Extraction of Mucilage and its Comparative Evaluation as a Binder
Received: 23-Jan-2023 / Manuscript No. cpb-23-88376 / Editor assigned: 24-Jan-2023 / PreQC No. cpb-23-88376 / Reviewed: 09-Feb-2023 / QC No. cpb-23-88376 / Revised: 13-Feb-2023 / Manuscript No. cpb-23-88376 / Published Date: 23-Feb-2023 DOI: 10.4172/2167-065X.1000312
Abstract
A binder holds powders together to form granules and also provides the cohesiveness required for binding of the granules under compression to form a tablet. Some natural excipients are currently available for pharmaceutical formulation. Aim of this study was to extract the mucilage from different plants and to evaluate them as a binder. Here an effort was made to investigate the efficacy of mucilage obtained from seed of Fenugreek and Flax as tablet excipient. A Precipitation of soaked seed in acetone technique was developed to optimize the extraction of mucilage from the seeds of Fenugreek and Flax plants. Seed of both plants have been extracted by conventional method. The extracted mucilage has been evaluated for various physicochemical properties. Tablets were manufactured using extracted mucilage as a binding agent and comparison was made against the tablets prepared with mucilage as standard binder on studying standard parameters like diameter, thickness, hardness, friability and disintegration. Mucilage could be used as a binding agent for at low concentrations. This can be used for sustaining drug release from tablets.
Keywords
Mucilage; Pharmaceutical excipients; Tablets; Physicochemical characterization
Introduction
Polysaccharides are polymeric carbohydrates molecules composed of long chains of monosaccharide units. They are isolated from terrestrial and marine plants or are principally the exogenous metabolites of some bacteria.
Plant derived polymers have evoked tremendous interest due to their diverse pharmaceutical applications such as diluents, binder, disintegrate in tablets, thickeners in oral liquids, protective colloids in suspensions, gelling agents in gels and bases in suppository they are also used in cosmetics, textiles, paints and paper-making. These polymers such as natural gums and mucilage are biocompatible, cheap and easily available and are preferred to semi synthetic and synthetic excipients because of their lack of toxicity, low cost, availability, soothing action and non-irritantnature.The Mucilage is a water-soluble edible adhesive material that constitutes carbohydrates and uranic acids units present in different parts of plants including the mucous epidermis of the outer layer of seeds, bark, leaves, and buds [1].
Recent trend towards the use of plant based and natural products demands the replacement of synthetic additives with natural ones [2]. These plant based polymers have been studied for their application in different pharmaceutical dosage forms like matrix controlled systems, film coating agents, buccal films, microspheres, nanoparticles, viscous liquid formulations like ophthalmic solutions, suspensions, implants and their applicability and efficacy has been proven [3-5].These have also been utilized as viscosity enhancers, stabilizers, disintegrants, [6] solubilisers, emulsifiers, [7] suspending agents, [8] gelling agents [9] and bio-adhesives and binders [10] in the above mentioned dosage forms [11]. The plant derived gums and mucilage comply with many requirements of pharmaceutical excipients as they are non-toxic, stable, easily available, associated with less regulatory issues as compared to their synthetic counterpart and inexpensive; also these can be easily modified to meet the specific need [12].
Introduction to Fenugreek & Flax seed
Fenugreek (Trigonella Foenum-Graecum L.), plant is widely distributed throughout the world and which belongs to the family Leguminosae.Trigonella Foenum-Graecum has long stalked leaves up to 5 cm long stipules triangular, lanceolate, leaflets about 2.5 cms long, obovate to obanceolate. The root is a mass of finger structures. The sissile axillary flowers are white or pale yellow. The thin, swordshaped pods are 10-15 cm (4-6 in), with a curved beak-like tip, each carrying 10-20 seeds. The plant radiates a spicy odour which persists on the hands after touching. Wild and cultivated varieties exist. If the seed is cut in a direction transverse to the side in which the hilum lies, so as to pass through both lobes of the seed, it will be found that the larger lobe contains two accumbent cotyledons - the smaller, the radical. Both are yellowish in colour, and surrounded by a darker, horny, translucent endosperm, which separates the radicle from the cotyledons. When it is soaked in water the endosperm swells and yields mucilage to the surrounding liquid. Entire seeds macerated in warm water burst their seed-coats by the swelling of the mucilage, and disclose the structure of the seed [13-15].
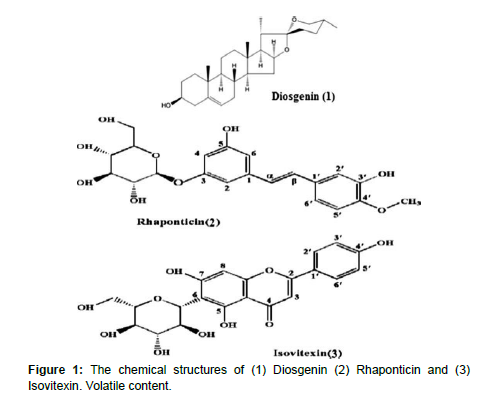
Phytochemistry of fenugreek seed
Fenugreek contains a number of chemical constituents including steroidal sapogenins. Diosgenin component has been found in the oily embryo of fenugreek. There are two furastanol glycosides, F-ring opened precursors of diosgenin that have been reported in fenugreek also as hederagin glycosides. Alkaloids such as trigocoumarin, nicotinic acid, trimethyl coumarin and trigonelline are present in stem. The mucilage is a standing out constituent of the seeds. There is about 28% mucilage; a volatile oil; 2 alkaloids such as trigonelline and Choline, 5% of a stronger-smelling, bitter fixed oil, 22% proteins and a yellow coloring substance are present in stem (Grieve, 1984). Fenugreek contains 23–26% protein, 6–7% fat and 58% carbohydrates of which about 25% is dietary fiber (Figure 1). Fenugreek is also a rich source of iron, containing 33 mg/100 g dry weight [16]. Chemical constituents of fenugreek purposes such as medicinal uses (anti-diabetic, lowering blood sugar and cholesterol level, anti-cancer, anti-microbial, etc.)
