Expression profiles and functional roles of BRCC3 and NLRP3 in malignant transformation of endometriosis
Received: 11-Jan-2023 / Manuscript No. cmb-23-86706 / Editor assigned: 13-Jan-2023 / PreQC No. cmb-23-86706(PQ) / Reviewed: 27-Jan-2023 / QC No. cmb-23-86706 / Revised: 01-Feb-2023 / Manuscript No. cmb-23-86706(R) / Accepted Date: 03-Feb-2023 / Published Date: 15-Feb-2023 DOI: 10.4172/1165-158X.1000256
Abstract
Objective: The mechanisms underlying the progression from endometriosis to endometriosis-associated ovarian cancer (EAOC) are poorly understood. Previous studies suggest that NLRP3 inflammasome plays an important role in inflammatory response associated with tissue damage or malfunction. The present study aimed to explore the role of NLRP3 inflammasome activation during the progression of endometriosis to EAOC.
Method: The clinical tissue samples of endometrium, endometriosis, and EAOC were collected. The expression patterns of NLRP3, caspase-1 and IL-1β, as well as BRCC3, an upstream regulator of NLRP3, were investigated. Furthermore, the functional role of BRCC3 in endometriosis malignant transformation into EAOC was explored.
Results: Our study showed that activation of NLRP3 inflammasome occurred in EAOC and endometriosis samples compared to the normal endometrium. NLRP3 expression was also positively correlated with FIGO stage, and negatively associated with tumor differentiation. Moreover, BRCC3 was significantly increased in EAOC and endometriosis, positively correlated with to NLRP3 in EAOC tissues. Functionally, our study demonstrated that BRCC3 overexpression significantly promoted cell proliferation, inhibited apoptosis, and enhanced migration and invasion of endometriosis cells. Conversely, knockdown of BRCC3 suppressed such effects in endometriosis cells. Mechanically, BRCC3 activated NLRP3 expression, and NLRP3 knockdown reversed the effects of BRCC3 on endometriosis cells.
Conclusion: In conclusion, BRCC3 could regulate NLRP3 expression, thereby inducing malignant transformation of endometriosis to EAOC.
Keywords
Endometriosis; Endometriosis-Associated Ovarian Cancer; BRCC3; NLPR3
Introduction
Endometriosis is an inflammatory gynecological disease affecting about 10% of reproductive-aged women and associated with chronic pelvic pain or infertility [1]. It has been well-established that endometriosis patients have an increased risk for developing the certain type of ovarian cancers, named as Endometriosis-Associated Ovarian Cancer (EAOC) [2]. A variety of cancer-related somatic mutations have been reported in endometrioma, leading to silencing of tumor suppressor genes (i.e., TP53 and PTEN) and activation of oncogenes (i.e., KRAS and PIK3CA) [3, 4]. However, it remains unknown about the molecular pathogenesis of EAOC.
The nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) is important in inflammatory response associated with tissue damage or malfunction [5-7]. It has been suggested that NLRP3 inflammasome is involved in the pathogenic mechanism of endometriosis [8, 9]. A recent study has found that NLRP3/Caspase-1/interleukin-1β (IL-1β)-mediated pyroptotic pathway is a critical event for the pathogenesis of endometriosis [10].
The upstream regulators responsible for NLRP3 inflammasome activation has been extensively studies6. BRCC3 is an E3 ubiquitin ligase, and its expression is associated with increased cell proliferation [11]. Several studies have reported that BRCC3 could posttranscriptionally regulate NLRP3 activation [12, 13]. However, it remained unknown whether BRCC3-NLRP3 axis is involved in the endometriosis and EAOC. Herein, we explored the expression pattern and clinical significance of NLRP3 and its upstream regulator BRCC3. Furthermore, the functional role of BRCC3-NLRP3 axis in the progression of endometriosis to EAOC was investigated.
Materials and Methods
Clinical samples
Participants were enrolled from the Maternal and Child Health Hospital of Changning District, Shanghai. The study design was approved by the ethics committee of the local hospital. A total of 45 samples were obtained from patients with normal endometrium (n=15), endometriosis (n=15), and EAOCs (n=15). Endometriomas and EAOC was diagnosed and confirmed by histological examination of laparoscopic biopsy.
