Research Article Open Access
Expression of Stem Cells Marker ALDH1 in Premalignant Lesions, Cancer, Benign Hyperplasia and Normal Duct of Human Breast
Aiping Shi1*, Xin Guan1, Lu Han1, Yi Dong2, Wenlong Li1, Angela Vantreese3, Lirong Bi1 and Peng Zhao11Department of Breast Surgery, The First Hospital of Jilin University, Changchun, Jilin, China
2Department of the Second Section Office of Breast Tumor, Jilin Cancer Hospital, Changchun, Jilin, China
3Ballare High School, Houston, USA
- *Corresponding Author:
- Aiping Shi
Department of Breast Surgery
The First Hospital of Jilin University
Changchun, Jilin, China
Tel: +008615804301451
E-mail: 13364308696@163.com
Received date: February 02, 2016 Accepted date: March 14, 2016 Published date: March 21, 2016
Citation: Shi A, Guan X, Han L, Dong Y, Li W, et al. (2016) Expression of Stem Cells Marker ALDH1 in Premalignant Lesions, Cancer, Benign Hyperplasia and Normal Duct of Human Breast . Adv Cancer Prev 1:107. doi:10.4172/acp.1000107
Copyright: © 2016 Shi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Cancer Prevention
Abstract
Purpose: To study the differences of ALDH1 expression in Invasive Ductal Carcinoma (IDC), Atypical Ductal Hyperplasia (ADH), Usual Ductal Hyperplasia (UDH) and normal ducts and their clinical significance.
Materials and Methods: Immunohistochemistry method was used to detect the expression of ALDH1 in 160 cases of IDC, 50 cases of ADH, 20 cases of UDH and 22 normal duct cases.
Results: ALDH1 was expressed in a few cells in most samples except in a few cases of ADH, UDH and normal ducts, in which ALDH1 expression showed extensive distribution. The positive rate of ALDH1 expression in luminal epithelial cytoplasm of IDC, ADH and UDH was 35%, 64% and 80%, while in myoepithelial cells and stroma of IDC, ADH and UDH was 40%, 52% and 70%. ALDH1 expression in luminal epithelial cytoplasm of IDC has correlation with OS and RFS of the breast cancer patients (P<0.05). However, in myoepithelial cells and the stroma of IDC, no correlation was found in OS and RFS (P>0.05). The chi-square test of ALDH1 expression in luminal epithelial cytoplasm of three kinds of tissues has shown statistical significance (P<0.05). Statistical significance was also found in myoepithelial cells and stroma of three kinds of tissues (P<0.05). We evaluated the semiquantitative expression of ALDH1 using a score based on positive cells rate and staining density in benign lesions. The score of ALDH1 in luminal epithelial cytoplasm expression is 3, 2, and 1 while myoepithelial cells and stroma expression are 3, 2, and 2 in ADH,UDH and normal ducts respectively. ALDH1 expression in either luminal epithelial cytoplasm or in myoepithelial cells and stroma in ADH is higher than that in UDH and normal duct, but there is no significance according to the Kruskal-Wallis rank sum test (P>0.05).
Conclusion: The positive expression of ALDH1 may play a role during the evolution of disease from ADH to breast cancer, as ALDH1 has a predicted value in outcome of breast cancer.
Keywords
Stem cells; Cancer; Hyperplasia; Carcinoma; Stroma
Introduction
Recent research demonstrates that tumors originate from the Cancer Stem Cells (CSCs) or Tumor Initiated Cells (TIC) [1], which are called breast cancer stem cellsin breast cancer. The breast cancer stem cells have the ability of infinite proliferation and multi-directional differentiation by themselves. They are also insensitive to chemotherapy or hormone therapy and are able to form tumors after primary treatment; therefore, there is a strong implication that the breast cancer stem cells may be the root of breast cancer recurrence and metastasis [2]. But where are cancer stem cells from? It is well known that normal stem cells or progenitors can transform malignantly to cancer stem cells by accumulating genetic mutation [3-5]. Tomasetti and Vogelstein [6] recently found a highly positive correlation (Spearman’s rho = 0.81; P<3.5 × 10−8) between the number of normal stem cell divisions in a tissue and the risk of cancer in that tissue. This implies that restricting the number of stem cell divisions will reduce the cancer risk [7]. However, this raises the concern of whether stem cells are more common in the mammary premalignant lesions than in normal ducts, UDH, or even cancer, as this may suggest that stem cell divisions are more frequent in premalignant lesion. Proliferation and divisions of stem cells in a premalignant lesion may lead the carcinogenesis.