Flaxseed (Linumu Sitatissimum L.) is a member of the genus Linum in the family Linaceae [17].Flaxseed contains some nutrients that may have various health benefits. Like other plant-based foods, flaxseed is rich in antioxidants. These can help prevent trusted Source disease by removing molecules called free radicals from the body. Free radicals occur as a result of natural processes and environmental pressures. If there are too many free radicals in the body, oxidative stress can develop, leading to cell damage and disease (Figure 2). Antioxidants help remove free radicals from the body. Flaxseed is a good source of lignin’s, which appear to have antioxidant properties (Table-1).
| S. no. | Chemical constituents of fenugreek |
|---|---|
| Alkaloids | Trimethylamine, Neurin, Trigonelline, Choline, Gentianine, |
| Carpaine and Betain | |
| Amino acids | Isoleucine, 4-Hydroxyisoleucine, Histidine, Leucine, lysine, l- |
| tryptophan, Arginine | |
| Saponins | Graecunins, fenugrin B, fenugreekine, trigofoenosides A–G |
| Sterodial | Yamogenin, diosgenin, smilagenin, sarsasapogenin, tigogenin, |
| Sapinogens | neotigogenin, gitogenin, neogitogenin, yuccagenin, saponaretin |
| Fibers | Gum, neutral detergent fiber |
| Flavonoids | Quercetin, rutin, vitexin, isovitexin |
Chemical constituents of Flaxseed
Consist of three groups of compounds in the flaxseeds, characterized by specific biological activity and functional properties: PUFA omega-3 family, soluble dietary fiber in the form of mucus, and lignin’s, which have phytoestrogen properties. Data on the chemical composition of flaxseed, obtained from various sources, are characterized by high variability. The flaxseeds contain 35-45% oil, which contains 9-10% of saturated fatty acids (politic and stearic), about 20% monounsaturated fatty acids (mainly oleic acid), and more than 70% alpha-linoleic fatty acids acid (Figure 3). The protein content in seeds of flax varies from 20-30%.Chemical constituents as Omega-3 fatty acid and alpha linoleic acid to help reduce the thickness of blood and reduce the chances of clots. Flaxseed was introduced to North America for oil and fibber production; it is finding use for its health benefits as a nutraceutical with anti-atherosclerosis, anti-cancer and anti-inflammatory and antiosteoporosis properties [18].
Extraction of Plant-Derived Mucilage
• Extraction method of hot water
• Enzymolysis method
• Extraction method of dilute alkali-water solution
• Solvent extraction
• Maceration extraction
• Other methods
• Precipitation of soaked seed in acetone
Extraction method of hot water:
This is the most widely used method in polysaccharide extraction currently, which principle is that most polysaccharides have bigger solubility in hot water. Polysaccharide is stable in hot water, so polysaccharide can receive the minimal damage using this extraction method. The usual practice is to extract 2–6 h using hot water. If extract is low in viscosity, the residue in the extract can be easily filtered. If extract is viscous, the residue can be removed using centrifugation [19,20].
Extraction method of dilute alkali-water solution:
Some acidic polysaccharides or high MW polysaccharides are not easily dissolved in hot water. Their solubility is generally bigger in dilute alkali solution than in hot water. Therefore, NaOH solution or NaCO3 solution of 5%–15% (w/w) is often used to extract instead of hot water. When using dilute alkali solution to extract, extraction temperature should be kept below 10°C. Otherwise, polysaccharides are prone to degradation. In practice, usually hot water is first used to extract polysaccharides, and then dilute alkali solution is used to extract the remaining polysaccharides in the obtained residue. So most polysaccharides in animal / plant material will be extracted in this way.
Enzymolysis method:
The crushed raw material of plants is suspended in water. According to the optimal reaction condition of composite enzymes to be used, the optimal temperature and pH are set first, and then a certain amount of composite enzymes will be added in the suspension and react for a period of time. The filtrate is the extract solution of polysaccharide after filtering the residue. This method has been applied in the preparation of some polysaccharide healthcare products. However, in practice, the widely used is the combined method of hot water extraction andenzymolysis, i.e., first use hot water to extract, and then use enzymes to extract. Thus the yield of polysaccharide can increase much.
Classification of carbohydrates: A carbohydrates is a biological molecules consists of (C, H, O) atoms. Usually hydrogen – oxygen atom ratio (2:1) as in water [21]. Polysaccharides are a kind of carbohydrates. A Polysaccharide substance extracted as a viscous or gelatinous solution from plant roots, seeds etc., and used in medicines and adhesives (Table-2).
| Polysaccharides | Mucilage |
|---|---|
| Seaweed extracts: Agars, aliginates, carrageenans. | Linumu Sitatissimum L. (Seed) |
| Higher plant cell wall insoluble: cellulose. | Spinaciaoleracea L. (Leaves) |
| Higher plant cell wall soluble: pectin. | Salvia Hispanica (seed) |
| Higher plant seeds: cereal starch, guar gum, locust | Psyllium seed (Husk) |
| bean gum. | |
| Higher plant tuberu& roots: potato starch, tapioca | Basella alba (Stem) |
| starch. |
Monosaccharides: Are the simplest carbohydrates. They cannot be broken down to smaller carbohydrates, it contain a single polyhydroxy aldehyde or ketone unit (e.g., glucose, fructose) [22].
