Research Article Open Access
Expression of P53 and P16 at Tumour Invasive Front in Oral Squamous Cell Carcinoma (OSCC)
Azizi SA1, Nik Mohd Abdul Nasser NFS1, Sailan AT2, Ajura AJ3 and Ibrahim N4*
2Department of Clinical Oral Biology, Faculty of Dentistry, The National University of Malaysia, Kuala Lumpur, Malaysia
3Stomatology Unit, Institute for Medical Research, Kuala Lumpur, Malaysia
4Department of Oral Medicine and Oral Pathology, Faculty of Dentistry, The National University of Malaysia, Kuala Lumpur, Malaysia
- *Corresponding Author:
- Dr. Norliwati Ibrahim
BDS MClinDent
Lecturer at the Department of Oral Medicine and Oral Pathology
Faculty of Dentistry
Universiti Kebangsaan, Malaysia
Fax: +60392897798
Tel: +60392897998
E-mail: norliibrahim@gmail.com
Received: October 21, 2015 Accepted: January 27, 2016 Published: January 30, 2016
Citation: Azizi SA, Nik Mohd Abdul Nasser NFS, Sailan AT, Ajura AJ, Ibrahim N (2016) Expression of P53 and P16 at Tumour Invasive Front in Oral Squamous Cell Carcinoma (OSCC). Cosmetol & Oro Facial Surg 2:105. doi:10.4172/jcofs.1000105
Copyright: © 2016 Azizi SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Cosmetology & Oro Facial Surgery
Abstract
Human papillomaviruses (HPV) tumorigenesis involves two major oncogenes, E6 and E7 of which E6 inactivates p53 while E7 indirectly leads to overexpression of p16.
Objectives: Correlation of expression of p53, p16 with clinicopathological and demographic parameters at tumour invasive front of oral squamous cell carcinoma (OSCC) were analyzed. Materials and Methods: Immunohistochemistry with p53 and p16 on paraffin embedded sections of 28 surgical cases of histopathologically confirmed OSCC and 10 biopsy cases of normal oral mucosa (NOM) was performed.
Results: p53 positivity was observed in 71.4% of OSCC with 32% showing strong intensity while only 20% of NOM were moderately positive (p=0.005). p16 expression was observed in 92.8% of OSCC with 50% showed strong intensity while 80% of NOM were positive with 60% showing strong intensity. Thus, the expression of p16 in OSCC and NOM is almost similar. No statistically significant difference was found between p53, p16 expressions and invasive front grading. However the intensity of p53 expression was significantly higher among Indians (p=0.03).
Conclusion: In oral mucosa, the p53 immunoexpression varies between cancerous or noncancerous tissue by mechanisms independent of p16 and we proposed that both expressions do not correlate with tumour cohesiveness.
Keywords
Oral squamous cell carcinoma; Oral cancer; P53; P16; Invasive front grading
Introduction
Oral squamous cell carcinoma (OSCC) has become one of the leading causes of death mostly in developing countries with worldwide estimated incidence of around 300,000 and constitutes more than 95% of oral cancer [1,2]. In addition, the 2008 National Cancer Registry also stated that squamous cell carcinoma was the predominant type of oral cancer of the tongue [3]. Indeed oral cancer has definitely become a health issue in the world including Malaysia.
Oral cancer has multifactorial aetiologies. Oral habits such as tobacco smoking, betel quid chewing and alcohol intake [4,5] are thought to be the three major risk factors of carcinogenesis, although, some patients may develop OSCC without exposure to these 3 factors. One of the prominent cofactors of OSCC is oncogenic HPV viruses [5]. Human papillomavirus (HPV) infection has a well-established association with cervical and anal cancer and hypothetically HPV viruses might play a role in malignant transformation of any squamous epithelia including oral mucosa. However, its role in oral cancer is less clear. Cervical cancer is mainly caused by the high-risk HPV type 16 and 18 which are also found in head and neck cancer (HNC) particularly HPV-16 [6]. Nevertheless, there are large variations of HPV prevalence in HNC in different parts of the world which ranges from 19 to 72% and this is most probably due to variation in methodologies of HPV detection [7,8].
