Research Article Open Access
Expression of the Sortilin Gene in Cultured Human Keratinocytes Increases in a Glucose-free Medium
| Kyoichi Matsuzaki1,2*, Miyuki Tomioka3, Yuichi Watabe3 | ||
| 1 Director, Department of Plastic and Reconstructive Surgery, Kawasaki Municipal Tama Hospital, Japan | ||
| 2 Associate Professor, Department of Plastic and Reconstructive Surgery, St. Marianna University School of Medicine, Japan | ||
| 3 Researcher, Department of Plastic and Reconstructive Surgery, St. Marianna University School of Medicine, Japan | ||
| Corresponding Author : | Kyoichi Matsuzaki Director, Department of Plastic and Reconstructive Surgery Kawasaki Municipal Tama Hospital 1-30-37 Shukugawara, Tama-Ku Kawasaki 214-8525 Japan Tel: +81-44-933-8111 Fax: +81-44-933-8362 E-mail: k4matsu@marianna-u.ac.jp |
|
| Received December 05, 2013; Accepted January 20, 2014; Published January 25, 2014 | ||
| Citation: Matsuzaki K, Tomioka M, Watabe Y (2014) Expression of the Sortilin Gene in Cultured Human Keratinocytes Increases in a Glucose-free Medium. Clin Res Foot Ankle S3:004. doi:10.4172/2329-910X.S3-004 | ||
| Copyright: © 2014 Matsuzaki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Clinical Research on Foot & Ankle
Abstract
Introduction: When proNGF binds to sortilin and p75, the cell death signal is transduced into the cell. This study examined how sortilin gene expression changes in cultured human epidermal cells depending on the glucose concentration in the culture medium. The results might help elucidate if sortilin in epidermal cells is involved in the delay of wound healing in diabetes.