Glucose: Glucose is a simple sugar and most abundant monosaccharide.
Fructose: Fructose is also known as ‘fruit sugar” a type of simple sugar occurs naturally in fruits, honey, sugar cane and vegetables.
Disaccharides: Disaccharides consist of two monosaccharide units linked together by a covalent bond (e.g., sucrose) [23].
Sucrose: Sucrose is made up of one molecule of glucose and one molecule of fructose joined together. It naturally produced in plant, from which table sugar is refined.
Maltose: Maltose, also known as malt sugar, is a disaccharide formed from two units of glucose joined with a bond.
Oligosaccharides: Oligosaccharides contain from 3 to 10 monosaccharide units (e.g., raffinose).
Raffinose: An oligosaccharide found in peas and beans; largely undigested until reaching the intestinal flora in the large intestine, releasing hydrogen, carbon dioxide, and methane.
Polysaccharides: Polysaccharides contain very long chains of hundreds or thousands of monosaccharide units, which may be either in straight or branched chains (e.g., cellulose, glycogen, starch) [24].
Starch: Starch is a storage carbohydrate consisting of glucose monomers in plants such as cereals, root vegetables and legumes. It is comprised of two polymers, namely amylose and amylopectin [25].
Cellulose: Cellulose is a polysaccharide consisting of a linear chain of several hundred to many thousands of β (1→4) linked D-glucose units. Cellulose is the most abundant organic polymer on Earth. Cellulose is mainly used to produce paperboard and paper. Cellulose for industrial use is mainly obtained from wood pulp and cotton.[26, 27]
Dextrin: Dextrin is a complex, branched glucan composed of chains of varying length. It is used medicinally as an antiplatelet to reduce blood viscosity. The straight chains consists of α-1,6glycosidic linkages between glucosemolecules, while branching begin from α-1, 3 linkages [28].
Pectin: Pectin is a structural polysaccharide heteropolysaccharide contained in the primary Cell walls of terrestrial plants. It is used in food as a gelling agent, particularly in jams and jellies [29].
Rationale
• For preparation of dosage form required excipients and most of excipient extract form plant different part like, leave, seed, stem and flower.
• Literature was show on the extraction content flaxseed mucilage and the yield can range from 3.5 to10.2% of the total seed weight [30].
• Mucilage from Fenugreek seeds by extraction, the percentage yield of mucilage was 10-15% w/w [31].
• Extract mucilage has use different properties i.e., binding agents, disintegrating agents, gelling agents, suspending agent and drug release retarding polymer.
• So mucilage as a gelling agent can be used as a binder in Pharmaceutical Industry & comparative studies was carried out.
• So study of fenugreek & Flax seed as a binder in Tablet formulation was carried out.
Aim: Extraction of Mucilage and its Comparative Evaluation as a Binder.
Objective
· To extract mucilage from fenugreek & Flax seed.
· To perform evaluation of mucilage.
· To prepared tablets with using mucilage as binding agent.
· To evaluated prepared tablets.
· To perform accelerated stability studies.
Literature Review Related To Mucilage
Rishabha Malviya et al. (2020) Techniques of Mucilage and Gum Modification and their Effect on Hydrophilicity and Drug Release. The present manuscript highlights the advantages, modifications of gum and mucilage, their effects on hydrophilicity and drug release as well as aspects of the natural gums which can be assumed to be bifunctional excipient because of their concentration-dependent effect on drug release and their high degree of swellability [32].
Jyoti Wadhwa et al. (2013) Potential of plant mucilages in pharmaceuticals and therapy. Mucilages, and in particular plant mucilages, have gained more attention over the last few decades due to their reputable medicinal properties. Some publications have appeared in reputable Scientific Journals that have made appreciable contributions to the discovery of the functions and utilizations of such naturally occurring products. Therapeutic value of mucilages has been extended to wound healing, diabetes, immunostimulation, cancer, angiotensin converting enzyme inhibition, stomachic and antioxidant properties. Based on their sustaining capacities as well as binding and gelling properties, mucilages have been proposed to be one of the most useful materials to modulate drug delivery. Chemical analysis reveals that generally these contain monosachrides along with a range of other organic and inorganic components. Although physiological properties of various plant mucialges have been described, it still remains uncertain as to which of the component(s) is responsible for these physiological properties. Further research needs to be done to unravel the myth surrounding the biological activities and the functional properties of them. This review presents an overview of the current status and knowledge on the applications of plant mucilages as therapeutic agent and pharmaceutical additives [33].
Nadia Bayar et al. (2016) Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. The chemical extraction and the characterization of polysaccharides from mucilage (MC), pectin (PC) and total pectic mucilage fraction (TFC) of Opuntia ficus indica cladodes as well as the evaluation of their antioxidant activities was investigated. The FTIR spectroscopic analysis revealed the presence of carboxyl and hydroxyl groups corresponding to polysaccharides. Uronic acid and the total sugar contents of PC were higher than those of TFC and MC whereas ash content of MC was considerably more important. In addition, the findings showed that all the samples had little protein content and low average molecular weight compared to the results mentioned in literature. Furthermore, MC reached not only the highest water (WHC) and oil holding (OHC) capacities (7.81g/g and 1.34g/g, respectively) but also the highest antioxidant properties (DPPH and ABTS scavenging activities, β-carotene bleaching inhibition activity and reducing power). However, PC had the strongest emulsifying and foaming properties. As for TFC, it had low WHC, OHC and emulsifying properties whereas it had higher foaming properties than MC and greater antioxidant properties compared to PC. These outcomes can encourage the use of PC as a surfactant and MC and TFC as natural antioxidants in food and pharmaceutical industries [34].