Immunohistochemistry (IHC) analysis
Immunohistochemistry (IHC) for caspase-1, NLRP3, IL-1β, and BRCC3 (Cell Signaling Technology, MA, USA) was performed. Specifically, caspase-1 (1:200), NLRP3 (1:200), IL-1β (1:200), and BRCC3 (1:200) were incubated overnight at 4 ℃, followed by secondary antibodies. Tissue sections were stained with diaminobenzidine and images were taken under a microscope.
Cell culture
Endometriosis cell line CRL-7566 was cultured in RPMI-1640 medium with 10% FBS. Cells were cultured at 37 °C with 5% CO2.
MTT assay
The cell proliferation was detected by MTT assay. To be brief, cells (3000 cells/well) were seeded in 96-well plates, and added with 10 μl MTT (Sigma, USA). After 5 h incubation, the medium was replaced with DMSO (100 μl). After being incubated for 20 min in the dark, the absorbance at 570 nm was measured using a micro plate reader.
Colony formation assay
Cells were seeded into 12-well plates, and cultured for 14 days. Survival cells were washed with PBS, stained with 2% crystal violet, and observed under a microscopy. All tests were performed in triplicate.
Transwell assays
Transwell chambers (Corning) were used for migration and invasion assays, precoated without or with matrigel (Sigma), respectively. The cells were cultured in the upper layer of the Transwell chambers for 48 h in an incubator at 37 °C. The migrated or invaded cells were fixed and stained with 0.1% crystal violet. Five fields were randomly selected and the numbers of stained cells were counted under a microscope.
RT-qPCR
Total RNAs were extracted by using TRIzol reagent (Thermo Fisher Scientific, USA), and reversely transcribed into cDNA utilizing PrimeScript RT reagent Kit (Takara, China). PCR program was conducted with an initial denaturation for 2 min at 95°C, followed by 28 cycles of 30 sec at 95°C and 60 sec at 60°C. β-actin was used as a control. The primer sequences were as follows: BRCC3 forward, 5′- GAGTCTGACGCTTTCCTCGTT -3′ and reverse, 5′- TGTATCATCGTTCAACTCCCCT -3′; and GAPDH forward, 5′- GTGACGTTGACATCCGTAAAGA -3 and reverse, 5′- GCCGGACTCATCGTACTCC -3′. Relative gene expression was calculated by using the 2−ΔΔCq method.
Flow cytometry
Cell apoptosis was detected with FITC-Annexin V and PI double labeling using an Annexin V-FITC/PI Apoptosis Detection Kit (Cell Signaling Technology, USA). Briefly, cells were suspended in a binding buffer and labeled with Annexin V-FITC and PI according to the manufacturer's instructions. After incubation for 15 min in the dark, the percentage of apoptotic cells was measured by flow cytometry. All tests were performed in triplicate.
Western blot analysis
Total protein samples were subject to 12% SDS-PAGE, and then transferred to a polyvinylidene difluoride membrane (Millipore, USA). After being blocked with 5% non-fat milk, the membranes were incubated overnight at 4°C with the primary antibodies against BRCC3, NLRP3, MMP2, MMP9, caspase-1, and IL-1β (Cell Signaling Technology), followed by incubation with the secondary antibodies. The protein signals were detected utilizing the enhanced chemiluminescent reagent (Millipore, Billerica, MA, USA).
Statistical analysis
All values were expressed as the mean ± Standard Deviation (SD). Data were analyzed using SPSS software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance or Student's t-test was used to determine the statistical significance of differences between groups. Values of P<0.05 were considered statistically significant.
Results
Demographic and disease characteristics
We collected 45 samples including normal endometrium (n=15), endometriosis (n = 15), and EAOCs (n = 15). The disease characteristics of this cohort were summarized in Table 1. The patients with EAOC were older than those with endometriosis (mean age of 54.3 versus 36.7 years old). Most patients with EAOC had an early stage (13 patients, 86.7%) and had tumors of clear cell and endometrioid histology (33.3% and 66.7%, respectively). Patients with EAOC and endometriosis have comparable times of pregnancies (mean time of 1.27 versus 1.4).
| EAOC | Endometriosis | P | |
|---|---|---|---|
| Age (mean, SD) | 54.3 | 36.7 | <0.05 |
| Pregnancy (n) | 1.27 | 1.4 | >0.05 |
| Abortion (n) | 0.33 | 0.6 | >0.05 |
| Length of tumor or cyst (cm) | 10.43 | 7.33 | <0.05 |
| CEA (ng/ml) | 1.68 | 1.87 | >0.05 |
| AFP (ng/ml) | 3.56 | 3.21 | >0.05 |
| Ca125 (U/mL) | 79.85 | 63.73 | <0.05 |
| Ca199 (U/mL) | 48.42 | 24.53 | <0.05 |
Table 1: Demographic and disease characteristics.