Materials and methods: Epidermal cells were grown in a serum-free, glucose-free culture medium for 24 h. The new medium was prepared with a glucose concentration of 0, 20, 40, 60, 100, 200, 400, or 800 mg/dL. The cells were grown in each medium for an additional 24, 48, and 72 h. At each culture time, the sortilin gene expression level at 100 mg/dL glucose was compared with the expression levels at other glucose concentrations.
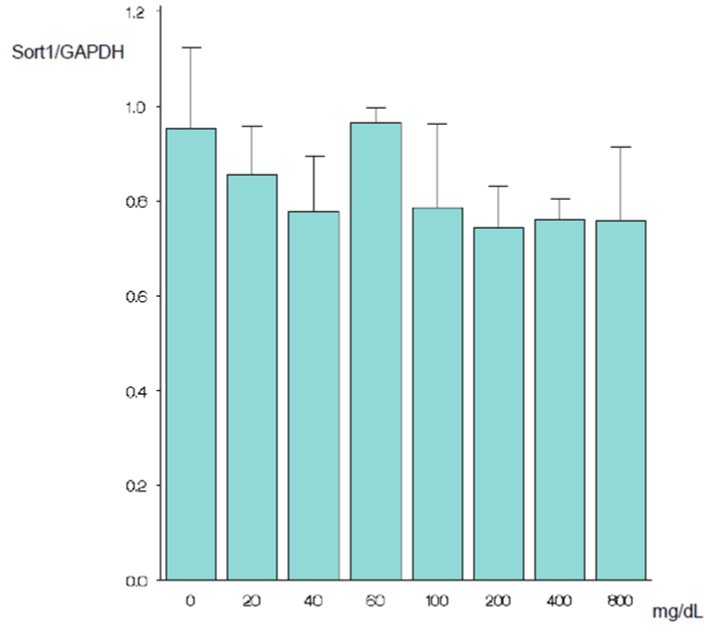
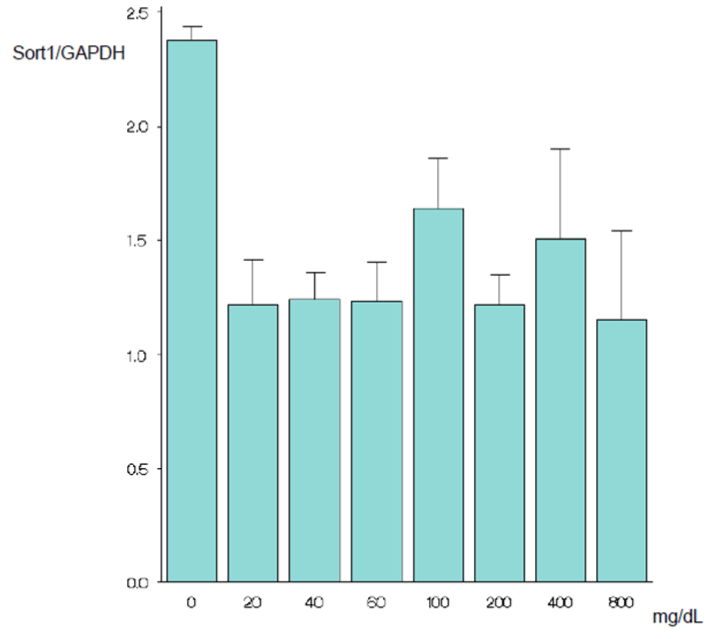
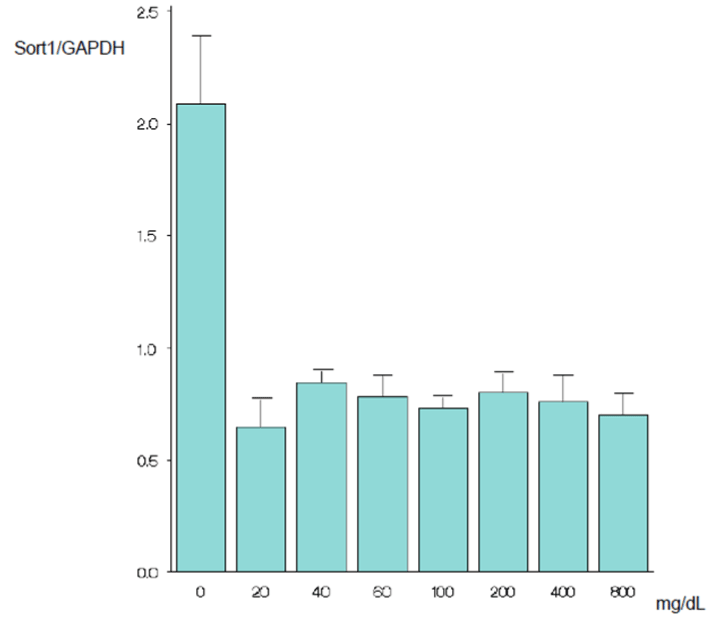
Results and discussion: When the cells were grown in the new medium for 24 h, the sortilin gene expression level at 100 mg/dL glucose did not differ significantly from the expression levels at other glucose concentrations. At 48 h of culture, only the sortilin gene expression level at 0 mg/dL glucose was significantly higher than the expression level at 100 mg/dL glucose (p=0.002). At 72 h of culture, only the expression level at 0 mg/dL glucose was significantly higher than the expression level at 100 mg/dL glucose (p<0.001). The results suggest that the sortilin gene levels of epidermal cells are not clinically problematic at low glucose concentrations, just as at high glucose concentrations, provided the glucose levels are sufficient to maintain biological activities.