Ibukunoluwa Fola Olawuyi et al. (2021) Application of plant mucilage polysaccharides and their techno-functional properties' modification for fresh produce preservation. The use of edible coating/ film to improve fresh produce's quality and shelf life is an old but reliable and popular method of preservation. Recently, plant-derived mucilage has been extensively used to prepare edible packages (MEPs). This review focuses on recent studies that characterize mucilages from different plants, and examine their specific applications as edible packages in preserving fruits and vegetables. Structure-function relations and corresponding influence on film-forming properties are discussed. This review also surveys the additive-modifications of MEPs techno-functional properties. MEPs from a range of plant sources are effective in preventing quality loss and improving the storability of various fruits and vegetables. The preservative mechanisms and essential techno- functional properties of MEPs required for fruit and vegetable packaging were summarized. The key findings summarized in this study will help promote the utilization of mucilages and draw attention to other novel applications of this valuable polymer [35].
Literature Review related to plant
Ali Nokhodchi et al. (2019) In-vitro evaluation of fenugreek mucilage as a potential excipient for oral controlled-release matrix tablets. In this study the effect of lactose on the release behaviour of propranolol hydrochloride from matrices formulated to contain the fenugreek mucilage also was investigated. An increase in concentration of the mucilage in matrices resulted in a reduction in the release rate of propranolol hydrochloride comparable to that observed with hypomellose matrices. The rate of release of propranolol hydrochloride from fenugreek mucilage matrices was mainly controlled by the drug: mucilage ratio. However, the mechanism of release from matrices containing drug: mucilage ratios of 1:1, 1:1.25, 1:1.5, and 1:2 remained the same. The kinetics of release, utilising the release exponent n, showed that the values of n were between 0.46-0.57 indicating that the release from fenugreek mucilage matrices was predominantly by diffusion. The presence of lactose in matrices containing mucilage increased the release rate of propranolol hydrochloride. This is due to a reduction in tortuoisity and increased pore size of channels caused by lactose through which propranolol diffuses and therefore diffusion of water into the tablet is facilitated [36].
Surya Narayan Ratha Adhikari et al. (2017) Buccal patches of atenolol formulated using fenugreek (Trigonella foenum-graecum L.) seed mucilage.The use of mucoadhesive natural polymers in designing mucoadhesive patch systems has received much attention. The developed atenolol-releasing buccal patches can be beneficial over the conventional drug delivery systems to decrease the dosing frequency and enhance patient compliance.
Sara Basiri et al. (2018) Flaxseed mucilage a natural stabilizer in stirred yogurt. Today, there is much interest in the use of natural ingredients in the food industry. Flaxseed mucilage (FSM) stands out for its health benefits and functional characteristics. The effect of FSM and its combination with carboxyl methyl cellulose (CMC) on quality properties of stirred yogurt were investigated. The addition of FSM and FSM + CMC to stirred yogurt increased the viscosity and decreased syneresis. Addition of FSM decreased the cohesiveness and increased the adhesiveness of the stirred yogurt, while its combination with CMC leads to decreased adhesiveness, increased cohesiveness and springiness. The gumminess and hardness of yogurt were reduced when supplemented with FSM and FSM + CMC. Sensory attributes were influenced by FSM and FSM + CMC; however, these were not deteriorated significantly during 21 days storage at 4°C. FSM has the potential as a natural stabilizer to improve the texture of stirred yogurt. Romain Roulard et al. (2016) Molecular investigations of flaxseed mucilage polysaccharides Experiments showed that the conformational structure of mucilage molecules was strongly influenced by ionic strength. Mucilage carbohydrates exhibited a spherical and compact structure in NaCl solution while they displayed a random-coil conformation in water.
Materials & Methods
Extraction of Plant-Derived Mucilage
Extraction of mucilage by Precipitation of soaked seed methods: Method of extraction was adapted by soaking About 100 g of Fenugreek & Flax seeds were soaked in 800ml of distilled water for 12 hrs. Then heated up to 50°C, 70°C or 90°C by using water bath and subsequently filtered. To the filtrate (800 ml) equivalent amount of acetone was added to allow precipitation of mucilage. White supernatant coagulant mass separated after precipitation by acetone was filtered through the muslin cloth. Precipitated mucilage was then spread on glass slab and dried in tray dryer at temperature not exceeding 120oC. Dried mucilage easily separated in the form of flakes from glass slab by spraying acetone over dried mucilage (Figure 4). Mucilage flakes were further dried at 60°c for 5 min. Mucilage obtained was converted into powder by size reduction (Table-3). Obtained powder was sieved using 40# sieve.
| Chemicals | Manufactured By |
|---|---|
| Methanol | Avantor Performance Material India Ltd. |
| Acetone | Molychem |
| CCS | Vishal Chem. Ltd. Mumbai |
| Talc | West Cost Laboratories Mumbai |
| Magnesium stearate | West Cost Laboratories Mumbai |
| Lactose | Clairofit India |
Evaluation of extract mucilage
Bulk and Tapped density
A pre-weighed, pre-sieved quantity of dried mucilage was poured into a graduated cylinder and the volume recorded. The cylinder was then tapped until powder bed volume reached a minimum value and the tapped volume recorded. The bulk and tapped densities were calculated.
Y=M D / V T
MD= refers to the mass of the dry soil and VT =refers to the volume of the dry soil. Y=is the bulk density of dry soil.
Calculate the tapped density (g/ml) using the formula mf/100 where mf is the mass of powder in the measuring vessel.
Carr's Index and Hausner’s Ratio: Carr's index and Hausner’s ratio were calculated from the bulk and tapped densities.
Hausner Ratio= Tapped bulk density/ Loose bulk density.