Immuno-histochemical analysis for NLRP3 expression in patients with EAOC and endometriosis
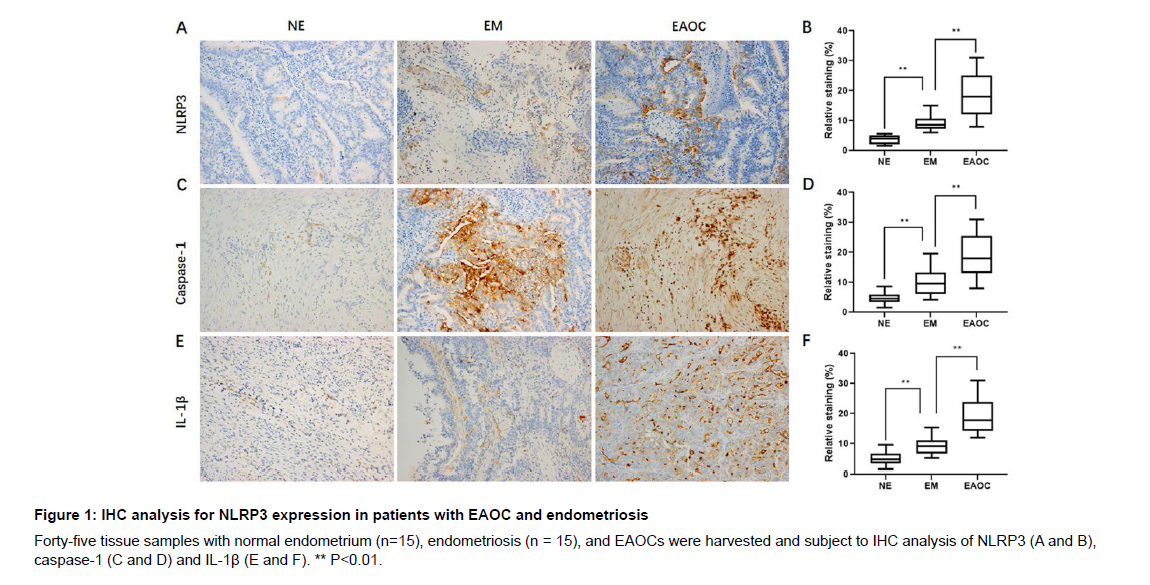
To evaluate the significance of NLRP3 inflammasome in malignant transformation from endometriosis, we immune-stained them with anti-caspase-1, anti-NLRP3, and anti-IL-1β antibodies. We found increased protein levels of NLRP3 (Figure 1A), caspase-1 (Figure 1C) and IL-1β (Figure 1E) in EAOC and endometriosis samples in comparison to normal endometrium. In addition, the protein levels of NLRP3 (Figure 1A), caspase-1 (Figure 1C) and IL-1β (Figure 1E) were elevated in EAOC when compared to those in the endometriosis samples. Quantification of NLRP3 (Figure 1B), caspase-1 (Figure 1D) and IL-1β (Figure 1F) levels in all samples showed a higher NLRP3 level in both EAOC and endometriosis samples when compared to normal endometrium samples. Consistently, quantified protein levels of NLRP3 (Figure 1B), caspase-1 (Figure 1D) and IL-1β (Figure 1F) was also higher than those in the endometriosis samples.
NLRP3 correlates with the clinicopathologic features in EAOC patients
We further examined the association of NLRP3 expression level with the clinical parameters in patients with EAOC, such as FIGO stage, differentiation, and biochemical index. NLRP3 expression level exhibited a significant positive correlation with FIGO stage (Figure 2A), and a negative association with tumor differentiation (Figure 2B). NLRP3 expression was not found significantly associated with tumor length and biochemical parameters.