| Keywords | |
| Diabetes; Wound healing; Diabetic neuropathy; Sortilin; Keratinocyte, High glucose; Low glucose; Nerve growth factor | |
| Introduction | |
| Nerve growth factor (NGF) is involved in the delay of wound healing associated with diabetic peripheral neuropathy. NGF stimulates neurite outgrowth and differentiation via a process mediated by the tyrosine kinase receptor TrkA. Even after neurons mature, NGF is involved in their survival and maintenance of their functions. In addition, NGF has a neuroprotective effect. Therefore, it inhibits neuronal cell death and is involved in nerve injury repair [1-3]. The NGF precursor proNGF binds to p75, a member of the tumor necrosis factor receptor family. In 2001, it was reported that cell death is induced when proNGF binds to the coreceptor sortilin and p75 [4]. In other words, it was shown that the effects of NGF and proNGF are almost the opposite of each other. | |
| Sortilin is a 95 kDa glycoprotein and a member of the mammalian type-I transmembrane receptor family containing a Vps10p domain [5]. Sortilin is present in intracellular membranes of the central nervous system. It is particularly abundant in the Golgi apparatus and is involved in intracellular molecular transport. ProNGF binds to sortilin and p75, a low-affinity neurotrophin receptor in the cell membrane. Subsequently, the cell death signal is transduced into the cell. For example, if there is a brain injury such as in stroke, the injured cells release proteins, and apoptosis is induced in the surrounding normal cells. Thus, neuronal cell death begins within a few minutes of the stroke. Sortilin plays an important role in this apoptotic command. It is thought to be involved in apoptosis that occurs subsequent to central nervous system diseases such as Alzheimer’s disease [6,7]. In 2010, it was reported that sortilin is also present in the cultured epidermal cells, and proNGF binds to sortilin and p75, resulting in cell death [7]. Our study examined how sortilin gene (SORT1) expression changes in cultured human epidermal cells depending on the glucose concentration in the culture medium. The results might help elucidate if sortilin is involved in the delay of wound healing in diabetes. | |
| Materials and Methods | |
| Epidermal cell culture | |
| Human epidermal cells were from excess normal skin obtained during surgery. The skin was collected from patients after informed consent and approval of the ethics committee of our institution. A growth supplement (Epilife® Defined Growth Supplement, Life technologies Corporation, Carlsbad, USA) was added to a medium for the growth of epidermal keratinocytes (EpiLife®, Life technologies Corporation, CA, USA). The epidermal cells were grown in this medium at 37°C in 5% CO2. These cells were subcultured on 6-well plates to 80% confluence and then cultured for 24 hours in a serumfree, glucose-free medium (HuMedia-KG2, KURABO INDUSTRIES LTD, Tokyo, Japan). This medium was used to prepare a new medium with a glucose concentration of 0, 20, 40, 60, 100, 200, 400, or 800 mg/ dL. The cells were grown in each medium for an additional 24, 48, and 72 hours. | |
| Expression of SORT1 | |
| An RNeasy Mini Kit (Qiagen, Venlo, Netherlands) was used to extract total RNA from epidermal cells cultured under the aforementioned conditions. A SuperScript® VILO™ cDNA Synthesis Kit (Life Technologies Corporation, Carlsbad, CA, USA) was used for reverse transcription of the total RNA into cDNA. A KAPA SYBR® FAST Universal qPCR Kit (KAPA BIOSYSTEMS, Boston, USA) was used for real-time PCR of the resulting cDNA. Rotor Gene Q (Qiagen, Venlo, Netherlands) was used for gene expression analysis. The following primers were used: SORT1 (forward) 5’-TTATCCTGGCCATCGTGGGATTGA-3’, (reverse) 5’-ATTAGTGTGGGAGGCTGTGTCCAA-3’; and GAPDH (forward) 5’-CATGTTCGTCATGGGTGTGAACCA-3’, (reverse) 5’-AGTGATGGCATGGACTGTGGTCAT-3’. | |
| Statistical analysis | |
| The Dunnett multiple comparison test was used to determine statistical differences at a particular culture time between the reactive mRNA levels (SORT1/GAPDH) at 100 mg/dL glucose and the reactive mRNA levels (SORT1/GAPDH) at other glucose concentrations. Statistical analysis software was SAS Ver. 9.4 (SAS Institute Inc., Cary, NC, USA). | |
| Results | |
| As mentioned above, the cells were cultured for 24 hours in a serum-free, glucose-free medium and then cultured further in the new medium. After 24 hours incubation in the new medium, the SORT1 expression level at 100 mg/dL did not differ significantly from the expression levels at other glucose concentrations (Figures 1A and 1B). After 48 hours incubation in the new medium, only the SORT1 expression level at 0 mg/dL glucose was significantly higher than the expression level at 100 mg/dL glucose (p=0.002) (Figures 2A and 2B). After 72 hours incubation in the new medium, only the expression level at 0 mg/dL glucose was significantly higher than the expression level at 100 mg/dL glucose (p<0.001) (Figures 3A and 3B). | |
