Expression and Viability of Tamoxifen-Resistant Breast Cancer Cells
Received: 27-May-2022 / Manuscript No. bccr-22-67248 / Editor assigned: 30-May-2022 / PreQC No. bccr-22-67248 / Reviewed: 13-Jun-2022 / QC No. bccr-22-67248 / Revised: 17-Jun-2022 / Manuscript No. bccr-22-67248 / Accepted Date: 23-Jun-2022 / Published Date: 24-Jun-2022 DOI: 10.4172/2572-4118.1000164
Abstract
Movement of 78-kDa glucose-controlled protein from endoplasmic reticulum to plasma film addresses a change in outlook past its conventional capability as an ER chaperone protein. Cell surface GRP78 applies novel flagging capabilities, and instruments basic its cell surface articulation are simply arising. Obtained tamoxifen obstruction of bosom malignant growth cells is went with raised degree of csGRP78. Subsequently, the tamoxifen-safe MCF7 bosom malignant growth cells addresses a clinically pertinent model to concentrate on components of csGRP78 articulation.
Keywords
Endoplasmic reticulum; Proline-rich region; Cancer cells
Introduction
Movement of 78-kDa glucose-directed protein from endoplasmic reticulum (ER) to plasma film addresses a change in perspective past its customary capacity as an ER chaperone protein. Cell surface GRP78 applies novel flagging capacities, and instruments fundamental its cell surface articulation are simply arising. Procured tamoxifen obstruction of breast disease cells is gone with raised degree of csGRP78. In this manner, the tamoxifen-safe MCF7 breast malignant growth cells address a clinically pertinent model to concentrate on systems of csGRP78 articulation [1]. Breast disease is among the main sources of malignant growth passings in ladies. Estrogen receptor-positive breast malignant growth represents around 70% of breast disease patients; in this way, the estrogen receptor adversary tamoxifen is the most broadly utilized adjuvant hormonal treatment.
Discussion
Tamoxifen-resistant MCF7 breast malignant growth cells were a caring gift from Dr. Rachel Schiff and refined as recently portrayed in phenol-red free RPMI 1640 medium containing 5% charcoal-stripped fetal ox-like serum, 2.5 μg/ml fungizone, 200 mM glutamine, 10 IU/ ml penicillin, and 10 μg/ml streptomycin enhanced with 100 nM 4-hydroxy tamoxifen [2]. The cells were confirmed by STR DNA profiling examination at the reagent center office in the USC Norris Comprehensive Cancer Center. Just mycoplasma-negative cells were utilized.
Purging of GST-labeled recombinant proteins
Plasmids containing GST-labeled full-length GRP78 or cancellation freaks were changed into E. coli. The statement of the GST-combination proteins was instigated with 4 mM isopropyl-β-D-thiogalactoside when the optical thickness of bacterial stock culture came to 0.5. Microorganisms were then brooded at 37°C and 200 rpm for 4 hours to permit the outflow of recombinant proteins [3]. Cells were then lysed in TBS containing 50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mg/ml lysozyme, 1% Triton X-100, and protease and phosphatase inhibitor mixed drinks. Bacterial cells were then sonicated for 4 minutes with 20 seconds on and 20 seconds off [4], trailed by centrifugation at 4°C and 11,500 rpm for 60 minutes. Supernatant was gathered and hatched with Glutathione-Sepharose 4B dots at 4°C for 12 hours. Recombinant GSTlabeled protein was eluted with newly arranged decreased glutathione at 4°C for 12 hours. The arrangement containing recombinant proteins was then cradle traded to TBS utilizing protein concentrators [5]. Recombinant proteins in TBS containing 15% glycerol were snap frozen in fluid nitrogen and afterward put away at −80°C.
Apoptosis in tamoxifen-resistant breast cancer Overexpression of GRP78 in growth sores from bosom disease patients has been related with the advancement of restorative opposition, and knockdown of GRP78 can resensitize tamoxifen-safe bosom malignant growth cells to tamoxifen [6]. Subsequently, we explored the possibility of usage of little peptide encoding the COOHterminal PRR of GRP78 to target potential protein communications interceded by the PRR of GRP78 in tamoxifen-safe bosom malignant growth cells [7]. We chose this area since it is in the middle among SBD and KDEL theme, and it safeguards a conformational and succession data notwithstanding the PPP grouping, as past examinations have shown that the flanking district of center proline-rich grouping likewise considerably added to the particularity and fondness of PRRintervened protein connections [8]. The incorporation of the arranging and secretory successions anticipated that the peptides would be confined to the ER as well as discharged into the medium.
To test the usefulness of the discharged peptides, we treated MCF7- LR cells with the cM reaped from the way of life media of transfected cells. Like cells transfected with the plasmids, cells treated with the cM gathered from the cells transfected with the plasmid containing wildtype PRR grouping (P), however not the mP succession [9], showed a huge decrease of CD44v level and a reduction in multiplication marker Cyclin D1. In any case, these decreases didn’t prompt difference in cell morphology and expansion in cell apoptotic markers. This was probable on the grounds that the constrained articulation would consistently supply the little peptides, while the sum in the gathered contingent media is restricting.