Atypical Ductal Hyperplasia(ADH) is a frequently detected precancerous lesion in the breast of individuals aged 40 -50. Autopsy studies detected moderate to severe hyperplasia in over 30% of women aged 45-54 [8-10]. ADH is a well-established precursor of breast cancer [11-14]. Women with ADH have approximately fivefold increased risk of developing breast cancer [13-17]. In addition, gene expression profiling of ADH, Ductal CarcinomaIn Situ (DCIS), and Invasive Ductal Carcinoma (IDC) showed that these three stages of breast cancer are highly similar to each other at the transcriptional level [18], further suggesting that ADH is a precursor during breast cancer evolution. However, very little is known about the stem cells profile in these precancerous lesions. To measure the number of stem cells and understand their proliferating state in ADH compared with UDH and normal duct is helpful in order to generate a strategy of cancer prevention.
Aldehyde Dehydrogenase 1 (ALDH1) is a basic substance used in the function and metabolism of the normal stem cells and cancer stem cells. A large number of clinical experiments indicate that breast cancer cells that possess high ALDH1 activity [19] contribute to recurrence and metastasis, leading to poor prognosis and lower survival rates. A series of reports prove that ALDH1 can be used as an ideal marker of breast cancer stem cells [20]. Dong Yi [21] has shown that patients with positive ALDH1 expression in primary tumors and in ALNM had significantly shorter Relapse-Free Survival (RFS) times and Overall Survival (OS) times compared to those whose tissues were ALDH1 negative. However, Bednarz-Knoll [22] reported ALDH1 (+) stroma in the primary tumor correlated to longer disease-free and metastasis-free times as well as overall survival, having an independent prognostic impact on DFS. Anwar [23] found that the ability of breast epithelial cells to undergo an epithelial-to-mesenchymal transition has been linked to the acquisition of stem cell properties and enhanced tumor invasion, metastasis, and resistance to available treatments [24]. ALDH1 is an ideal marker of breast stem cells. We found that the pattern of ALDH1 expression is in both cytoplasm and matrix. As we know, stem cells have a characteristic undergoing EMT. If we detect ALDH1 in the myoepithelial cells and mesenchymal cells, we could assume there are cancer stem cells of these cells, we could also assume the cancer stem cells come from breast epithelial cells which undergo an epithelial-to-mesenchymal transition. So the expression of ALDH1 in the myoepithelial cells and mesenchymal cells may play a role in Epithelial-Mesenchymal Transition (EMT). To inhibit stem cell division and cells undergoing epithelial-to-mesenchymal transition in ADH is an attractive target for therapy and breast cancer prevention. In this present study, we attempt to explore the significance of ALDH1 expression and localization in the mammary tumor and in ADH.
Tissue selection and preparation
The ethics committee of the of Jilin University approved this study and written consent was obtained for the use of these samples in research. This study used an immunohistochemical method to detect ALDH1 expression in the pathological specimens of 160 breast cancer patients who underwent modified radical mastectomy between October 2003 and December 2008. All of the patients were females between the ages of 31 to 70 (median: 49 years), and the follow-up period ranged from 9 to 89 months (mean follow-up period: 59 months). Stage TNM was classified according to the NCCN Clinical Practice Guidelines in Oncology (version 2015). The postoperative information was recorded. Detailed patient information is summarized in Table 1. Patients who died of other diseases were excluded from this study. 50 specimens of ADH patients, 20 specimens of UDH patients and 22 specimens of normal duct patients were obtained from patients who had surgeries between 2010 and 2014 (Table 2) in the First Hospital of Jilin University, China. Immediately after the surgical dissection was finished, all tissues were fixed in formalin overnight. The resulting paraffin-embedded tissue blocks were used for the study of ALDH1 expression in ADH and UDH in human breasts. Hematoxylin and Eosin (H&E)-stained slides were evaluated by Dr.Lirong Bi from the First Hospital of Jilin University with the microscope of Olympus IX51 and tissue specimens that demonstrated one or more pathological lesions (UDH and ADH) were selected. Pure ADH (n=50) and UDH (n=20) tissues were biopsies or surgical resection specimens from noncancerous breasts (i.e., without DCIS or IDC)
| Information | N (%) |
|---|---|
| Age ≤ 50 | 94 (58.8) |
| Age>50 | 66 (41.2) |
| Pre-menopause | 99 (61.9) |
| Post-menopause | 61 (38.1) |
| Tumor stage | |
| T1 | 55 (34.4) |
| T2 | 95 (59.4) |
| T3 | 2 (1.2) |
| T4 | 8 (5.0) |
| N1 | 79 (49.4) |
| N2 | 57 (35.6) |
| N3 | 24 (15) |
| Patients with distant metastases | 42 (26.3) |
| Bone | 4 (2.5) |
| Brain | 2 (1.3) |
| Live | 7 (4.4) |
| Lung | 12 (7.5) |
| More than two sites | 17 (10.6) |
| Deaths | 37 (23.0) |
Table 1: The postoperative information.