Angle of Repose: The angle of repose was determined by the fixed height funnel method. Stop pouring the material when the pile reaches a predetermined height or the base a predetermined width. Rather than attempt to measure the angle of the resulting cone directly, divide the height by half the width of the base of the cone. The inverse tangent of this ratio is the angle of repose (Table-4).
| Instrument | Model | Manufactured By |
|---|---|---|
| UV visible Spectrophotometer | UV 1900i | Shimadza |
| Tablet Dissolution Test Apparatus | DS8000 | Lab India |
| Disintegration Test Apparatus | 2737 | Systronics |
| Digital Balance | _ | Retouch |
Determination of swelling index (SI) of seed mucilage
Swelling index of Fenugreek & Flax seed mucilage was determined by using modified method elsewhere reported. One gram of Fenugreek & Flax seed mucilage extracted was accurately weighed and transferred to a 100ml stopper measuring cylinder. The initial volume of the powder in the measuring cylinder was noted. The volume was made up to 100 ml mark with distilled water. The cylinder was shaken gently and set aside for 24 hr. The volume occupied by the mucilage sediment was noted after 24 hr.Where xo is the initial height of the powder in graduated cylinder and xt denotes the height occupied by swollen gum after 24 hr. The content from the measuring cylinder from the above test were filtered through a muslin cloth and the water was allowed to drain completely into a dry 100 ml graduated cylinder. The volume of water collected was noted and the difference between the original volume of the mucilage and the volume drained was taken as water retained by sample and was referred to as water retention capacity or water absorption capacity [37].
Testing of mucilage polysaccharide
Ruthenium red test
A small quantity of extract was suspended with water and added ruthenium red solution. Formulation of pink colour showed the presence of gum and mucilage.
For analytical estimation of ruthenium red, 1 g of ruthenium red crystals is dissolved in 100 ml of distilled water. The solution is heated slowly to 60°C and cooled before storage in a dark bottle at 4°C in a refrigerator. Dilutions of the stock solution are made freshly using distilled water. The wavelength for maximum absorption for ruthenium red dye solution is found to be 535 nm using UV-visible spectrometer (Table-5).
Identification tests |
Name of tests |
|---|---|
| Test for mucilage | Ruthenium red test |
| Test for Mucilage | O-toluidine test |
The o-toluidine test for complex carbohydrates (polysaccharides)
First hydrolyzing the sample with sulfuric acid and testing the hydrolizate for the presence of monosaccharides using the o-toluidine solution in the same manner as the simple carbohydrate test. This test which is published as the “complex carbo-hydrate (gum) test” for the identification of binders in ethnographic materials. This test was included as the“test for complex carbohydrates using o-toluidine” in Odegaardet al.2000, these early versions stated that a blue green color reaction indicated a positive result for complex carbohydrates. However, because many gums and mucilage are composed of both aldo- pentoses and aldohexoses, this color reaction is not accurate.
Gelling Capacity: According to a study the 1g of powder mucilage is taken in10ml of measuring cylinder and water is add to 10ml, and then allows to keep for 15min. After that allow 1g of weight to suspend from the top and see when it get set the bottom time interval can be noted.
Preparation of Diclofenac tablets
Diclofenac and all other ingredient pass through the sieve no. 40. Diclofenac, excipients and disintegrant are mixed in mortar and mix it well by pestle. To the above mixture talc was added, mix it well. The tablets were prepared by direct compression on a rotary tablet press, fitted with concave punches of 8mm diameter. The turret was rotated at a fixed speed of 30 rpm (Table-6).
Ingredients |
Batch I (Fenugreek mg) | Batch II ( Flax Seed mg) |
|---|---|---|
| API | 50 | 50 |
| Lactose | 160 | 160 |
| Mucilage | 25 | 25 |
| Cross carmellose sodium | 7.5 | 7.5 |
| Magnisium Stearate | 5 | 5 |
| Talc | 2.5 | 2.5 |
| Total | 250 | 250 |
Hardness and Friability test
Hardness Test is also referred as Crushing Strength of tablet. Define as the load required crushing the tablet when it is placed on its edge. For Hardness Test Portable Hardness Tester was used. Tablet hardness should lie in between 4 to 10 kg/cm2.
Friability testing is a method, which is employed to determine physical strength of compressed and uncoated tablets upon exposure to mechanical shock and attrition. It tells how much mechanical stress tablets are able to withstand during their manufacturing, distribution and handling by the customer.
Friabilator or Roche Friabilator is used for friability testing.
For the test 6.5g weight of tablets were selected from, and their initial weights altogether were recorded. Roche Friability is operated at 25 rpm for 4 min (100 revolutions).After these the tablets are taken out and dusted properly and weighted again. The % friability is calculated.
Tablet that loses less than 1.0% of their weight are generally considered acceptable.
% Friability = Initial weight – Final weight / Initial weight x 100
Disintegration time (DT)
The time required for disintegration of 6 tablets per batch was carried out in USP Disintegration Test Apparatus containing 900 mL HCL buffer (pH1.2) at 37+0.5˚C. The mean DT was calculated.
In-vitro Dissolution studies
The drug release study was carried out using paddle type dissolution test apparatus at 37±0.50 C and at 50 rpm using 900mL 0.1N Hydrochloride (pH1.2) as dissolution medium. Five milliliters sample solution was withdrawn at predetermined time intervals, filtered through a 0.45 micron membrane filter, diluted suitably, and analysed spectro photometrically at 276 nm using a Shimazdu-1700 UV-Visible double beam spectrophotometer. Equal amounts of fresh dissolution medium were replaced immediately after withdrawal of a test sample. The percentage drug dissolved at different time intervals was calculated using regression equation generated from the standard curve.
