Review Article Open Access
Extraction of Proteases from Medicinal Plants and their Potential as Anti- Viral Targets
Amit Gupta1, Ankit P Shah2 and Sushama R Chaphalkar11Department of Immunology and Virology, Vidya Pratishthan’s School of Biotechnology (VSBT, Research centre affiliated to Savitribai Phule Pune University), Baramati, Maharashtra, India
2MAEER’s Maharashtra Institute of Pharmacy (MIT), Pune, India
- Corresponding Author:
- Amit Gupta
Department of Immunology and Virology
Vidya Pratishthan’s School of Biotechnology (VSBT)
Baramati, Baramati, Maharashtra, India
E-mail: amitvsbt@gmail.com, amitgupta@vsbt.res.in
Received Date: May 05, 2016; Accepted Date: May 13, 2016; Published Date: May 20, 2016
Citation: Gupta A, Shah AP, Chaphalkar SR (2016) Extraction of Proteases from Medicinal Plants and their Potential as Anti-Viral Targets. J Biotechnol Biomater 6:228. doi:10.4172/2155-952X.1000228
Copyright: © 2016 Gupta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Medicinal plants especially leaves are used in traditional medicine for rapid anti-viral therapy against infectious diseases. Protease, a potential candidate in medicinal plants is not so for studied in leaves. So an attempt was made to determine the protease activity of various medicinal plants especially leaves. Buffers of different pH range were used for extraction of the leaves to identify the best buffer for extraction of protease. Firstly, protein from fresh plant leaves of these medicinal plants were determined and then evaluated its protease activity using crude enzyme of protein (leaves) against specific protein antigen i.e. Bovine serum albumin (BSA). Thereafter, exposure of these proteases (acid or basic) on virally infected human whole blood samples determined through flow cytometry. The results showed that protease at particular pH of PBS buffer range of these medicinal plant leaves on virally infected human whole blood samples showed anti-viral activity.
Keywords
Medicinal plants; Leaves; Protein; Protease; Flow cytometer
Introduction
Development of vaccines against intracellular infectious diseases e.g. polio, mumps, smallpox etc. have been controlled but infections like HIV have been difficult to target because of variation in genotypes [1]. As per literature, infectious diseases have widely been treated using various medicinal plants and about 25% of current medicines originated from medicinal plant products [2,3]. Numerous medicinal plants are known for their magical medicinal properties and serve as an indispensable reservoir for drug discovery against infectious diseases [4,5]. In this regard, separation of these active metabolites using HPTLC technique have enabled researchers to find out the active compounds of medicinal plants as antiviral agents and to overcome the provocation of emerging infectious disease in human population [6]. There is a wide range of medicinal plants which are being used to extract compounds from plant products that are being used for their antiviral activity [3,6]. In view of this, viral infections are still painful to threat and some remained calamitous diseases in spite of antiviral drug research over decades. For this purpose, medicinal plant proteases have emerged as new targets for antiviral intervention and showed that proteases play a interpretative role in the life cycle of many viruses by effecting or splitting the high-molecular-weight viral polyprotein predecessors to generate functional products or by catalyzing the processing of the structural proteins indispensable for assembly and morphogenesis of virus particles e.g. liver diseases (HCV) [6-8].
In general, antiviral drugs extracted from medicinal plant products in the form of proteases that may stop the development and propagation of a virus without causing an appropriate damage in the host cell [9,10]. Inspite of this, major achievement i.e. more than 30 new drugs are approved to fight against AIDS virus but its number is limited group of pathogens e.g. herpes simplex virus (HSV), varicellazoster virus (VZV), human cytomegalovirus (HCMV), influenza virus and hepatitis B and C viruses (HBV and HCV, respectively) [6,11,12].