Carcinogenic effect of HPV is attributed to two major virally encoded oncogenes E6 and E7 in which they inactivate p53 and retinoblastoma protein pRb respectively. Tumour suppressor gene p53 plays a role in apoptosis, genomic stability, and inhibition of angiogenesis in which its inactivation by E6 leads to uncontrolled cell division and eventually tumour formation. While E7 binds to and degrades pRb, releasing E2F, causing the cell to enter S-phase, resulting in cell-cycle disruption, proliferation and malignant transformation leading to overexpression of p16 tumour suppressor protein p16INK4A which inhibits phosphorylation of the Rb-E2F complex. Thus, P16 and p53 may serve as a biomarker in OSCC related to HPV infection [9-11].
The overexpression of p53 at the deep tumour invasive front of OSCC has been found to be associated with the histology grade of malignancy [12]. Studies by Kato et al. [13] also showed a high positivity of p53 at the tumour invasive front in OSCC. Among other genes involved in carcinogenesis, p53 is the most significant and its mutation indicates tumour progression and prognosis. p53 positivity also indicates high risk of tumour recurrence and it may be associated with poor prognosis in OSCC. Therefore, the expression of p53 protein at the invasive front of OSCC is important to determine the tumour cell characteristics and prognosis [13]. However, correlation between p53 expression and clinicopathological and demographical parameters are not well established. Furthermore, the correlation of p16 expression in OSCC with tumour invasive front grading system showed different findings and inconclusive [14].
Broder’s grading system has been widely used but revealed poor correlation with the outcome with no prognostic value [15]. The modified grading system: ‘invasive front grading’ is our main focus study area where it exhibits better correlation with disease progression, metastasis and prognosis [16]. Grading system of invasive front type 1 and 2 are considered as ‘cohesive’ type whereas type 3 and 4 are known as non-cohesive [17]. The importance of tumour cohesion is that the molecular events of carcinogenesis such as increased cell proliferation and angiogenesis at the tumour invasive front of various carcinomas may reflect tumour prognosis better than other parts of tumour [18]. In addition, histology characteristics of tumour at the invasive front area are also related to the clinical behaviour of OSCC [15], hence the clinicopatholigical and demographical informations were included in this study.
The aims of this study are to analyze pattern of expressions of p53 and p16 proteins at tumour invasive front of OSCC and to correlate them with clinicopathological and demographical parameters.
Materials and Methods
Tissue samples
28 formalin fixed, paraffin embedded surgical specimens of oral squamous cell carcinoma (OSCC) were retrieved from Stomatology Unit of Institute for Medical Research (IMR) and 10 biopsy cases of normal oral mucosa (NOM) were obtained from department of Oral Pathology and Medicine, Faculty of Dentistry, Universiti Kebangsaan Malaysia, from year 2000 to 2011. The histopathology diagnoses of squamous cell carcinoma with related Broder’s classifications and tumour invasive front grading were obtained from the histopathology reports and further reconfirmed by two oral pathologists/authors independently. Most of the slides (98%) were classified similarly by both investigators. The demographic and clinical records were also collected from the histopathology report of respective cases. This study was approved by the Ethical Committee of the School of Medicine, Universiti Kebangsaan Malaysia.
Immunohistochemistry
All the blocks of formalin-fixed, paraffin embedded specimens were cut at 4-μm thickness and mounted on silanized slides. Immunohistochemical studies were performed using commercially available monoclonal antibodies which are anti-p16INK4a obtained from abcam® and anti-p53 from DAKO. Immunohistochemical staining was conducted using DakoCytomation Chemmate System according to manufacturer’s instructions. Dewaxed slide sections were heated using water bath and pressure cooker for p16 and p53 respectively. Antigen retrieval was performed with Citrate buffer pH6 for p16 and Tris-EDTA pH 9 for p53, followed by incubation with 6% hydrogen peroxide. The sections were then incubated in primary antibodies at dilution of 1:500 for p16 and 1:100 for p53 within 30 minutes. Sections were exposed to chemmate Envision polymer for 30 minutes followed by DAB substrate incubation for 10 minutes and counterstained in hematoxylin. The sections were dehydrated, cleared and mounted. A confirmed p53 positive OSCC case was used as p53 positive control. While for p16, section of cervical carcinoma was used as positive control. Negative control staining was carried out for both p16 and p53 without incubation with primary antibody. Finally, all the stained slides were visualized using light microscope for immunoreactivity assessment with comparison with their H&E counterparts.