Immuno-histochemical analysis for BRCC3 expression in patients with EAOC and endometriosis
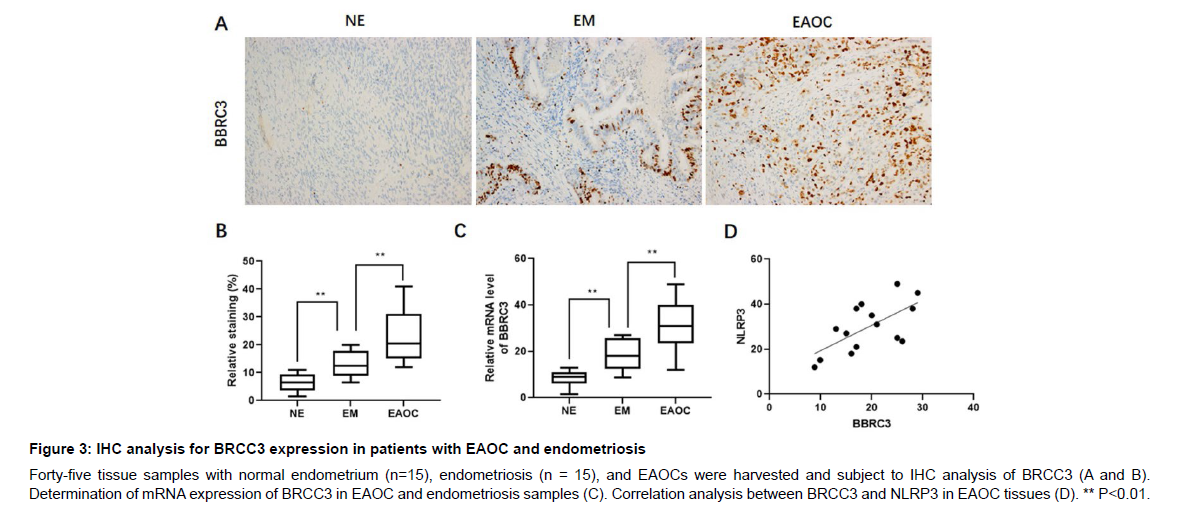
BRCC3 has been reported to be an upstream regulator of NLRP3 [13], we therefore evaluated the clinical significance of BRCC3 and its association with NLRP3 in these tissue samples. We found that BRCC3 protein level was significantly increased in EAOC and endometriosis samples compared to that of normal endometrium (Figure 3A and B). In addition, mRNA expression of BRCC3 was also elevated in EAOC and endometriosis samples. Particularly, the protein and mRNA levels of BRCC3 in EAOC samples were dramatically higher than those in the endometriosis samples (Figure 3C). Furthermore, correlation analysis showed that BRCC3 was positively related to NLRP3 in EAOC tissues (Figure 3D).
BRCC3 promotes malignant transformation of endometriosis
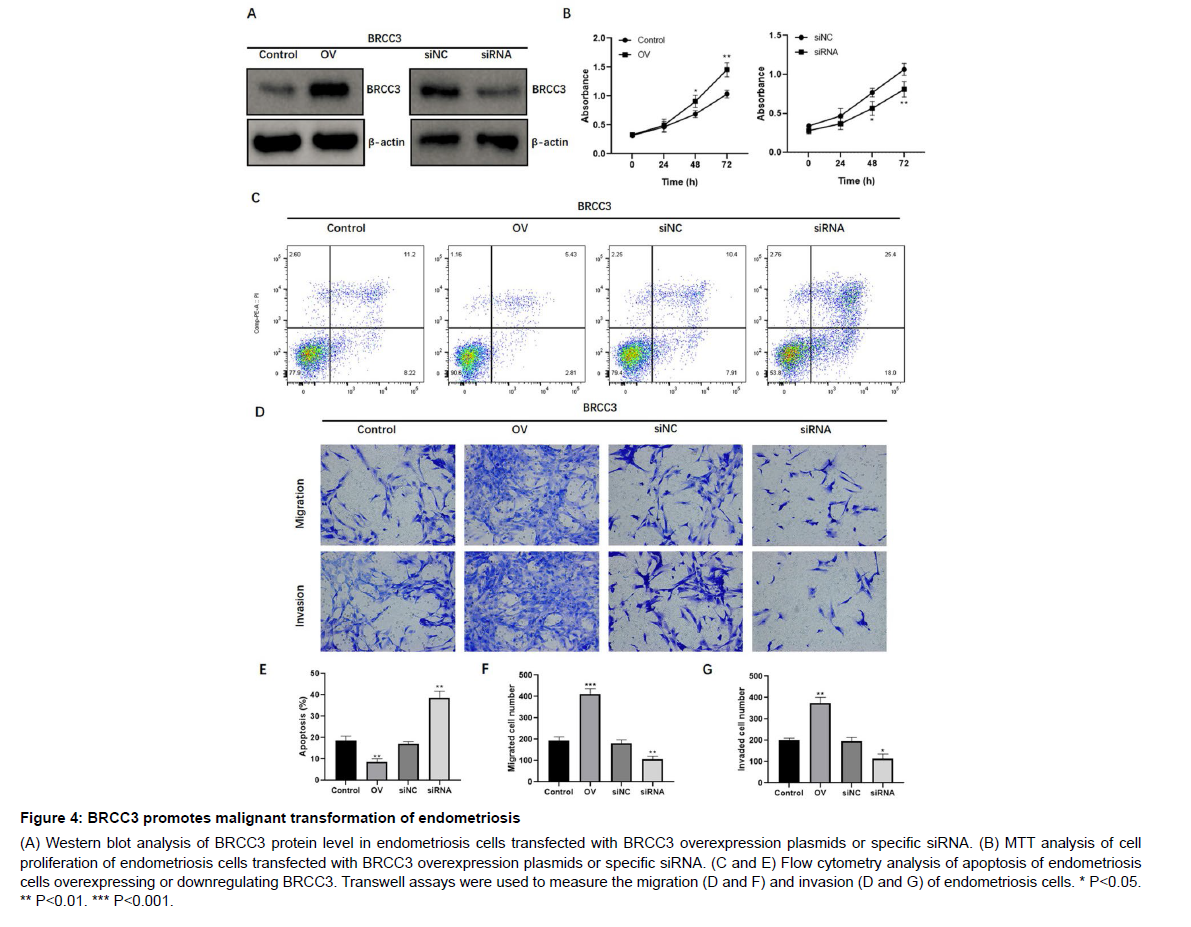
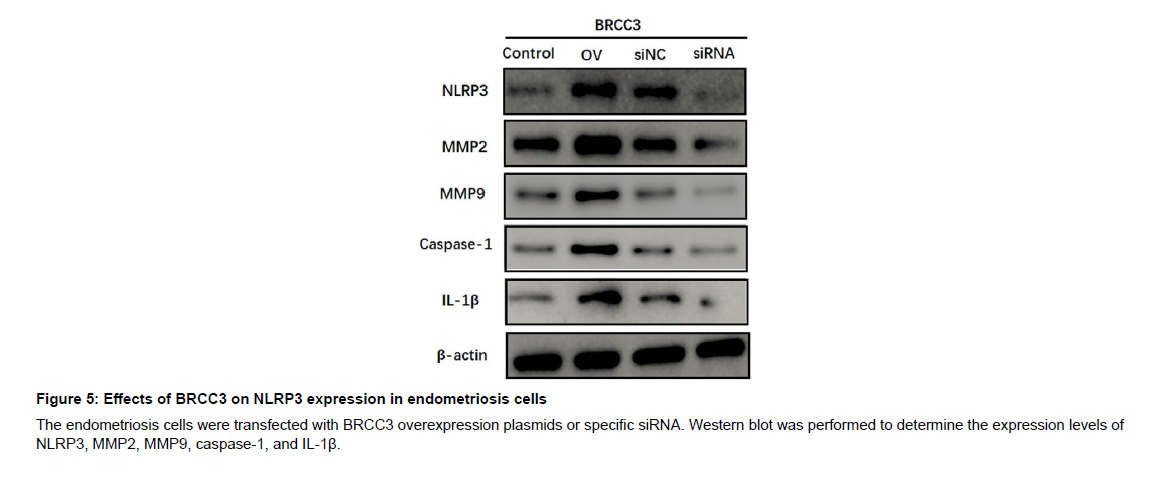
To explore the functional role of BRCC3 in endometriosis malignant transformation, the endometriosis cell line CRL-7566 was transfected with BRCC3 overexpression plasmids or specific siRNA, respectively (Figure 4A). By conducting cell proliferation assay, we found that overexpression of BRCC3 in CRL-7566 cells notably increased cell proliferation (Figure 4B), while knockdown of BRCC3 significantly decreased cell proliferation (Figure 4B). In addition, BRCC3 overexpression significantly inhibited apoptosis whereas inhibition of BRCC3 promoted apoptosis in CRL-7566 cells (Figure 4C and E). Transwell assays revealed that BRCC3 dramatically enhanced the migration and invasion, while knockdown of BRCC3 suppressed such effects in endometriosis cells (Figure 4D, F and G). Furthermore, western blot analysis showed that overexpression of BRCC3 significantly increased the expression levels of NLRP3, MMP2/9, caspase-1, and IL- 1β, whereas depletion of BRCC3 suppressed their protein expression (Figure 5). Collectively, these data showed that BRCC3 could induce malignant changes of endometriosis cells.