Recently, pharmaceuticals used proteases as drug or in the form of formulation for the treatment of various diseases. These are based largely on the production of small molecules identified through HPTLC or synthesized by microbes. It includes various hormones, antibiotics, analgesics etc. Previously, researchers mostly focused on plant proteins in the form of large or complex molecules and tried to use as therapeutic agents [13,14]. The first protein i.e. Insulin was used to treat diabetes that is more common cardiovascular disease in all over the world and it was a major breakthrough in that era of biotechnology [13,14]. Now a day, scientists focused on those proteases (crude enzyme of protein against specific protein antigen) extracted from medicinal plants and is responsible for breaking down the simple or complex protein that is responsible for causing intracellular infections.
One of the most important groups of industrial enzymes i.e. Proteases (Figure 1) that conducts proteolysis (protein catabolism by hydrolysis of the peptide bonds) and showed several physiological processes and determine the potential of proteolytic enzyme that are required or essential e.g. digestion of food proteins, protein turnover, cell division, blood clotting cascade, signal transduction, processing of polypeptide hormones, apoptosis etc. [10,15]. In view of this, proteases are physiologically needed for living organisms and are normally reported in plants, animals and microorganism. For protease production, use of medicinal plants is totally dependent on the availability of land for agriculture and certain climatic conditions [16]. The most familiar examples of plant proteases i.e. Papain, bromelain, keratinases etc.; animal origin e.g. pancreatic trypsin, chymotrypsin, pepsin, rennin etc. and microorganisms preferred both the enzymes from plant and animal sources and showed all the characteristics desired for their biotechnological applications [10,15]. Major types of proteases and their sources are listed in Figure 1.
Examples of Proteases Extracted From Medicinal Plants That Are Currently or Still Under Investigation
The current study establishes a flow cytometry method for detecting the viral infections in patient (human) blood samples that keep the records of infected cells and how much improvement will occur after exposure of proteases extracted from medicinal plants using specific protein antigen (Bovine serum albumin, BSA) and crude enzyme of protein test candidates. In an effort to achieve this objective, firstly isolate the protein (determined through Nanodrop) from medicinal plants using Tris HCl and ice cold acetone [16,17]. Thereafter, protease determination was done calometrically using BSA as substrate. In this study, crude enzyme extract of protein was assayed by using 1% BSA dissolved in citrate buffer (pH 7). For these studies, add equal quantity of BSA and crude enzyme extract in test tube and allowed to stand for 2h. Afterwards, TCA solution was added to stop the enzymatic reaction and then centrifuging the samples at high speed. The supernatant was collected and add equal quantity of NaOH (sodium hydroxide) solution in comparison with TCA (trichloroacetic acid) solution [17]. Incubate all these samples at room temperature. Afterwards, Folins colins reagent