Immunohistochemical assessment
The sections were initially scanned at low power (4x and 10x objective magnifications) where three representative areas of OSCC at tumour invasive front and three representative areas of NOM at the epithelium were chosen with at least 200 cells counted for each areas. Images were then taken at higher magnification of 20x objective and manual counting were proceeded for these three representative areas of each case by imposing a total of 35 grids over the images. Systematic random sampling of grids were done and the immunoreactive score was expressed as a percentage of the total cell count. All slides were evaluated by 2 persons and the kappa value was 0.9. If the differences of the results were more than 10%, the slides were re-evaluated. The evaluation of expression for each slide includes the positivity of staining and intensity of the expression of both p16 and p53 proteins. Positive immunohistochemistry expression of p16 is defined as a nuclear and cytoplasmic staining of tumour cells while the expression of p53 is shown by nuclear staining of tumour cells. A tumour was recorded positive if moderate or strong staining occurs in more than 10% of tumour cells. Intensity of proteins expression was graded as no staining (0), weak staining (1), moderate staining (2) or strong staining (3) [19].
Demographic characteristics of the samples
There were 28 cases of surgical neck dissection of OSCC and 10 biopsy cases of NOM from patients with no OSCC. In NOM we were looking only at the epithelium area of mucous extravasation cysts specimens. The mean age for OSCC was 57.64 ± 13.62. Females constitute the most of OSCC cases (67.9%), while Indian ethnic group constitute the majority of the OSCC cases (35.7%). Table 1 shows the demographic characteristics of each patient with OSCC.
| OSCC 28 (100%) | Valuea | ||
|---|---|---|---|
| Age | Mean | 57.64 ± 13.62 | |
| Range | 27-77 | ||
| Gender | Female | 19 (67.9%) | |
| Male | 9 (32.1%) | ||
| Ethnicity | Indiana | 10 (35.7%) | P=0.03 |
| Malay | 7 (25%) | ||
| Others | 6 (21.4%) | ||
| Chinese | 5 (17.9%) | ||
aIndians with OSCC showed significantly higher p53 intensity (p=0.03) using Spearman’s rho analysis
Table 1: Demographic characteristics of the OSCC samples.
Clinicopathologic characteristics of the OSCC
The majority of OSCC cases were graded as well differentiated OSCC (53.6%) followed by moderately (42.9%) and poorly (3.5%) differentiated based on Broder’s grading. In term of pattern of invasion, cohesive type cases (85.7%) were more than noncohesive type cases (14.3%). Most of the OSCC cases had tumour greatest dimension less than 50 mm (60.7%) compared to the greatest dimension of more than 50 mm (39.3%). Tumour thickness showed almost equal percentage where tumour thickness of less than 11 mm was 53.6% and tumour thickness of more than 11 mm was 46.4%. The majority of cases had no bone invasion (92.9%). Perineural invasion was also absence (71.4%) in most of the cases. Lymphoproliferative invasion was presence in 89.3% of cases while absence of intravascular invasion was 64.3%. Table 2 shows the clinicopathologic characteristics of each patient with OSCC.
| Characteristic | Grades/groups | No (%) |
|---|---|---|
| Broder’s grading | Well differentiated OSCC | 15 (53.6%) |
| Moderately differentiated OSCC | 12 (42.9%) | |
| Poorly differentiated OSCC | 1 (3.5%) | |
| Pattern of invasion | Cohesive | 24 (85.7%) |
| Pattern 1 | 10 (35.7%) | |
| Pattern 2 | 14 (50%) | |
| Noncohesive | 4 (14.3%) | |
| Pattern 3 | 3 (10.7%) | |
| Pattern 4 | 1 (3.6%) | |
| Tumour greatest dimension | £50μμ | 17 (60.7%) |
| >50μμ | 11 (39.3%) | |
| Tumour thickness | £11μμ | 15 (53.6%) |
| >11μμ | 13 (46.4%) | |
| Bone invasion | Presence | 2 (7.1%) |
| Absence | 26 (92.9%) | |
| Perineural invasion | Presence | 8 (28.6%) |
| Absence | 20 (71.4%) | |
| Lymphoproliferative invasion | Presence | 25 (89.3%) |
| Absence | 3 (10.7%) | |
| Intravascular invasion | Presence | 10 (35.7%) |
| Absence | 18 (64.3%) |
Table 2: Clinicopathologic characteristics of the OSCC samples.