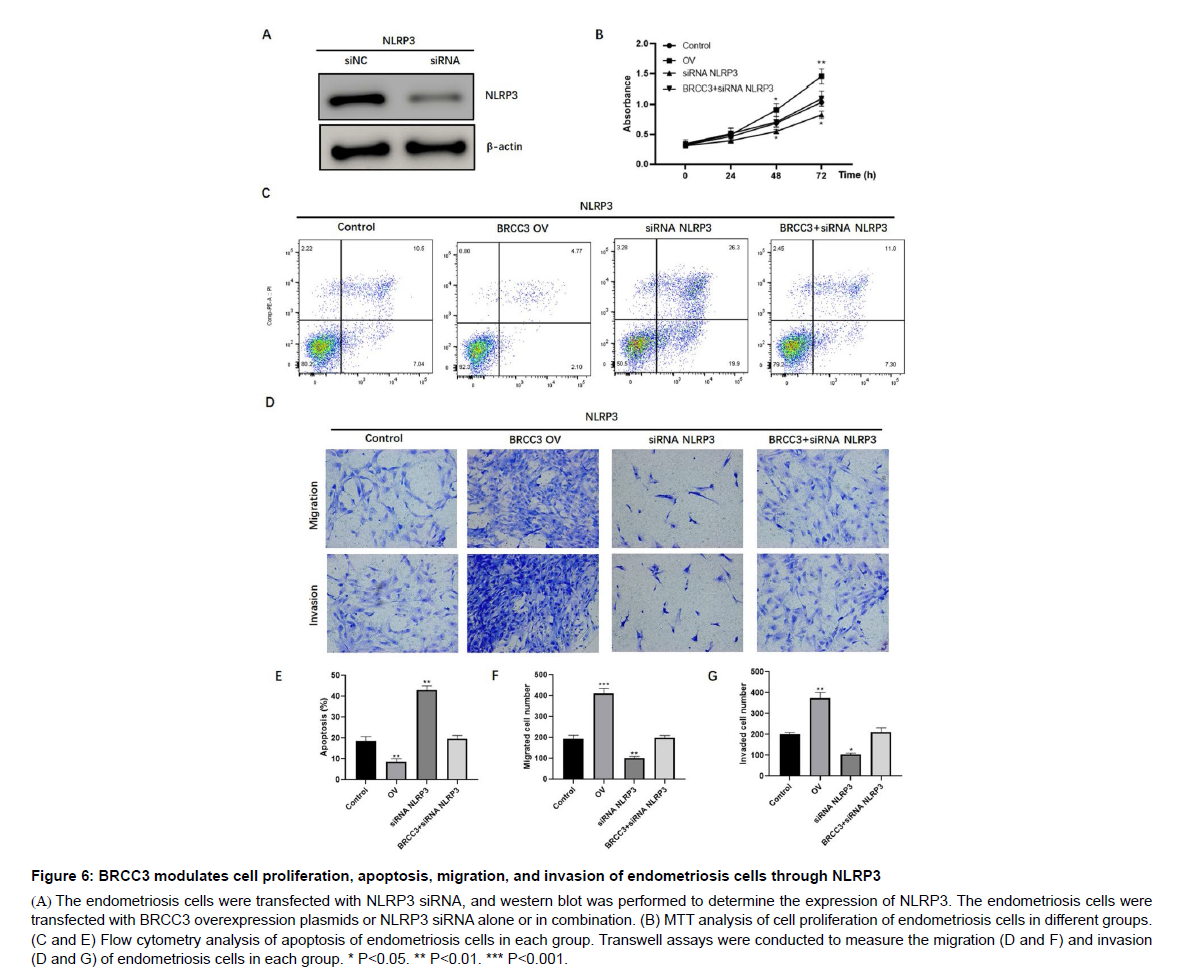
BRCC3 modulates cell proliferation, apoptosis, migration, and invasion of endometriosis cells through NLRP3
Considering the regulative role of BRCC3 on NLRP3 expression, we speculated that BRCC3 regulates endometriosis malignant transformation via NLRP3. To validate such hypothesis, we firstly knocked down of NLRP3 by siRNA (Figure 6A). Consequently, depletion of NLRP3 significantly decreased cell proliferation, and reversed BRCC3-induced overexpression of CRL-7566 cells (Figure 6B). Moreover, overexpression of BRCC3 could inhibit apoptosis (Figure 6C and E), and enhance migration (Figure 6D and F) and invasion (Figure 6D and G) of CRL-7566 cells; these effects were abrogated by NLRP3 knockdown (Figure 6C-G). These results suggest that BRCC3 promotes the malignant phenotype of endometriosis cells through NLRP3.