was added and the intensity of blue colour was measured at 700 nm within half an hour using spectrophotometer. In view of this, protease undergoes kinetic studies (i.e. temperature) and still remains active at 45ºC. For identification of these proteases using HPTLC (high performance Thin layer chromatography) was performed on silica gel 60 F254, 20 X 10 cm TLC plates (Merck, Darmstadt, Germany) with n-Butanol: acetic acid: water (4:1:1(v/v) as a mobile phase. Sample application was done with CAMAG-Linomat V automated spray on band applicator equipped with 100 μl syringe and operated with following settings: band length 47 mm, solvent front position 91 mm, application volume 80 μl, number of tracks (3), distance between tracks (60 mm). The development of TLC plate by using n-Butanol: acetic acid: water (4:1:1 v/v) solvent system using 20 x 10 twin trough solvent chamber. After development, the plates were dried using hair drier for 10 min and the results were calculated by studying the densitometric evaluation of each chromatogram. A CAMAG TLC Scanner 3 was used to densitometrically identify the bands using WIN CATS software (Version 1.2.3) with scanning speed 20 mm/s at multiple wave lengths [18]. The photodocumentation was carried out by CAMAG-Reprostar3 (Canon powershot G2) at 254 nm and 366 nm (data not shown). Following are the proteases extracted from various medicinal plants (leaves) dissolved in PBS of respective pH buffer (only active candidate data is mentioned) using crude enzyme of protein from test candidates against specific protein antigen i.e. BSA as shown below-
Azadirachta indica
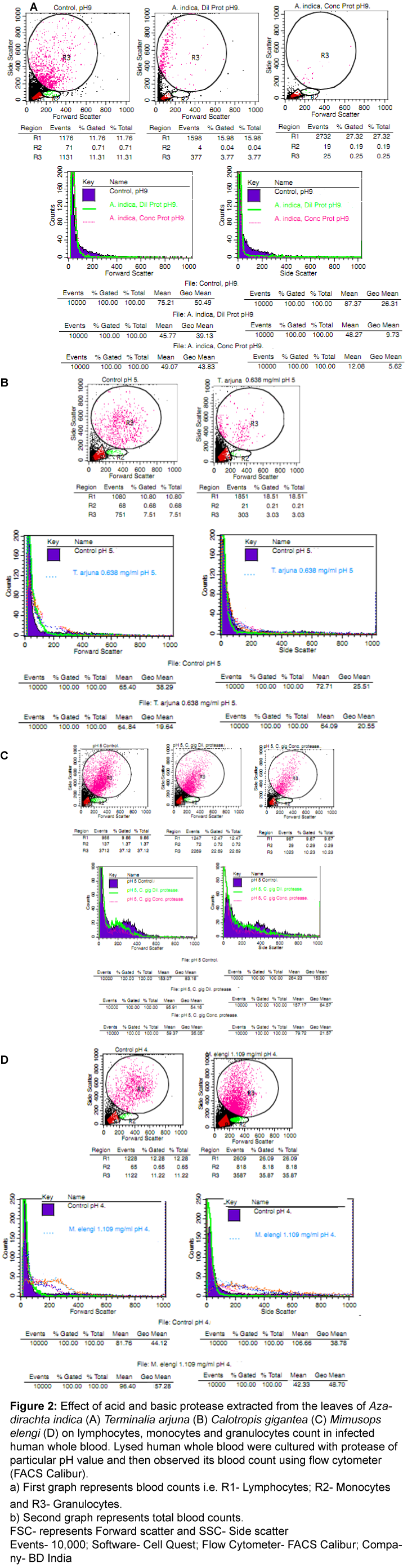
It belongs to family Meliaceae and showed various medicinal uses i.e. antipyretic, blood purifies of detoxifiers and also used for manufacturing of many health and beauty care products including bath powders, soaps, shampoos, cream and lotions. It shows antiviral, antineoplastic, antibiotic, anti-inflammatory, antiseptic, insecticidal, anti-hyperglycemic activity etc. [17,19]. The results showed that Azadirachta indica showed protein (23.28 mg/ml) and protease (3.423 mg/ml) content using PBS buffer (pH 9) in fresh mature plant leaves. In Azadirachta indica, the flow cytometric results showed that the maximum effect of basic proteases was observed at 3.423 mg/ml (pH 9; Figure 2A). In this case, basic protease showed enhancement of lymphocytes but slightly increased in the level of monocytes count and granulocytes count at the same concentration and returned to its normal blood profile as compared to infected control sample. Similarly, forward (shape and size) and side (granularity of the cell) scatter showed slightly enhancement or decline after treatment with these proteases extracted from Azadirachta indica.
Figure 2: Effect of acid and basic protease extracted from the leaves of Azadirachtaindica (A)Terminaliaarjuna (B) Calotropisgigantea (C) Mimusopselengi (D) on lymphocytes, monocytes and granulocytes count in infected human whole blood.Lysed human whole blood were cultured with protease of particular pH value and then observed its blood count using flow cytometer (FACS Calibur).
a) First graph represents blood counts i.e. R1- Lymphocytes; R2- Monocytes and R3- Granulocytes.
b) Second graph represents total blood counts.