Development of Calibration Curve of Diclofenac sodium
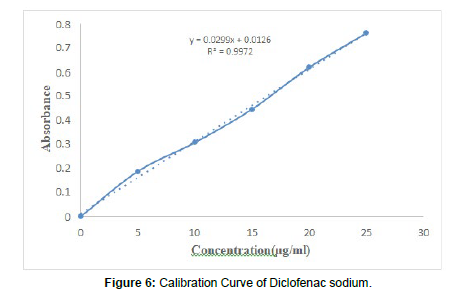
The method employs estimation by straight line equation obtained from calibration curve of diclofenac sodium. The method obeys Beer's law. Calibration curve for Diclofenac sodium. The dilutions were made from Standard Stock solution to get concentration of 05, 10, 15, 20, and 25μg/ml respectively. These solutions were scanned from 400 to 200 nm and values was integrated in the range of 270-282nm. The calibration curve was plotted between Absorbance values against concentration (Table 7).
| Properties | Fenugreek seed | Flax seed |
|---|---|---|
| Color | Yellow | Brown |
| Odor | Nutty flavour, bunt sugar | Nutty flavour |
| Yield | 6.4%w/w | 15.2%w/w |
| Gelling Capacity | 53 sec. | 1.08 sec. |
Assay of Diclofenac sodium tablets
Triturate single tablet from each fenugreek and flax tablets. By calculating the average weighed sample powder equivalent to 10mg of diclofenac sodium was Transfer into volumetric flask containing 10ml water. After preparation of standard and tablet solutions, strength of solution 100 ppm in 100 ml absorbance of the sample preparation and standard preparation in 1cm cell at the wavelength of maximum absorbance at about 276 nm, using a UV spectrophotometer, using the blank solution. Calculate the quantity in mg, of Diclofenac.
Accelerated stability Studies
Main aim of accelerated stability study to predict the stability profile of a drug product that prediction of self-life of the product before launching into market. Final formulation was wrapping in aluminium folia and store at 40±2°Cand 75 ±5% relative humidity. Sample was withdraw predetermine time interval 0 days and 150 days [38].
Result and Discussion
Seeds of Fenugreek & Flax contain mucilage around the outer layer. The major problem of mucilage is that it swells but does not separate easily from the seeds. Therefore effective method was developed by using precipitation of soaked seeds in acetone. Period of 12 hours was sufficient for mucilage to swell completely and then swollen mucilage layer without crushing the seed. Due to which pure mucilage filtrate can be obtained without affecting internal part of the seed and therefore yield of mucilage was also increased. The method developed was made cost effective by reusing distilled acetone from the filtrate which minimized the cost of solvent (Table 8). Acetone increased the rate of precipitation and therefore less amount of solvent was required to precipitate the mucilage in larger quantity. Acetone being more volatile in nature was completely removed and no traces of solvent were found in dried the mucilage. The mucilage was dried at 60°C which reduced the time for extraction. The drying temperature did not affect the mucilage stability (Figure 5). Organoleptic characteristic of mucilage data is tabulated in from the results it was observed that Quality of the mucilage obtained was very good in terms of color, texture, odor and yield as compared (Table 9).
| Flow Properties | Fenugreek seed | Flax seed |
|---|---|---|
| Bulk Density(kg/m3) | 0.192 | 0.24 |
| Tap Density(kg/m3) | 0.208 | 0.251 |
| Carr's Index | 7.69 | 6.25 |
| Hausner’s Ratio | 1.08 | 1.066 |
| Angle of repose | 36.8 | 33.69 |
| Properties | Fenugreek seed | Flax seed |
|---|---|---|
| Swelling index (SI) | 1.9 | 3.8 |
Evaluation of Flow properties of extracted seed mucilage
The flow properties of dried mucilage are shown in Carr’s index, Hausner’s ratio and angle of repose were selected as flow indicating parameters. They reflect the particle size, surface characters and moisture content of the mucilage (Table 10). Flow properties of extracted mucilage datais tabulated. From the result it was observed that mucilage extracted from the exhibited average flow properties.
Determination of swelling index of seed mucilage
Mucilage on coming in contact with water hydrates and swells forming thick and viscous solution. Swelling index of extracted mucilage from method was compared and results obtained. The particle size of mucilage obtained from method was reduced by passing it through 40# sieve (Table 11). Due to its small particle size each particle swell to greater extent which results in higher swelling index. Mucilage extracted from Method swell to greater extent as compared to mucilage extracted from other methods. The mucilage with higher swelling index swell to greater extend forming sticky and viscous solution and can be used as gelling, suspending, binding and disintegrating agent in different pharmaceutical formulations (Table 12).
Sr. No |
Name | Average Disintegration Time |
|---|---|---|
| 1 | Fenugreek seed | 3 min 10 sec |
| 2 | Flax seed | 4 min 27 sec |
| Concentration | Absorbance |
|---|---|
| (µg/ml) | |
| 0 | 0 |
| 5 | 0.1872 |
| 10 | 0.3071 |
| 15 | 0.4452 |
| 20 | 0.618 |
| 25 | 0.76 |
| Time (min) | Absorbance | Concentration (μg/ml) | conc*d(1 0) | Con (mg/ml) | Conc(mg/5 ml) | Conc (mg/900ml) | cumulitive release | cum% release |
|---|---|---|---|---|---|---|---|---|
| (µg/ml) | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0.2043 | 6.63103 | 66.31 | 0.00663 | 5.96793 | 5.9679 | 59.6793 | 59.6793 |
| 10 | 0.2592 | 8.52413 | 85.243 | 0.00852 | 7.6717 | 7.7048 | 77.048 | 77.0487 |
| 15 | 0.3082 | 9.2137 | 92.137 | 0.0102 | 9.1924 | 9.235 | 92.3503 | 92.3503 |
Hardness Test
Hardness test was performed on different manufacturer tablets and on in-house tablet as per Indian Pharmacopoeia. Result of hardness test of different manufacturer tablets were within range of 4 – 10 kg/ cm2.
