Research Article Open Access
Exposure to Acute and Chronic Ethanol in Developmental Stage of Chick can Change the Brain Homocysteine and Leptin
| Zahra Farahani1, Mahnaz Taherianfard2* and Saied Nazifi3 | |
| 1Maternal, fetal and neonatal research center, Tehran University of medical sciences, Tehran, Iran | |
| 2Department of physiology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran | |
| 3Dept of Clinical Pathology, School of Vet Med, Shiraz University, Shiraz, Iran | |
| *Corresponding Author : | Dr. M. Taherianfard Dept of Physiology School of Veterinary Medicine Shiraz University, Zip code: 71345 P.O. Box: 1731, Shiraz, Iran Tel: 0098-711-2286950 Fax: 0098-711-2286940 E-mail: taherian@shirazu.ac.ir |
| Received December 24, 2012; Accepted February 05, 2013; Published February 08, 2013 | |
| Citation: Farahani Z, Taherianfard M, Nazifi S (2013) Exposure to Acute and Chronic Ethanol in Developmental Stage of Chick can Change the Brain Homocysteine and Leptin. Biochem Physiol 2:107. doi:10.4172/2168-9652.1000107 | |
| Copyright: © 2013 Farahani Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Aim: Ethanol intake decreases food intake in rat, and one of the possible mediators of this alcohol effect is leptin. On the other hand, the concentration of total plasma homocysteine is a well-established indicator for the risk of cardiovascular disease, and seems to be related to ethanol consumption. So, the aim of the present study was to investigate the effect of exposure to acute (70%) and chronic (10%) evaporated ethanol on 1) brain leptin and homocysteine concentration on the 15th day of embryonic development of chick; 2) brain leptin and homocysteine concentration immediately after hatch of chick; 3) serum leptin concentration immediately after hatch of chick.
Methods: 60 fertilized eggs were used. Eggs were divided into 3 groups, 1) Control; 2) acute exposure to ethanol 3) chronic exposure to ethanol. Homocysteine was measured by using enzyme-linked assay, and leptin was measured with the chick leptin radioimmunoassay kit.
Results: Data showed that exposure to acute and chronic ethanol significantly (P<0.05) decreased brain homocysteine concentration on the 15th day of embryonic stage of chicken, but did not have any effect on brain homocysteine concentration immediately after hatch Exposure to acute and chronic ethanol significantly (P<0.05) increased brain leptin on the 15th day of embryonic stage, brain leptin immediately after hatch of chick and plasma leptin immediately after hatch of chick.
Conclusion: Present results indicated that exposure to acute and chronic ethanol by evaporation in embryonic stage of chicken can change the brain homocysteine, brain leptin and serum leptin.
| Keywords |
| Acute ethanol; Chronic ethanol; Homocysteine; Leptin; embryonic stage; Chicken |
| Introduction |
| Leptin is a 16 KDa polypeptide hormone (167 amino acids). The OB gene codes for hormones are secreted mainly by adipose tissue, placenta, fetal tissue and membranes, and stomach. Leptin reaches the brain, crossing the blood-brain barrier or the chroroid-cerebrospinal fluid barrier and informs the brain about the size of the fat stores [1]. Leptin has a wide variety of central and peripheral actions such as reproduction, food intake, energy expenditure, lipid metabolism, immune system, blood pressure and angiogenesis [2,3]. Recent evidence has shown leptin as a risk factor for vascular disease. It may be an important link between cardiovascular disease and obesity. Leptin has a procoagulant, atherosclerotic role and platelet aggregation effect [4]. |
| Homocysteine (Hcy) is a sulfur-containing amino acid produced from food methionine metabolism in the body. It can be converted to methionine or cysteine by remethylation or trans-sulforation cycles with some enzymes (MS, MTHFR, SAM) and cofactors (B6, B12). Folate as a methyl group donor is essential in the remethylation cycle too. Hcy is transported from blood brain barrier into the CSF and brain, although human neural cells are capable of producing Hcy [5]. Insufficient folic acid, B6, B12 and impairment in enzymes functions cause hyper-homocysteinemia. Hcy>12 μ mol/dl has been shown as a risk factor for vascular disease, brain athrophy and neurodegenrative disease [6]. Hyper-Homocysteinemia has been linked to atherosclerosis and thrombosis [7]. |