| Information | ADH | UDH | Normal duct |
|---|---|---|---|
| Number of samples | 50 | 20 | 22 |
| Age at diagnosis (yrs) | 46.2 | 45.9 | 46.1 |
| Age at last pregnancy (yrs) | 25.8 | 26.3 | 26.2 |
| No. of full-term pregnancies | 2.1 | 2.4 | 2.2 |
| Pre-menopause | 37 | 16 | 17 |
| Post-menopause | 13 | 4 | 5 |
Table 2: Patients who had surgeries between 2010 and 2014.
Immunohistochemical staining
The following antibodies were used for immunohistochemical analysis: Purified anti-aldehyde dehydrogenase 1-A1/anti-ALDH1A1 (clone 44, 1:200 dilution, BD Biosciences, CA, USA) and S-P IHC reagent (MaiXin Biotechnology Company, Fuzhou, China). Histological examination of the slice was performed by two experienced pathologists. Formalin-fixed specimens embedded in paraffin were processed for immunohistochemistry. Normal human liver tissue sections were used as positive comparisons. 3 mm-sections were treated in sodium citrate buffer (pH 6.0) under high pressure in a pressure cooker and cooled at room temperature for 3 min.
After inhibiting the endogenous peroxidase activity with 3% H2O2, non-specific binding was blocked by incubating the slides with nonimmune serum for 15 min, then by incubating the sections with the primary antibody at 4°C overnight. Next, the sections were incubated with a secondary antibody for each marker at 37°C for 15 min, then with the avidin-biotin-peroxidase complex solution at 37°C for 15 min. The antigen-antibody reaction was visualized with DAB at room temperature without light for 5 min and counterstained with hematoxylin.
Assessment of ALDH1
Two previous studies [20,25-27] have shown that the ALDH1 expressions were strongest in the cytoplasm of epithelial cells. For this study, the positive expression rate of ALDH1 in the cytoplasm of luminal epithelial cells, myoepithelial cells, and stroma cells was counted. We also scored the ALDH1 staining in ADH, UDH, and normal ducts on a scale from 0 to 3 according to percentage of ALDH1 positive cells and the density of ALDH1. For the percentage of ALDH1 positive cells: 0 - negative (0-1% of cells showed ALDH1 staining); 1 - positive (1-10% of cells showed ALDH1 staining); 2-positive (10-50% of cells showed ALDH1 staining); and 3-positive (more than 50% of cells showed ALDH1 staining). For the density of ALDH1: 0 is negative, 1 is a weak stain, 2 is moderately staining, and 3 is a strong stain. The total number of positive cells and the density were calculated as the score of ALDH1 expression.
Statistical analysis
The associations between ALDH1 expression and the status of the cancer were tested using the Chi-square test and Kruskal-Wallis rank sum test. The Relapse-Free Survival (RFS) and Overall Survival (OS) rates were calculated using the Kaplan–Meier method. The statistical significance of the differences between RFS and OS curves were determined using the log-rank test. Statistical analyses were carried out using the SPSS statistical software (version 19.0, USA). The significance level of P<0.05 was considered to be statistically significant. As for the semiquantitative assay of ALDH1 expression, the Kruskal-Wallis rank sum test was used because the data did not conform to the normality test and the single factor of analysis of variance could not be applied. Each group of data is expressed with a median (interquartile range).