| Discussion | |
| Epidermal cells produce and secrete NGF in the wound healing process. The secreted NGF causes nerve fibers to sprout and nerves to regenerate, and proliferation and migration of epidermal cells are stimulated in an autocrine fashion [2,3,8,9]. Many reports have investigated the involvement of NGF in the delay of wound healing of diabetic ulcers [1-3,10,11]. Epidermal cells also produce proNGF and have p75 and sortilin. Therefore, an autocrine cell death pathway can be activated. When epidermal cells are grown in a high glucose medium, cell proliferation, differentiation, and mobility are inhibited [12-14]. We speculated that the SORT1 expression levels increase when glucose concentration increases in the medium. In our preliminary experiment, we grew epidermal cells at different glucose concentrations (0, 100, 200, 400, and 800 mg/dL) and examined the SORT1 expression levels. The results were contrary to our prediction, and the SORT1 expression level at 100 mg/dL did not differ significantly from the expression level at higher glucose concentrations. The preliminary experiment had shown that the SORT1 expression levels for 48-hour and 72-hour cultures were higher only at 0 mg/dL glucose compared with the expression levels at 100 mg/dL glucose (unpublished data). Thus, additional glucose concentrations of 20, 40, and 60 mg/dL were used to examine the SORT1 expression levels between 0 mg/dL and 100 mg/dL glucose. The results showed no change in the expression level between 0 mg/dL and 100 mg/dL and the expression level was higher only at 0 mg/dL. In our present study, the cells were cultured in a serum-free, glucose-free culture for 24 hours after the confluence of cultured epidermal cells reached 80%. Subsequently, the cells were grown for 24, 48, and 72 hours in a new medium prepared with a glucose concentration of 0, 20, 40, 60, 100, 200, 400, or 800 mg/dL. Therefore, the cells grown at 0 mg/dL glucose were exposed to a glucose-free environment for a total of 48, 72, or 96 hours. | |
| Glucose concentrations have a major effect on cell activities. Cytokines have been the focus of most studies to date on the delay of wound healing due to hyperglycemia. Diabetes inhibits the migration of epidermal cells because of a decreased level of TGF-β1 [15]. Proliferation and migration of epidermal cells are also inhibited due to decreased EGF produced by the platelets and a decreased response of epidermal cells to EGF [16]. In addition, it has been reported that endothelial progenitor cell mobilization and homing to the wound site are inhibited because SDF-1α production is decreased in epidermal cells [17]. However, no report has focused on sortilin in the delay of wound healing due to high glucose. When diabetes worsens, blood glucose level becomes difficult to control using insulin, and hypoglycemia can result in coma. Poor nutrition, such as that causing hypoglycemia, negatively affects wound healing [18]. In our study, the SORT1 expression level at 100 mg/dL glucose did not differ significantly from the expression levels at low glucose concentrations (20, 40, and 60 mg/dL). In other words, our results suggest that the SORT1 glucose levels of epidermal cells are not clinically problematic at low glucose concentrations, just as at high glucose concentrations, provided the glucose levels are sufficient to maintain biological activities. Further studies are necessary to examine the expression of the proteins sortilin, proNGF, p75, apoptosis cell makers, and necrosis cell markers in epidermal cells and in peripheral nerves. The results might elucidate the involvement of epidermal cell death mediated by sortilin, proNGF, and p75 in diabetic foot ulcers. | |
| Conclusion | |
| In cultured epidermal cells, the SORT1 expression level at a total culture time of 72 hours was significantly higher at 0 mg/dL glucose in the medium compared with at 100 mg/dL glucose. The expression level at a total culture time of 96 hours was significantly much higher. If the medium was not glucose-free, the SORT1 expression levels at 100 mg/dL did not differ significantly from the expression levels at high or low glucose concentrations. Therefore, the results suggest that the SORT1 expression levels of epidermal cells are not clinically problematic, provided the glucose levels are sufficient to maintain biological activities. | |
| Acknowledgements | |
| This work was supported by JSPS KAKENHI Grant Number 23592659. | |
| References | |
References
- value="1" id="Reference_Titile_Link">Yiangou Y, Facer P, Sinicropi DV, Boucher TJ, Bennett DL, et al. (2002) Molecular forms of NGF in human and rat neuropathic tissues: decreased NGF precursor-like immunoreactivity in human diabetic skin. J Peripher Nerv Syst 7: 190-197.