Hardness of Fenugreek and Flax seed In-house was found to be 3 and 4.5 kg/cm2 respectively.
Friability Test
Friability test was performed using Roche Friabilator on 6.5 gof in-house tablet as per Indian Pharmacopoeia. % Friability of different manufacturer tablets and in-house tablet was not more than 1% and passes the friability test (Table 13).
Time (min) |
Absorbance | concentration (μg/ml) | conc*d (10) | Con (mg/ml) | Conc(mg/5 ml) | conc(mg/9 00ml) | cumulitive release | cum% release |
|---|---|---|---|---|---|---|---|---|
| (μg/ml) | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0.2123 | 6.906897 | 69.0689 | 0.006907 | 0.034534 | 6.2162 | 6.2162 | 62.162 |
| 10 | 0.2782 | 9.17931 | 91.7931 | 0.009179 | 0.045897 | 8.2613 | 8.2959 | 82.9591 |
| 15 | 0.3224 | 9.370345 | 93.034 | 0.010703 | 0.053517 | 9.6331 | 9.679 | 96.79 |
Result of Fenugreek and Flax seed was found to be was found to be 0.591% and 0.421% respectively.
Disintegration Test
The tablet passes the test if all units have disintegrated. Is one or two tablets fails to disintegrate, repeat the test for 12 additional units, not less than 16 out of tablet 18 tablets should disintegrate within the specific limit.As per IP Uncoated tablet limit should be within 15min (Table 14).
| Name | Strength | % Drug Content |
|---|---|---|
| Flax seed | 50mg | 102.5712 |
| Fenugreek seed | 50mg | 101.4716 |
Development of Calibration Curve of Diclofenac sodium: The method employs estimation by straight line equation obtained from calibration curve of diclofenac sodium and obeys Beer's law (Table15). Data straight line was 5-25μg/ml and R2 was found 0.997 in 0.1N HCL (pH1.2) (Figure 6).
| Evaluation | Initial (0 days) | After (15 days) |
|---|---|---|
| Dissolution Fenugreek seed (%) after | 92.35 | 92.063 |
| (30min) | ||
| Dissolution Flaxseed (%) after | 96.79 | 96.66 |
| (30min) | ||
| % Drug Content Fenugreek seed | 101.4716 | 100.98 |
| % Drug Content Flaxseed | 102.5712 | 101.982 |
Conclusion
Present study was to extract the mucilage from different seed of plant to evaluate them as a binder. Here an effort was made to investigate the efficacy of mucilage obtained from seed of Fenugreek and Flax as tablet excipient. A Precipitation of soaked seed in acetone technique was developed to optimize for extraction of mucilage from the seeds of Fenugreek and Flax. Various physico-chemical properties like solubility, swelling ratio, flow property and compressibility were measured.
Mucilage data results
Here the mucilage Yield of fenugreek seed & Flax seed was found to be 6.4% w/w & 15.2%w/w. Similarly fenugreek seed & Flax seed Swelling index (SI) was 1.90, 3.80.Gelling Capacity was 53sec, 1.08sec. Bulk Density (kg/m3) was 0.192, 0.24.Tap Density(kg/m3) 0.208, 0.251. Carr's Index was 7.69, 6.25.Hausner’s Ratio was 1.08, 1.066.Angle of repose was 36.8 & 33.69 reported.
The Diclofenac tablets were prepared by passing ingredient through the sieve no. 40. And direct compression on a rotary tablet press and evaluated for various quality control parameters like hardness, friability and disintegration time. Drug releases from Diclofenac tablets were performed.
Tablets data
Here the evaluation parameter of fenugreek & Flax seed tablet was found to be, Hardness Test was 3kg/cm2&4.5 kg/cm2. Friability Test was 0.591%, 0.421. Disintegration Time was 3min 10sec & 4min 27sec. Assay (%Drug) was 101.47 & 102.57 reported.
Also data of accelerated study & its comparative evaluation of fenugreek & flax seed tablet after 15 day can be said that the above formulation is stable.
Hence, from the comparative study we can conclude that flax seed mucilage can be used as binder in future as an excipient in pharmaceutical industry.
References
- Baveja SK, Rangarao KV, Arora J (1998) “Introduction of natural gums and mucilage as sustaining materials in tablet dosage forms”. Indian J Pharm Sci 50: 89-92.
- Dharmendra S, Surendra JK (2012) Natural excipient - a review. IJPBA 3: 1028- 1034.
- Pandey R, Khuller GK (2004) Polymer based drug delivery systems for mycobacterial infections. Curr drug deliv 1: 195-201.
- Chamarthy SP, Pinal R (2008) Plasticizer concentration and the performance of a diffusion-controlled polymeric drug delivery system. Elsevier 331: 25-30.
- Alonso-Sande M, Teijeiro-OsorioD, Remunan-Lopez C, Alonso M (2009)Glucomannan, a promising polysaccharide for biopharmaceutical purposes. Eur J Pharm Biopharm 72: 453-462.
- Shrinivas K, Prakesh K, Kiran HR, Prasad PM (2003) Study of Ocimumbasilicum and Plantago ovate as disintegrants in the formulation of dispersible tablets. Indian J Pharm Sci 65: 180-183.
- Verma PRP, Razdan B (2003) Studies on Leucaenaleucocephala seed gum: emulsifying properties. J SciInd Res 62: 198-206.
- Ibezim C, Khanna M, Sing S (2000) A study of suspending properties of Anacardiumaccidentale gum. J SciInd Res 59: 1038-1043.