Results
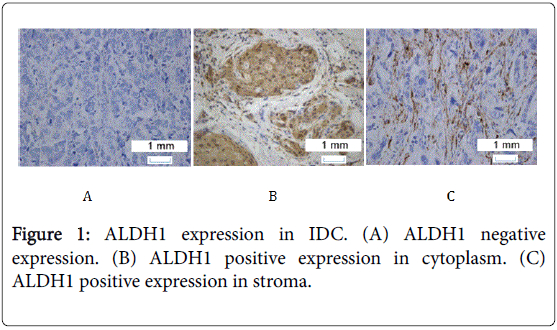
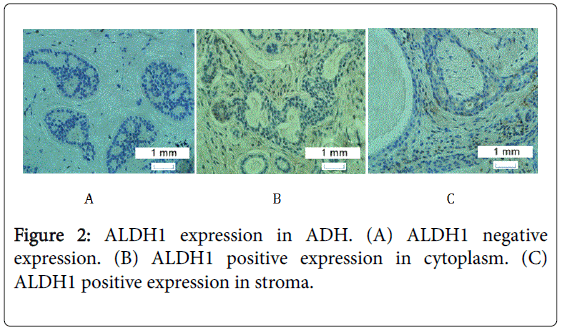
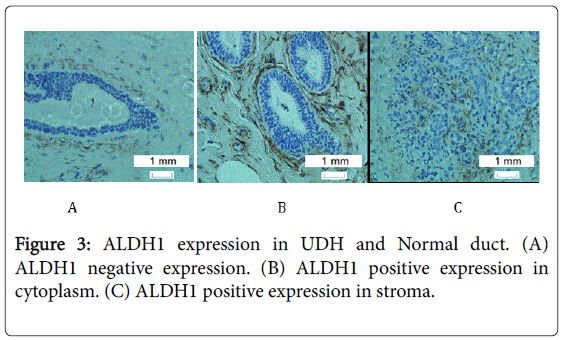
ALDH1 was expressed in a few cells in most samples except in a few cases of ADH, UDH and normal ducts, in which ALDH1 expression showed extensive distribution which was shown in Figures 1-3.
The expression of ALDH1 in the cytoplasm of IDC, ADH, and UDH is 35%, 64%, and 80%, respectively. Chi-square tests of ALDH1 expression in the cytoplasm of three kinds of tissues has statistical significance (P<0.05 shows in Table 3).
| ALDH1 positive expression | ALDH1 negative expression | Expression rate | χ2 | P | |
|---|---|---|---|---|---|
| IDC | 56 | 104 | 0.35 | 23.63 | <0.001 |
| ADH | 26 | 24 | 0.52 | ||
| UDH | 16 | 4 | 0.8 |
Table 3: Chi-square tests of ALDH1 expression in the cytoplasm of three kinds of tissues hasstatistical significance.
The expression of ALDH1 in the stroma of IDC, ADH and UDH
The expression of ALDH1 in the stroma of IDC is 40%, in the stroma of ADH is 52% and in the stroma of UDH is 70%. Chi-square test of ALDH1 expression in the stroma of three kinds of tissues has statistical significance (P<0.05 shows in Table 4).
| ALDH1 positive expression | ALDH1 negative expression | Expression rate | χ2 | P | |
|---|---|---|---|---|---|
| IDC | 64 | 96 | 0.4 | 7.65 | 0.022 |
| ADH | 26 | 24 | 0.52 | ||
| UDH | 14 | 6 | 0.7 |
Table 4: Chi-square test of ALDH1 expression in the stroma of three kinds of tissues has statistical significance.
The difference of ALDH1 expression in the cytoplasm of ADH, UDH and normal duct
Kruskal-Wallis rank sum test of ALDH1 expression in the cytoplasm of three kinds of tissues has no statistical significance (P>0.05 shows in Table 5).
| Groups | Median | X2 | P | |
|---|---|---|---|---|
| ALDH1 expression in cytoplasm | ADH(n=50) | 3(2) | 3.661 | 0.16 |
| UDH(n=20) | 2(4) | |||
| Normal duct(n=22) | 1(4) |
Table 5: Kruskal-Wallis rank sum test of ALDH1 expression in the cytoplasm of three kinds of tissues has no statistical significance
The difference of ALDH1 expression in the stroma of ADH, UDH, and normal duct
Kruskal-Wallis rank sum test of Table 4 ALDH1 expression in the stroma of three kinds of tissues has no statistical significance (P>0.05 shows in Table 6).