Discussion
Although endometriosis is usually considered benign, its biological behaviors exhibit some common features with ovarian malignancies, such as cell proliferation, apoptosis, migration, and invasion [14]. It has been widely acknowledged that endometriosis may increase the risk of epithelial ovarian cancer. However, it remains unknown about the pathological mechanism of endometriosis malignant transformation to EAOC [15]. This study evaluated the expression and functional role of NLRP3 inflammasome in endometriosis. Our findings showed that activation of NLRP3 inflammasome and its upstream BRCC3 occurred and induced malignant transformation of endometriosis cells.
Endometriosis and ovarian cancer share abnormal immunological and apoptotic features in the peripheral blood and peritoneal fluids [14, 16]. Several genes are found to be associated with tumor development in endometriotic tissues from patients with endometriosis [14, 17-19]. Inflammatory responses play critical roles for tumorigenesis and are also capable of disturbing the tumor responses to therapy. It has been found that inflammasome-related genes (e.g., NLRP3, IL-1, TLR1, TNF) are differentially expressed in endometriosis and EAOC, and correlated with poor progression-free survival, suggesting a vital role of inflammasome in EAOC carcinogenesis [20]. NLRP3 is a complex protein involved in the inflammatory responses. Our study found that caspase-1, NLRP3, and IL-1β were significantly activated in EAOC and endometriosis compared to normal endometrium. NLRP3 expression was also correlated with clinical parameters in patients with EAOC, such as FIGO stage and differentiation.
Increasing evidence has revealed that BRCC3, an upstream regulator of NLRP3, promotes inflammasome activation by deubiquitinating NLRP3 [13, 21-23]. Our data suggested that BRCC3 levels were upregulated in EAOC and endometriosis, positively correlated with NLRP3. Deregulation of BRCC3 is associated with tumor progression [24, 25]. To unravel the functional role of BRCC3 in endometriosis cells, we performed in vitro assays with manipulating BRCC3 in the endometriosis cell line. Data showed that BRCC3 overexpression induced cell overgrowth, inhibited apoptosis, and enhanced migration and invasion of endometriosis cells. Conversely, knockdown of BRCC3 suppressed such effects in endometriosis cells. Furthermore, we found that BRCC3 activated NLRP3 expression, and NLRP3 knockdown could abrogate the effects of BRCC3 on endometriosis cells, suggesting that RCC3-NLRP3 axis is critical for inflammasome activation and malignant transformation in endometriosis.
Conclusions
In conclusion, the present study demonstrates that BRCC3 could regulate NLPR3 expression, and promote cell proliferation, migration, and invasion, and inhibit apoptosis rate. The RCC3-NLRP3 axis might be a key target to prevent the malignant conversion of endometriosis to EAOC.
Abbreviation
Endometriosis-Associated Ovarian Cancer, EAOC; Nucleotidebinding domain, leucine-rich-containing family, pyrin domaincontaining- 3, NLRP3; caspase-1 recruitment domain, ASC; interleukin- 1β, IL-1β; Immunohistochemistry, IHC; BRCA1/BRCA2 Containing Complex Subunit 3, BRCC3
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research was supported by Changning District Science and Technology Committee Project (CNKW2018Y18).
Ethics statement
The protocol was approved by the Institutional Review Board of Changning Maternity and Infant Health Hospital.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
References
- Vercellini P, Viganò P, Somigliana E, Fedele L (2014) Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 10: 261-275.
- Herreros-Villanueva M, Chen CC, Tsai EM, Er TK (2019) Endometriosis-associated ovarian cancer: What have we learned so far? Clin Chim Acta 493: 63-72.
- Ruderman R, Pavone ME (2017) Ovarian cancer in endometriosis: an update on the clinical and molecular aspects. Minerva Ginecol 69: 286-294.
