in healthy volunteers. Poster presented at the European Respiratory Society, 15-19 September 2007, Stockholm, Sweden.
- O'Connor D, Adams WP, Chen ML, Daley-Yates P, Davis J, et al. (2011) Role of Pharmacokinetics in Establishing Bioequivalence for Orally Inhaled Drug Products: Workshop Summary Report. J Aerosol Med Pulmon Drug Deliv 24: 119-135.
- Hollander M, Wolfe DA (1999) Non parametric statistical methods. 2nd edition, Wiley Interscience, New York.
- Hauschke D, Steinijans VW, Diletti E (1990) A distribution-free procedure for the statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol 28: 72-78.
- Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ (2002) Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross over study in healthy volunteers. Chest 122: 510-516.
- De Backer W, Devolder A, Poli G, Acerbi D, Monno R, et al. (2010) Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv 23: 137-148.
- Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, et al. (2009) Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med 103: 41-49.
- Derom E, Pauwels RA (2005) Pharmacokinetic and pharmacodynamic properties of inhaled beclometasone dipropionate delivered via hydrofluoroalkane-containing devices. Clin Pharmacokinet 44: 815-836.
- Daley-Yates PT, Price AC, Sisson JR, Pereira A, Dallow N (2001) Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol 51: 400-409.
- Boobis AR (1998) Comparative physicochemical and pharmacokinetic profiles of inhaled beclomethasone dipropionate and budesonide. Respir Med 92: 2-6.
- Silkstone VL, Corlett SA, Chrystyn H (2002) Determination of the relative bioavailability of salbutamol to the lungs and systemic circulation following nebulization. Br J Clin Pharmacol 54: 115-119.
- Chrystyn H (2001) Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol 51: 289-299.
- Singh D, Collarini S, Poli G, Acerbi D, Rusca A (2009) Effect of the Aerochamber Plus spacer on the lung bioavailability of Foster: results from a randomized study. Br J Clin Pharmacol.
- Eklund A, Tronde A, Johannes-Hellberg I, Gillen M, Borgstrom L (2008) Pharmacokinetics of budesonide and formoterol administered via a series of single-drug and combination inhalers: four open-label, randomized, crossover studies in healthy adults. Biopharm Drug Dispos 29: 382-395.
- Lipworth BJ (1992) Risks versus benefits of inhaled beta 2-agonists in the management of asthma. Drug Saf 7: 54-70.
- Salpeter SR, Ormiston TM, Salpeter EE (2004) Cardiovascular effects of ß-agonists in patients with asthma and COPD. Chest 125: 2309-2321.
- Singh D, Piccinno A, Borrill Z, Poli G, Acerbi D, et al. (2008) Tolerability of high cumulative doses of the HFA Modulite beclomethasone dipropionate/formoterol combination inhaler in asthmatic patients. Pulm Pharmacol Ther 21: 551-557.
- Kramer JM (2009) Balancing the Benefits and Risks of Inhaled Long-Acting Beta-Agonists. The Influence of Values. N Engl J Med 360: 1592-1595.
- Sears MR (2009) Safety of long-acting ß-agonists: are new data really required? CHEST 136: 604-607.
- Salpeter SR, Wall AJ, Buckley NS (2010) Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med 123: 322-328.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 6738
- [From(publication date):
November-2012 - Sep 18, 2024] - Breakdown by view type
- HTML page views : 2315
- PDF downloads : 4423
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