- value="2" id="Reference_Titile_Link">Barker AR, Rosson GD, Dellon AL (2006) Wound healing in denervated tissue. Ann Plast Surg 57: 339-342.
- value="3" id="Reference_Titile_Link">Radtke C, Rennekampff HO, Reimers K, Vogt PM, Kocsis JD (2013) Paracrine loop of keratinocyte proliferation and directed neuritic outgrowth in a neuroepithelial coculture. Ann Plast Surg 70: 162-167.
- value="4" id="Reference_Titile_Link">Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945-1948.
- value="5" id="Reference_Titile_Link">Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77: 579-586.
- value="6" id="Reference_Titile_Link">Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, et al. (2011) Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol 68: 99-106.
- value="7" id="Reference_Titile_Link">Kiss M, Dallos A, Kormos B, Sántha P, Dobozy A, et al. (2010) Sortilin is expressed in cultured human keratinocytes and is regulated by cutaneous neuropeptides. J Invest Dermatol 130: 2553-2560
- value="8" id="Reference_Titile_Link">Di Marco E, Mathor M, Bondanza S, Cutuli N, Marchisio PC, et al. (1993) Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem 268: 22838-22846.
- value="9" id="Reference_Titile_Link">Pincelli C, Marconi A (2000) Autocrine nerve growth factor in human keratinocytes. J Dermatol Sci 22: 71-79.
- value="10" id="Reference_Titile_Link">Bennett NT, Schultz GS (1993) Growth factors and wound healing: Part II. Role in normal and chronic wound healing. Am J Surg 166: 74-81.
- value="11" id="Reference_Titile_Link">Ordoñez G, Fernandez A, Perez R, Sotelo J (1994) Low contents of nerve growth factor in serum and submaxillary gland of diabetic mice. A possible etiological element of diabetic neuropathy. J Neurol Sci 121: 163-166.
- value="12" id="Reference_Titile_Link">Spravchikov N, Sizyakov G, Gartsbein M, Accili D, Tennenbaum T, et al. (2001) Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes 50: 1627-1635.
- value="13" id="Reference_Titile_Link">Terashi H, Izumi K, Deveci M, Rhodes LM, Marcelo CL (2005) High glucose inhibits human epidermal keratinocyte proliferation for cellular studies on diabetes mellitus. Int Wound J 2: 298-304.
- value="14" id="Reference_Titile_Link">Lan CC, Wu CS, Kuo HY, Huang SM, Chen GS (2009) Hyperglycaemic conditions hamper keratinocyte locomotion via sequential inhibition of distinct pathways: new insights on poor wound closure in patients with diabetes. Br J Dermatol 160: 1206-1214.
- value="15" id="Reference_Titile_Link">Wang XJ, Han G, Owens P, Siddiqui Y, Li AG (2006) Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc 11: 112-117.
- value="16" id="Reference_Titile_Link">Rafehi H, El-Osta A, Karagiannis TC (2011) Genetic and epigenetic events in diabetic wound healing. Int Wound J 8: 12-21.
- value="17" id="Reference_Titile_Link">Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, et al. (2007) Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 117: 1249-1259.
- value="18" id="Reference_Titile_Link">Blondet JJ, Beilman GJ (2007) Glycemic control and prevention of perioperative infection. Curr Opin Crit Care 13: 421-427.
Figures at a glance
 |
 |
 |
 |
 |
 |
|||||
| Figure 1A | Figure 1B | Figure 2A | Figure 2B | Figure 3A | Figure 3B |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14690
- [From(publication date):
specialissue-2014 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 10088
- PDF downloads : 4602
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