FSC- represents Forward scatter and SSC- Side scatter
Events- 10,000; Software- Cell Quest; Flow Cytometer- FACS Calibur; Company- BD India
Terminalia arjuna
It belongs to family Combretaceae and showed various medicinal uses i.e. bark is rich in high in Coenzyme Q10, which reduces blood pressure. Bark of Arjuna is processed with milk to prepare a heart tonic. It is also beneficial in healing fractures quickly. Use Arjuna for the treatment of circulatory problems including treat kidney, liver and gall bladder problems. Arjuna tea is traditionally used to relieve pain in the kidneys and to break kidney stones, as well as for protection of liver cirrhosis [20,21]. In this case, protein (3.971 mg/ml) and protease (0.638 mg/ml) content using PBS buffer (pH 5). The flow cytometric results revealed that the maximum effect (Figure 2B) of acid protease was observed at 0.638 mg/ml (pH 5). Acid protease showed enhancement of lymphocytes, monocytes and granulocytes count and then returned to its normal blood profile. Similarly, forward and side scatter showed inhibition after treatment with acid protease extracted from Terminalia arjuna.
Calotropis gigantea
It belongs to family Apocynaceae and showed various medicinal uses i.e. dried whole plant is a good tonic, expectorant, depurative and anthelmintic. The flowers are bitter, digestive, astringent, stomachic, anthelmintic, and tonic. Traditionally calotropis is used alone or with other medicinally to treat common disease such as fevers, rheumatism, indigestion, cough, cold, eczema, asthma, elephantiasis, nausea, vomiting, diarrhoea etc. [22,23]. In this case, protein (3.878 mg/ml) and protease (0.763 mg/ml) content using PBS buffer (pH 5). The flow cytometric results revealed that the maximum effect (Figure 2C) of acid protease was observed at 0.763 mg/ml (pH 5) and showed decline in lymphocytes, monocytes and granulocytes count Similarly, forward and side scatter showed rapidly decline after treatment with acid proteases extracted from Calotropis gigantea. Overall, the results showed that these proteases showed significant anti-viral activity against infected human whole blood.
Mimusops elengi
It belongs to family Sapotaceae and showed various medicinal uses i.e. bark, flowers, fruits, and seeds are used in Ayurvedic medicine in which it is purported to be astringent, cooling, anthelmintic, tonic, and febrifuge. It is mainly used for dental ailments such as bleeding gums and loose teeth [3]. In this case, protein (5.199 mg/ml) and protease (1.109 mg/ml) content using PBS buffer (pH 4). The flow cytometric results revealed that the maximum effect (Figure 2D) of acid protease was observed at 1.109 mg/ml (pH 4). Acid protease showed enhancement of lymphocytes, monocytes and granulocytes count as compared to infected control. Similarly, forward and side scatter showed inhibition after treatment with acid protease extracted from Mimusops elengi. Overall, the results showed that these proteases showed significant effect against infected human whole blood and tried to return its normal blood profile.
In addition, protease extracted from medicinal plants using variable concentration of PBS buffer (pH ranging from 3 to 9). These proteases (acid, basic and neutral) showed many fascinating medicinal properties (immunomodulatory, anti-inflammatory etc.) and also involved in various applications especially for biotechnology. However the isolation and purification of proteases from these medicinal plants will assist us to recognize the mechanism of various disease models
Conclusion
The present study helps to identify the protease (acid and basic) activity in leaves of these medicinal plants against specific protein antigen, BSA. These medicinal plant leaves showed more protease activity against infected human whole blood samples and responsible for its anti-viral properties. However the isolation and purification of proteases from this plant and in vitro and in vivo testing of the enzyme on various pathogenic micro micro-organisms will help us to understand the anti-viral potential of medicinal plant leaves.
Acknowledgement
AG: Writing of the manuscript including data collection and revised the manuscript critically for important intellectual content. AG, APS and SRC read and approved the final manuscript.