- Guwthamarajan K, Kulkarni TG, Vijayakumar RS, Suresh B (2003) Evalution of Borassusflabellier mucilage as gelling agent. Indian drugs 40: 640-644.
- Kulkarni, Gowthamarajan T G, Brahmajirao BG (2002) Evaluation of binding properties of selected natural mucilage. JSIR 61: 529-532.
- Tiwari PN, Kulmi GS (2004) Performance of Chandrasur (Lepidiumsativum) under different levels of nitrogen and phosphorus. J Med Aromatic Plant Sci 26: 479-481.
- Avachat AM, Dash RR, Shrotriya SN (2011) Recent investigation of plant based gums, mucilages and resins in novel drug delivery system. IJPR 45: 86-99.
- Basu SK (2006) Seed production technology for fenugreek (Trigonellafoenum- graecum L.) in the Canadian. Master of Science Thesis. DeparT Biological Sci Uni of Lethbridge Alberta Canada 202-269.
- Huang H, Zhang S (2006) Flaxseed nutrient composition and application in food industry. Food Res Dev 27: 147-149.
- Morris DH (2003) Flax: A Health and Nutrition Primer. 3rd ed Flax Council of Canada Winnipeg MB Canada 11-33.
- Shim Y, Y GUI, B Arnison PG, Wang Y, Reaney MJT (2014) Flaxseed (Linumusitatissimum L.) bioactivecompounds and peptide nomenclature: A review. Trends Food Sci Technol 38: 5-20.
- Dwek R (1996) Glycobiology, “Towards understanding the function of sugars”. Chemical Review 96: 683- 720.
- Ma F Wang, R Li, X Kang, W Bell A W (2020) “ Physical properties of mucilage polysaccharides from dioscoreaOppositaThunb”. Food Chem 311.
- Amin El S , Poleologu (1973) “Phytochemical and Biological Studies of Some Polysaccharides Isolated From Aloe, Tamarindus, Opuntia, and Citrus”. Res 27 (2): 447-50.
- Whistler RL (1965) "Methods in carbohydrate chemistry" Academic press New York London 5: 18-52.
- GaurhariMandal, Rinaghosh , Amalendu Das (1983) “Charactrisation of polysaccharides of Aloe barbadensis Miller: part 111-structure of an acidic oligosaccharide”. Indian J chem 22: 890-893.
- Andrade LA Nunes, CA Pereira J (2015) “Relationship between the chemical components of taro rhizome mucilage and its emulsifying property”, Food Chem 178: 331-338.
- Mirhosseini H, Amid BT (2012) “A Review study on chemical composition and molecular structure of newly plant gum exudates and seed gums”. Food Res Int 46: 387-398.
- Xin T, Zhang F, Jiang Q, Chen C, Huang D, et al. (2012) “Extraction, purification and antitumor activity of a water-soluble polysaccharide from the roots of Polygala tenuifolia”. Carbohydrate Polymers 90: 1127- 1131.
- Huang Y, Chaw C, Hsiang T Y (2012) Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem 134: 1967-1972.
- Peng Y, Zhang L, Zhang Y, Xu X, Kennedy JF (2005) Solution properties of water- insoluble polysaccharides from the mycelium of Ganodermatsugae. Carbohydrate Polymer 9: 351-356.
- AliNokhodchi, Hossein Nazemiyeh, Afagh Khodaparast, Tarifeh Sorkh-Shahan, Hadi Valizadeh (2008) An in vitro evaluation of fenugreek mucilage as a potential excipient for oral controlled-release matrix tablet. 34 (3): 323- 329.
- Rishabha Malviya (2020) Techniques of Mucilage and Gum Modification and their Effect on Hydrophilicity and Drug Release. Recent Pat Drug Deliv Formul. 14(3): 214-222.
- Waghmare R, Moses JA, Anandharamakrishnan C (2022) Mucilages, “Sources, extraction methods, and characteristics for their use as encapsulation agents”. Crit Rev Food Sci Nutr 62: 1-22.
- Tavares LS, Junqueira (2017) “Cold extraction method of chia seed mucilage ( Salvia hispanica L.): effect on yield and rheological behavior”.
- Ibukunoluwa Fola Olawuyi (2021) Application of plant mucilage polysaccharides and their techno-functional properties' modification for fresh produce preservation. Carbohydr Polym 15(272): 118-371.
- Ali Nokhodchi (2008) An in vitro evaluation of fenugreek mucilage as a potential excipient for oral controlled-release matrix tablet. Drug Dev Ind Pharm 34(3): 323-329.
- Surya Narayan Ratha Adhikari (2017) Buccal patches of atenolol formulated using fenugreek (Trigonella foenum-graecum L.) seed mucilage. Polim Med 47(1): 5-11.
- Sara Basiri (2018) Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr Polym 187: 59-65.
- Romain Roulard (2016) Molecular investigations of flaxseed mucilage polysaccharides. Int J Biol Macromol 86: 840-847.
- Lucas Silveira Tavares (2018) Cold extraction method of chia seed mucilage (Salvia hispanica L.): effect on yield and rheological behavior. J Food Sci Technol 55: 457-466.
- Whistler RL (1965)" Methods in carbohydrate chemistry" Academic press New York London 5: 18.
- Kulkarni, Gowthamarajan TG, Brahmajirao BG (2002) Evaluation of binding properties of selected natural mucilages. JSIR 61: 529-532.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Gupta M (2023) Extraction of Mucilage and its Comparative Evaluation as a Binder. Clin Pharmacol Biopharm, 12: 312. DOI: 10.4172/2167-065X.1000312
Copyright: © 2023 Gupta M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2909
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2575
- PDF downloads: 334