Results
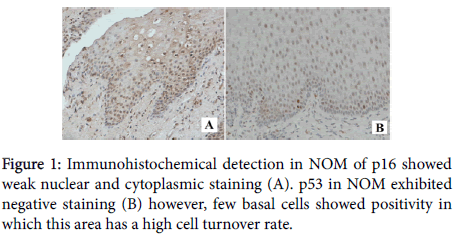
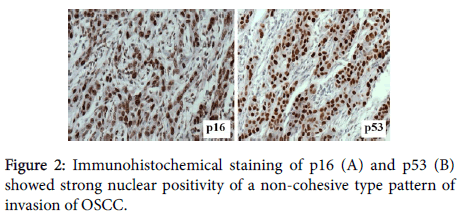
A total of 38 specimens of OSCC and NOM were included in this study. Immunostaining for p16 and p53 were carried out on the specimens (Figures 1 and 2: objective magnification x20) and their staining characteristics were correlated with the selected clinicopathologic and demographic characteristics.
Distribution of immunohistochemical staining
For p53, ANOVA results showed that there was a significant difference for p53 positivity in OSCC tissue against the NOM (p=0.04). In addition, there were significant differences using Mann Whitney analysis for p53 positivity (p=0.005) and intensity (p=0.008) in OSCC against the NOM (Table 3).
| Histological tissue type | Valuea | |||||
|---|---|---|---|---|---|---|
| OSCC | NOM | |||||
| p16b | p53a | p16b | p53a | |||
| Positivity | Positive | 26 (92.9%) | 20 (71.4%) | 8 (80%) | 2 (20%) | 0.005a |
| Negative | 2 (7.1%) | 8 (28.6%) | 2 (20%) | 8 (80%) | ||
| Intensity | No staining | 2 (7.1%) | 8 (28.6%) | 2 (20%) | 8 (80%) | 0.008a |
| Weak staining | 1 (3.6%) | 5 (17.9%) | 0 | 0 | ||
| Moderate staining | 11 (39.3%) | 6 (21.4%) | 2 (20%) | 2 (20%) | ||
| Strong staining | 14 (50%) | 9 (32.1%) | 6 (60%) | 0 | ||
aAnalysis using Mann Whitney for p53.
bNo significant difference was found for p16 positivity and intensity between OSCC and NOM.
Table 3: Comparison of staining positivity and intensity among the histological tissue of OSCC and NOM.
When correlating demographic parameters with p53 protein expression using Spearman’s rho, we found that the intensity of p53 expression was correlated with ethnicity where it was significantly higher in Indians (p=0.03).
However, for p16 expression, there was no correlation found between OSCC and NOM. The p16 correlation with clinicopathological and demographic data was also insignificant.
For both proteins, there was no significant difference found between expressions and invasive front grading. Other findings using Spearman’s rho correlations showed that females exhibiting larger tumour greatest dimension (p=0.003) and Indians tend to have greater tumour thickness (p=0.03). OSCC occurrence in older age was also significant in female (p=0.045). Greater tumour thickness was significant in Indian and other ethnicity than in Malays and Chinese (p=0.03).
Discussion
Squamous cell carcinoma of the head and neck showed heterogeneity at histological, biological and clinical level and as a result, it is difficult to predict the outcome of this malignancy. Therefore, it is crucial to find molecular markers that define tumour subgroups with homogenous behavior and HPV may represent one of them.
In this study, high p53 expression was found in the OSCC samples (71.4%) which is consistent with other studies, regardless of the tumour classifications [20,21].