References
- Ala PJ, Huston EE, Klabe RM, Jadhav PK, Lam PY, et al. (1998) Counteracting HIV-1 protease drug resistance: structural analysis of mutant proteases complexed with XV638 and SD146, cyclic urea amides with broad specificities. Biochemistry 37: 15042-15049.
- Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R (2015) The Application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. International Journal of Clinical Medicine 6: 635-642.
- Gupta A, Shah AP, Chabukswar AR, Chaphalkar SR (2016) Extraction of proteases from leaves of Mimusops elengi and its immunopharmacological applications. Indo American Journal of Pharmaceutical Sciences 3: 211 – 220.
- Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years.J Nat Prod 70: 461-477.
- Tyler VE (1999) Phytomedicines: back to the future.J Nat Prod 62: 1589-1592.
- Ashfaq UA, Idrees S (2014) Medicinal plants against hepatitis C virus.World J Gastroenterol 20: 2941-2947.
- Jahan S, Ashfaq UA, Khaliq S, Samreen B, Afzal N (2012) Dual behavior of HCV Core gene in regulation of apoptosis is important in progression of HCC.Infect Genet Evol 12: 236-239.
- Tilak JC, Adhikari S, Devasagayam TP (2004) Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin.Redox Rep 9: 219-227.
- López-Otín C, Overall CM (2002) Protease degradomics: a new challenge for proteomics.Nat Rev Mol Cell Biol 3: 509-519.
- Mahajan RT, Badgujar SB (2010) Biological aspects of proteolytic enzymes: A Review. Journal Pharm Res 3: 2048-2068.
- Schnitzler P, Schön K, Reichling J (2001) Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture.Pharmazie 56: 343-347.
- Ryu KJ, Lee SW (2003) Identification of the most accessible sites to ribozymes on the hepatitis C virus internal ribosome entry site.J Biochem Mol Biol 36: 538-544.
- Larrick JW, Thomas DW (2001) Producing proteins in transgenic plants and animals.Curr Opin Biotechnol 12: 411-418.
- Gavilondo JV, Larrick JW (2000) Antibody engineering at the millennium.Biotechniques 29: 128-1, 134-6, 138 passim.
- Roy JJ, Sumi S, Sangeet T, Abraham E (2005) Chemical modification and immobilization of Papain. Journal Chem Technol Biotechnology 80: 184-188.
- Devaraj KB, Gowda LR, Prakash V (2008) An unusual thermostable aspartic protease from the latex of Ficus racemosa (L.).Phytochemistry 69: 647-655.
- Gupta A, Chaphalkar SR (2015) Analytical studies of protease extracted from Azadirachta indica. World Journal of Pharmaceutical research 4: 1391-1398.
- Gupta AK, Tandon N, Sharma M (2003-2012) Quality Standards of Indian Medicinal Plants. Volume 1-10, ICMR, New Delhi.
- Gupta A, Chaphalkar SR (2015) Immunoadjuvant potential of Azadirachta indica against rabies, hepatitis and DPT vaccine antigen. International Journal of Medical and pharmaceutical sciences 5: 1-5.
- Gupta A, Khamkar PR, Chaphalkar SR (2014) Inhibition of nitric oxide production and proinflammatory cytokines by aqueous extract of Terminalia arjuna in human peripheral blood mononuclear cells. International Journal of Pharmaceutical and Biological Science Archive 2: 29-33.
- Gupta A, Chaphalkar SR (2015) Immunopharmacological activity of saponin from Terminalia arjuna and Prosopis spicigera. Journal of Pharmacological Reports 1: 1-4.
- Gupta A, Chaphalkar SR (2016) Antidiabetic activity of Calotropis gigantea in human whole blood. Journal of Disease and Global Health 6: 107-112.
- Gupta A, Chaphalkar SR (2016) Inhibition of antigen specific T cell population using Calotropis gigantea and Terminalia arjuna. Journal of Biology and Nature 5: 14-19.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16572
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 15216
- PDF downloads : 1356