Results
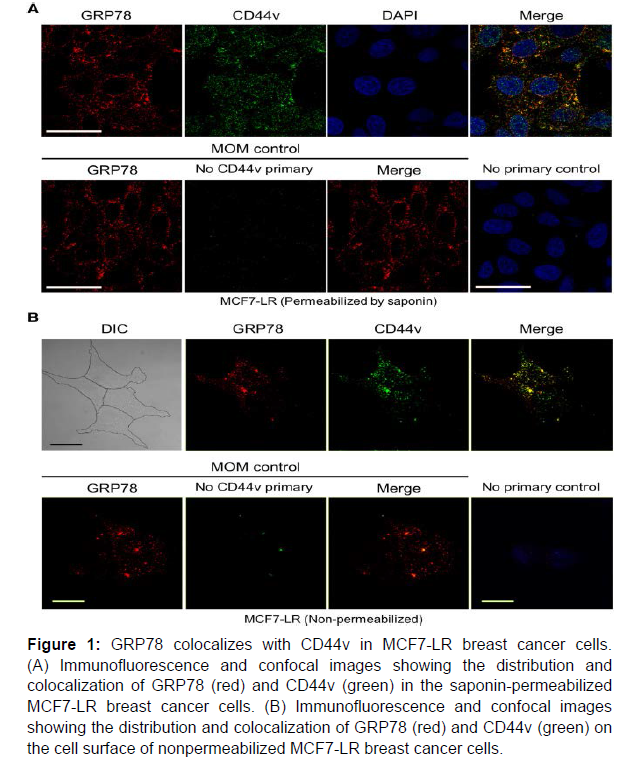
In permeabilized MCF7-LR cells, immunofluorescent staining and confocal microscopy showed that endogenous GRP78 was communicated bounteously in the perinuclear true to form for its ER protein collapsing capability. As a transmembrane protein, CD44 is combined in the ER and deals to the cell surface. CD44v, a variation isoform of CD44 containing variable exon 3 to 10 that is plentifully communicated in the MCF7-LR cells, was distinguished in the cytosolic and perinuclear locale as well as inside the core [10]. Colocalization of GRP78 and CD44v was distinguished in the perinuclear area. Since GRP78 is a significant chaperone protein in the ER, almost certainly, CD44v is a collapsing substrate of GRP78. In nonpermeabilized cells, GRP78 and CD44v were distinguished on the cell surface and showed significant colocalization. These outcomes propose that GRP78 and CD44v colocalize both in the ER compartment and on the cell surface.
Figure 1: GRP78 colocalizes with CD44v in MCF7-LR breast cancer cells. (A) Immunofluorescence and confocal images showing the distribution and colocalization of GRP78 (red) and CD44v (green) in the saponin-permeabilized MCF7-LR breast cancer cells. (B) Immunofluorescence and confocal images showing the distribution and colocalization of GRP78 (red) and CD44v (green) on the cell surface of nonpermeabilized MCF7-LR breast cancer cells.
Conclusion
Taken together, we recognized that the COOH-terminal polyproline succession of GRP78 is another developmental component acquired by higher eukaryotic organic entities, and our biochemical and cell concentrates on upheld the point of view that this grouping harbors already unidentified flagging capacities in managing cell surface articulation of GRP78 and STAT3 enactment in tamoxifen-safe breast malignant growth cells. The essential and clinical ramifications of these discoveries warrant lively examination later on.
Acknowledgement
The authors are grateful to the Division of Cancer Epidemiology & Genetics, National Cancer Institute for providing the resources to do the research on cancer.
Conflicts of Interest
The authors declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
References
- Dobroff AS, Angelo SD, Eckhardt BL, Ferrara F, Staquicini DI, et al. (2016) Towards A Transcriptome-Based Theranostic Platform for Unfavorable Breast Cancer Phenotypes. Proc Natl Acad Sci USA 113: 12780-12785.
- Bakewell SJ, Rangel DF, Ha DP, Sethuraman J, Crouse R, et al. (2018) Suppression of Stress Induction of the 78-Kilodalton Glucose Regulated Protein (Grp78) in Cancer by It-139, An Anti-Tumor Ruthenium Small Molecule Inhibitor. Oncotarget 9: 29698-29714.
- Zoller M (2011) CD44: Can A Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule?. Nat Rev Cancer 11: 254-267.
- Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, et al. (2013) Selective Inhibition of Tumor Oncogenes by Disruption of Super-Enhancers. Cell 153: 320-334.
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, et al. (2006) Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA 295: 2492-2502.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, Taylor C, Arriagada R, et.al (2011) Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 378: 1707-1716.
- Mavragani IV, Nikitaki Z, Kalospyros SA, Georgakilas GA (2019) Ionizing Radiation and Complex DNA Damage: From Prediction to Detection Challenges and Biological Significance. Cancers (Basel) 11: 1789.
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, et.al (2007) The Interaction Between HMGB1 and TLR4 Dictates the Outcome of Anticancer Chemotherapy and Radiotherapy. Immunol Rev 220: 47-59.
- Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, et.al (2020) Detection of Immunogenic Cell Death and Its Relevance for Cancer Therapy. Cell Death Dis 11: 1013.
- Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, et.al (2011) The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon-Dependent Innate and Adaptive Immunity. Cancer Res 71: 2488-2496
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google scholar , Crossref
Citation: Cao J (2022) Expression and Viability of Tamoxifen-Resistant Breast Cancer Cells. Breast Can Curr Res 7: 164. DOI: 10.4172/2572-4118.1000164
Copyright: © 2022 Cao J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1725
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1387
- PDF downloads: 338