In the present study, expression of p16 in OSCC was 92.9% which was higher compared to other studies done by Abrahao et al. [20] and Ohta et al. [22] where they found 43.3% from 30 cases and 61.4% from 44 cases showed p16 positivity in OSCC, respectively. In contrast, most of the 35 OSCC samples were absent of p16 (89%) as stated by Adel et al. [22]. They suggested that these tumours may be involved in early tumourigenesis as inactivation of p16 seems to be an early stage in the development of OSCC resulting in loss of p16 expression.
In relation to expression of p53 in 10 NOM samples, only 2% of samples showed positive p53 staining in this study. Similarly, Yanamoto et al. [23] and Schoelch et al. [21] found that there was no p53 expression in all 10 and 8 NOM samples, correspondingly. In contrast to p53, 80% of NOM samples showed positive p16 staining in current study. Other recent study [20] also showed p16 overexpression in NOM however they showed much less percentage of positivity. While another study showed no expression of p16 in NOM [24]. This variation in results was postulated because of p16 is involved in cell cycle regulation and its expression is therefore influenced by cell turnover.
Inconsistent results were obtained from recent studies regarding expression of p53 and p16 at tumour invasive front. Few recent studies showed significant results that p53 was high at tumour invasive front [13,25]. However, similarly to this study, a significant correlation was not found between p53 and p16 expressions and pattern of invasion [14].
Present study also showed that the intensity of p53 was significantly higher in Indian ethnicity. There was no recent study which correlated both parameters. A study stated that there was overexpression of p53 in premalignant and malignant oral lesions of Indian patients who consumed betel, areca nut and/or tobacco due to the mutation of p53 gene [26]. Therefore, the correlation between p53 intensity and Indian ethnic could be influenced by these habits.
Consistent with other studies, we showed that there was no significant correlation found between the expression of p53 and p16 with clinicopathological and demographic characteristics [19,27,28]. In contrast, Ng et al. [29] showed expression of p53 was higher in poorer differentiation OSCC and in younger patients. While Yanamoto et al. [23] found significant correlation between p53 expression with tumour size and histological differentiation of OSCC samples.
The variation of results between studies, for both p53 and p16 protein expressions in relation to clinicopathological and demographical parameters is probably due to small sample size and uneven distribution of related characteristics of each cases. In addition, habits, TNM staging and survival rate parameters were difficult to retrieve and not included in most studies including this current one, where they can be effectively correlated with the expression of p53 and p16.
Conclusion
In the oral mucosa, the p53 immunoexpression changes according to the cancerous or noncancerous type of tissue by mechanisms independent of p16 and it is proposed that both expressions do not correlate with tumour cohesiveness.
References
- Johnson N (2001) Tobacco use and oral cancer: a global perspective. J Dent Educ 65: 328-339.
- Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45: 309-316.
- Lim GC, Azura D (2008) National Cancer Patient Registry--a patient registry/clinical database to evaluate the health outcomes of patients undergoing treatment for cancers in Malaysia. Med J Malaysia 63: 55-56.
- Wynder EL, BrossIJ (1957) Aetiological factors in mouth cancer; an approach to its prevention. Br Med J 1: 1137-1143.
- Johnson NW (2003) Aetiology and risk factors for oral cancer. In: ShahJP, JohnsonNW, JG Batsakis Oral Cancer London: Martin Dunitz, an imprint of the Taylor and Francis Group.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467-475.
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630-5636.
- Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, et al. (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers.J Natl Cancer Inst 100: 407-420.
- Watanabe S, Kanda T, Yoshiike K (1989) Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol 63: 965-969.
- Jeon S, Lambert PF (1995) Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. ProcNatlAcadSci U S A 92: 1654-1658.
- Nemes JA, Deli L, Nemes Z, Márton IJ (2006) Expression of p16(INK4A), p53, and Rb proteins are independent from the presence of human papillomavirus genes in oral squamous cell carcinoma.Oral Surg Oral Med Oral Pathol Oral RadiolEndod 102: 344-352.
- Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tanaka T, et al. (2005) The relationship of the histologic grade at the deep invasive front and the expression of Ki-67 antigen and p53 protein in oral squamous cell carcinoma. J Oral Pathol Med 34: 602-607.
- Kato K, Kawashiri S, Yoshizawa K, Kitahara H, Okamune A, et al. (2011) Expression form of p53 and PCNA at the invasive front in oral squamous cell carcinoma: correlation with clinicopathological features and prognosis. J Oral Pathol Med 40: 693-698.
- Tokman B, Gultekin SE, Sezer C, Alpar R (2004) The expression of p53, p16 proteins and prevalence of apoptosis in oral squamous cell carcinoma. Correlation with mode of invasion grading system.Saudi Med J 25: 1922-1930.
- Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, et al. (1989) New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinomas. J Oral Pathol Med 18: 432-437.
- Woolgar JA (2006) Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol 42: 229-239.
- Helliwell TR, Woolgar JA (2000) Minimum dataset for head and neck carcinoma. London:Royal College of Pathologists 1-23.
- Bryne M, Boysen M, Alfsen CG, Abeler VM, Sudbø J, et al. (1998) The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Res 18: 4757-4764.
- Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW, et al. (2009) Scoring mechanism of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med 7: 25.
- Abrahao AC, Bonelli BV, Nunes FD, Dias EP, Cabral MG (2011) Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentially malignant disorders. Braz Oral Res 25: 34-41.
- Schoelch ML, Regezi JA, Dekker NP, Ng IOL, McMillan A, et al. (1999) Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol 35:333-342.
- Ohta S, Uemura H, Matsui Y, Ishiguro H, Fujinami K, et al. (2009) Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 107: 81-91.
- Yanamoto S, Kawasaki G, Yoshitomi I, Mizuno A (2003) Expression of p53R2, newly p53 target in oral normal epithelium, epithelial dysplasia and squamous cell carcinoma. Cancer Lett 190: 233-243.
- Buajeeb W, Poomsawat S, Punyasingh J, Sanguansin S (2009) Expression of p16 in oral cancer and premalignant lesions. J Oral Pathol Med 38: 104-108.
- Angiero F, Berenzi A, Benetti A, Rossi E, Del Sordo R, et al. (2008) Expression of p16, p53 and Ki-67 proteins in the progression of epithelial dysplasia of the oral cavity. Anticancer Res 28: 2535-2539.
- Kaur J, Srivastava A, Ralhan R (1994) Overexpression of p53 protein in betel- and tobacco-related human oral dysplasia and squamous-cell carcinoma in India. Int J Cancer 58: 340-345.
- Yuen PW, Chow V, Choy J, Lam KY, Ho WK, et al. (2001) The clinicopathologic significance of p53 and p21 expression in the surgical management of lingual squamous cell carcinoma. Am J Clin Pathol 116: 240-245.
- Yuen PW, Man M, Lam KY, Kwong YL (2002) Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol 55: 58-60.
- Ng IO, Lam KY, Ng M, Regezi JA (1999) Expression of p21/waf1 in oral squamous cell carcinomas--correlation with p53 and mdm2 and cellular proliferation index. Oral Oncol 35: 63-69.
--
Relevant Topics
- Blepharoplasty
- Bone Anchored Hearing Aids
- Chemical peel
- Cleft Surgery
- Congenital Craniofacial Malformations
- Cosmetic Facial Surgery
- Craniofacial Surgery
- Dental Orofacial Surgery
- Dentoalveolar Surgery
- Head and Neck Reconstruction
- Injectable Cosmetic Treatments
- Lip Reconstruction
- Mandibular Nerve Surgery
- Maxfax Surgery
- Maxillofacial Surgery
- Neck Liposuction
- Oral and Maxillofacial Surgery
- Oral Surgery Surgeon
- Orofacial Surgery Braces
- Pediatric Maxillofacial Surgery
- Rhytidectomy
- Sleep Apnea Orofacial Surgery
- Temporomandibular Joint Disorders
- Upper Jaw Surgery
Recommended Journals
Article Tools
Article Usage
- Total views: 11702
- [From(publication date):
June-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10687
- PDF downloads : 1015