| Groups | Median | X2 | P | |
|---|---|---|---|---|
| ALDH1 expression in stroma | ADH(n=50) | 3(4) | 2.151 | 0.341 |
| UDH(n=20) | 2(4) | |||
| Normal duct(n=22) | 2(5) |
Table 6:Kruskal-Wallis rank sum test of Table4 ALDH1 expression in the stroma of three kinds of tissues has no statistical significance
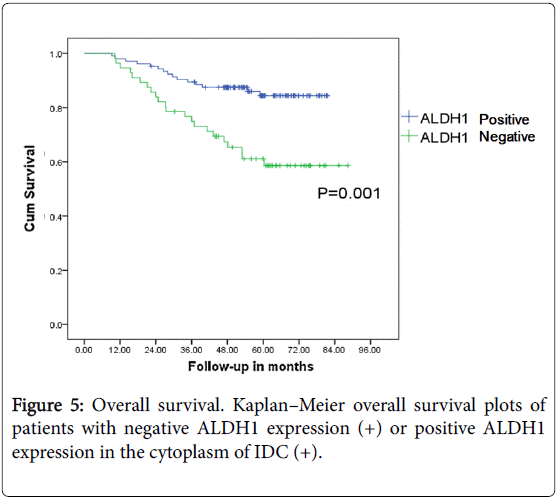
The survival analysis of ALDH1 expression in the cytoplasm of IDC
ALDH1 was expressed in the cytoplasm of 56 cases of IDC (35%) and in 64 stroma cases (40%). Positive ALDH1 expression in the cytoplasm of cancer cells is related to the prognosis of the breast cancer patients (P<0.05) (Figures 4 and 5).
The survival analysis of ALDH1 expression in the stroma of IDC
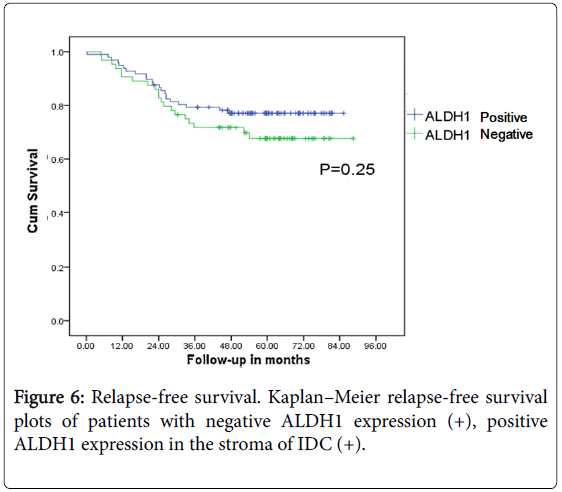
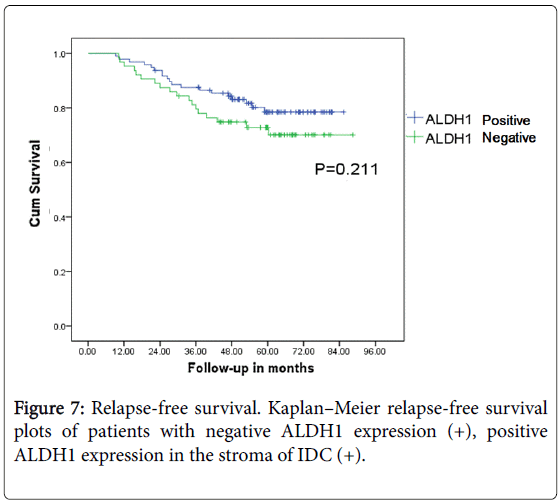
ALDH1 expression in cytoplasm of the IDC is 56 cases (35%) while that in stroma is 64 cases (40%). Positive ALDH1 expression in stroma of cancer cells is not related with the prognosis of the breast cancer patients (P>0.05) (Figures 6 and 7).
Discussion
Breast cancer is regarded as one of the most common malignant tumor nowadays. Data shows that breast cancer has surpassed cervical cancer as the most prevalent malignant tumor among females in China [28]. In America and in European countries, 25% to 30% of all malignant tumors in women are breast cancer, accounting for 8% - 10% of all malignant tumor cases in our country [29]. Because of the increasing incidences of breast cancer, more and more women are beginning to pay close attention to breast health. The high risks of ADH developing into breast precancerous lesions are becoming more and more important, and determining the final result of precancerous lesions has become the focus of the mammary gland disease today.