- Murakami K, Kotani Y, Nakai H, Matsumura N (2020) Endometriosis-Associated Ovarian Cancer: The Origin and Targeted Therapy. Cancers (Basel) 12: 1676.
- Song N, Li T (2018) Regulation of NLRP3 Inflammasome by Phosphorylation. Front Immunol 9: 2305.
- Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci 20: 3328.
- Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, et al. (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17: 588-606.
- Bullon P, Navarro JM (2017) Inflammasome as a Key Pathogenic Mechanism in Endometriosis. Curr Drug Targets 18: 997-1002.
- Zhou F, Li C, Zhang SY (2020) NLRP3 inflammasome: a new therapeutic target for high-risk reproductive disorders? Chin Med J (Engl) 134: 20-27.
- Hang Y, Tan L, Chen Q, Liu Q, Jin Y (2021) E3 ubiquitin ligase TRIM24 deficiency promotes NLRP3/Caspase-1/IL-1β-mediated pyroptosis in endometriosis. Cell Biol Int 45: 1561-1570.
- Boudreau HE, Broustas CG, Gokhale PC, Kumar D, Mewani RR, et al. (2007) Expression of BRCC3, a novel cell cycle regulated molecule, is associated with increased phospho-ERK and cell proliferation. Int J Mol Med 19: 29-39.
- Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331-338.
- Rao Z, Chen X, Wu J, Xiao M, Zhang J, et al. (2019) Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination. Front Immunol 10: 2783.
- Prowse AH, Manek S, Varma R, Liu J, Godwin AK, et al. (2006) Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer 119: 556-562.
- Anglesio MS, Yong PJ (2017) Endometriosis-associated Ovarian Cancers. Clin Obstet Gynecol 60: 711-727.
- Haidarali E, Vahedi A, Mohajeri Sh, Mostafidi E, Azimpouran M, et al. (2016) Evaluation of the Pathogenesis of Tumor Development from Endometriosis by Estrogen Receptor, P53 and Bcl-2 Immunohistochemical Staining. Asian Pac J Cancer Prev 17: 5247-5250.
- Xu XR, Wang X, Zhang H, Liu MY, Chen Q (2018) The clinical significance of the combined detection of serum Smac, HE4 and CA125 in endometriosis-associated ovarian cancer. Cancer Biomark 21: 471-477.
- Barreta A, Sarian LO, Ferracini AC, Costa LBE, Mazzola PG, et al. (2019) Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometrioid carcinomas (type I) and endometriosis. Hum Pathol 85: 72-81.
- Andersen CL, Boisen MM, Sikora MJ, Ma T, Tseng G, et al. (2018) The Evolution of Estrogen Receptor Signaling in the Progression of Endometriosis to Endometriosis-Associated Ovarian Cancer. Horm Cancer 9: 399-407.
- Chang CM, Wang ML, Lu KH, Yang YP, Juang CM, et al. (2017) Integrating the dysregulated inflammasome-based molecular functionome in the malignant transformation of endometriosis-associated ovarian carcinoma. Oncotarget 9: 3704-3726.
- Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331-338.
- Cheng X, Xu S, Zhang C, Qin K, Yan J, et al. (2020) The BRCC3 regulated by Cdk5 promotes the activation of neuronal NLRP3 inflammasome in Parkinson's disease models. Biochem Biophys Res Commun 522: 647-654.
- Singh M, Kumari B, Yadav UCS (2019) Regulation of oxidized LDL-induced inflammatory process through NLRP3 inflammasome activation by the deubiquitinating enzyme BRCC36. Inflamm Res 68: 999-1010.
- Zhang F, Zhou Q (2018) Knockdown of BRCC3 exerts an anti-tumor effect on cervical cancer in vitro. Mol Med Rep 18: 4886-4894.
- Hu Y, Zhang Y, Ding M, Xu R (2020) Long noncoding RNA TMPO-AS1/miR-126-5p/BRCC3 axis accelerates gastric cancer progression and angiogenesis via activating PI3K/Akt/mTOR pathway. J Gastroenterol Hepatol 36: 1877-1888.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Liu Y, Yang Y, Wu WQ, Zhang WY, Huang YH, et al. (2023) ExpressionProfiles and Functional Roles of BRCC3 and NLRP3 in Malignant Transformationof Endometriosis. Cell Mol Biol, 69: 256 DOI: 10.4172/1165-158X.1000256
Copyright: © 2023 Liu Y, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2200
- [From(publication date): 0-2023 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1907
- PDF downloads: 293