Cancer stem cells have become one of the biggest issues in the field of medical research in recent years. In the early 1960s, Lapidot [30] first reported human malignant tumor stem cells in people with CD34+CD38- phenotype of acute myelogenous leukemia stem cells.
Then, a large number of studies found tumor stem cells in a variety of malignant tumors. Cancer stem cells, which occupy about 2% of the tumor cells, are insensitive to all sorts of auxiliary treatments; this can lead to the recurrence and metastasis of breast cancer. Therefore, marking the small number of cancer stem cells, finding the corresponding target, and matching the target therapy for a malignant tumor is very important for an effective, radical cure. Breast cancer stem cells are similar to breast epithelial stem cells; however, they also have unlimited proliferation and malignant differentiation ability, which is linked to the growth, recurrence, and metastasis of a tumor. In 2007, Balicki [31] used ALDH1 to identify and isolate breast cancer stem cells, which suggested that ALDH1 may be an ideal and reliable marker for breast cancer stem cells. At the same time, Ginestier etc [19] found that there only 500 ALDH1 positive cells were needed to develop the cells of Nonobese Diabetic/Severe Combined Immunodeficiency (NOD/SCID) mice into a tumor. This accounted for only 2% of the cancer cells in the mice, but this small number of cells has unlimited self-renewal and malignant differentiation ability, thus making ALDH1 one of the ideal markers of breast cancer stem cells.
According to the results of our study, the positive expression rate of ALDH1 in the cytoplasm of IDC, ADH and UDH was 35%, 64% and 80%, while in the stroma of IDC, ADH and UDH, the expression rates were 40%, 52% and 70%, respectively. We also detected ALDH1 expression in myoepithelial cells and observed that ALDH1 positive stem cells in the mammary gland had properties of basal-like cells [32]. The expression of ALDH1 in the cytoplasm of IDC has a correlation with the OS and RFS of the breast cancer patients (P<0.05), but the expression of ALDH1 in the stroma of IDC has no correlation with the OS and RFS of the breast cancer patients (P>0.05). This differs from the study of Bednarz-Knoll [22]. Dr. Schwartz et al. [33] reported ALDH1 expression was higher in stroma components of benign compared with cancerous lesions, but in our study the difference of ALDH1 expression in the stroma of three kinds of tissues has no statistical significance. So what ALDH1 expression in stroma really mean? This needs to be explored. 35% of the IDC sample showed ALDH1 expression, which is consistent with Ginestier’s report [19]. This is lower than the ALDH1 expression in benign and normal epithelial cells (P<0.05). It is well known that stem cells and cancer stem cells share a same marker ALDH1. The cells with positive ALDH1 in IDC are cancer stem cells while ALDH1 cells in ADH and UDH are disposed to be normal stem cells. ALDH1 positive tumors are associated with high histological grade, ERBB2 over-expression and absence of estrogen and progesterone receptor expression [19]. There is a higher trend of the expression of ALDH1 in ADH than in UDH and normal ducts. However, because the sample size was limited, it did not show significant difference, which shows that ALDH1 positive stem cells may play an important part in the process of the evolution of hyperplasia of mammary glands to breast cancer. This information may provide a promising way to help prevent breast cancer.
Acknowledgments
We wish to thank Dr. Yi Li (Breast Center, Baylor College of Medicine, Houston, TX, United States) for his constructive advice and guidance. We also thank the Pathology Core Facility at the First Hospital of Jilin University for tissue processing.
References
- Wicha MS, Liu S, Dontu G (2006) Cancer stem cells: an old idea--a paradigm shift. Cancer Res 66: 1883-1890.
- Desheng Sun. The study of breast cancer stem cells.
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, et al. (2006) Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442: 818-822.
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105-111.
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, et al. (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 351: 657-667.
- Tomasetti C, Vogelstein B (2015) Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347: 78-81.
- Lopez-Lazaro M (2015) Understanding why aspirin prevents cancer and why consuming very hot beverages and foods increases esophageal cancer risk, Controlling the division rates of stem cells is an important strategy to prevent cancer. Oncoscience 2: 849-856
- Bartow SA, Pathak DR, Black WC, Key CR, Teaf SR (1987) Prevalence of benign, atypical, and malignant breast lesions in populations at different risk for breast cancer. A forensic autopsy study. Cancer 60: 2751-2760.
- Nielsen M, Jensen J, Andersen J (1984) Precancerous and cancerous breast lesions during lifetime and at autopsy, A study of 83 women. Cancer 54: 612-615.
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, et al. (2010) Postmenopausal hormone therapy: an Endocrine Society scientific statement. J ClinEndocrinolMetab 95: s1-s66.
- Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. Journal of the National Cancer Institute55:231-273.
- Allred DC, Mohsin SK (2000) Biological features of premalignant disease in the human breast. J Mammary Gland BiolNeoplasia 5: 351-364.
- Page DL, Dupont WD, Rogers LW, Rados MS (1985) Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer 55: 2698-2708.
- Page DL, Dupont WD (1993) Anatomic indicators (histologic and cytologic) of increased breast cancer risk. Breast Cancer Res Treat 28: 157-166.
- Dupont WD, Page DL (1985) Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 312: 146-151.
- Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K (2015) Atypical hyperplasia of the breast--risk assessment and management options. N Engl J Med 372: 78-89.
- Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, et al. (2005) Benign breast disease and the risk of breast cancer. N Engl J Med 353: 229-237.
- Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, et al. (2003) Gene expression profiles of human breast cancer progression. ProcNatlAcadSci U S A 100: 5974-5979.
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, et al. (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1: 555-567.
- Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, et al. (2005) Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells 23: 752-760.
- Dong Y, Bi LR, Xu N, Yang HM, Zhang HT, et al. (2013) The expression of aldehyde dehydrogenase 1 in invasive primary breast tumors and axillary lymph node metastases is associated with poor clinical prognosis. Pathol Res Pract 209: 555-561.
- Bednarz-Knoll N, Nastaly P, Zaczek A, Stoupiec MG, Riethdorf S, et al. (2015) Stromal expression of ALDH1 in human breast carcinomas indicates reduced tumor progression. Oncotarget 6:26789-26803.
- Anwar TE, Kleer CG (2013) Tissue-based identification of stem cells and epithelial-to-mesenchymal transition in breast cancer. Hum Pathol 44: 1457-1464.
- LiliHuo, Hui Li, Feng Wei, Hua Zhao, XiubaoRen (2014) Clinical significance of FoxP3 and the correlation of FoxP3 expression with epithelial-mesenchymal transition in breast cancer. Chinese Journal of Clinical Oncology 41: 158-161
- Mieog JS, de Kruijf EM, Bastiaannet E, Kuppen PJ, Sajet A, et al. (2012) Age determines the prognostic role of the cancer stem cell marker aldehyde dehydrogenase-1 in breast cancer. BMC Cancer 12: 42.
- Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, et al. (2009) Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 15: 4234-4241
- Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, et al. (2011) Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology 59: 776-780
- Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, et al. (2006) Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia 20: 2147-2154.
- Zhang B (2011) Brief description of rules in diagnosis and treatment of breast cancer. Chinese Journal of Minimally Invasive Surgery 12.
- Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, et al. (2006) Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia 20: 2147-2154.
- Balicki D (2007) Moving forward in human mammary stem cell biology and breast cancer prognostication using ALDH1. Cell Stem Cell 1: 485-487.
- Shehata M, Teschendorff A, Sharp G, Novcic N, Russell IA, et al. (2012) Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14: R134.
- Schwartz T, Stark A, Pang J, Awuah B, Kleer CG, et al. (2013) Expression of aldehyde dehydrogenase 1 as a marker of mammary stem cells in benign and malignant breast lesions of Ghanaian women. Cancer 119: 488-494.
Relevant Topics
- 3D Mammograms
- Alternative Cancer Medicines
- Aromatase Inhibitors
- Breast Reconstruction Surgery
- Cancer and Nutrition
- Cancer Prevention from Nuts
- Cancer Screening
- Chemoprevention
- Dietary Supplements
- Exercise and Cancer
- Naturopathic Treatments
- Oncoplastic Surgery
- Scintimammography
- Stem Cell Transplants for Cancer Prevention
Recommended Journals
Article Tools
Article Usage
- Total views: 12256
- [From(publication date):
May-2016 - Dec 26, 2024] - Breakdown by view type
- HTML page views : 11530
- PDF downloads : 726