| Ethanol has weakly charged molecules that move easily through the cell membrane, rapidly equilibrating between blood and tissue. Alcohol, at low doses can have some beneficial effects such as decreased rates of myocardial infarction, stroke, gallstone, and possibly vascular or Alzheimer’s dementia, but the consumption of more than 2 standard drinks per day increases the risk for health problems in many organ systems and hormonal changes [8]. Ethanol intake by changes in adipose tissue and BMI can affect leptin concentration [9]. Some studies have indicated an inverse relation between alcohol use and leptin level [10] . In other investigations Ethanol is shown as a powerful inducer of hyperleptinemia in both animals and humans. Alcohol intake has the potential to alter body weight as it is energy-dense and may also alter eating behavior at higher levels of consumption. Each of these lifestyle factors, therefore, has the ability to alter adipose tissue mass, possibly via leptin [9]. On the other hand, short-term and chronic ethanol intake influences Hcy concentration by changes in the methylation pathway, folate and cofactors concentration [11]. Ethanol–induced increase in serum Hcy levels has been observed in active alcoholics. Exogenous ethanol caused elevated endogenous brain Hcy level, reduced S-Adenosyl methionine (SAM) levels, and increased S-Adenosyl homocysteine (SAH) levels, which correlated to increased brain caspase-3 activities [12]. |
| The reported sequences of leptin from human, cow, pig, sheep, mouse, rat, dog, and chicken show a high degree of sequence conservation. This similarity suggests a common function or mechanism of hormone across species [13]. On the other hand, in previous studies exogenous ethanol caused a 1.6 fold increase in chick brain Hcy level at 11 days of development [12] . The present study was investigated the effect of exposure to acute (70%) and chronic (10%) evaporated ethanol on brain leptin and Hcy concentration on the 15th day of embryonic development of chick, brain and serum leptin, and Hcy concentration immediately after hatch of chick. 15th day that we used in present study was according to our previous study [14]. |
| Materials and Methods |
| All procedures were approved by the Animal Care Committee of the Iran Academy of Veterinary Medicine. Sixty fertile, pathogenfree, cubb eggs were purchased from Fars Poultry Center. More than twenty eggs (natural fertility is rarely 100%) of each group (acute, chronic exposure, control) were incubated under standard conditions (75% humidity at 37oC). Humidity and temperature were checked by thermometer and a hygrometer. Incubator turning (3 times a day) was considered essential in the early stages. For the last 3 days of incubation when the bird was preparing to hatch, turning was stopped. |
| Test groups: eggs were divided into three groups 1-control group (eggs incubated in normal condition, n=20), 2-chronic group (eggs incubated while the water in incubator was replaced by 10% ethanol, n=20), and 3- acute group (eggs incubated while the water in incubator was replaced by 70% ethanol at the 6th, 13th and 20th days). In all groups half of the eggs were examined on the 15th day of developmental stage and the other half were examined immediately after hatching of chick. |
| For the extraction of brain HCY and leptin, single whole brain was mixed with a phosphate buffer solution after homogenization, the mixture was centrifuged, and the brain extract was prepared for HCY and leptin. HCY were assayed by Liquid stable 2-part HCY reagent cobas mira plus and leptin was assayed by chicken leptin ELISA kit from Cosabio Inc. |
| The data were analyzed by SPSS program (version 18) and using one way ANOVA and Tukey as post hoc test. Significant level was considered to be P<0.05. |
| Results |
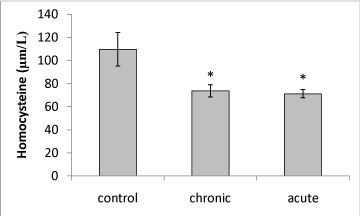
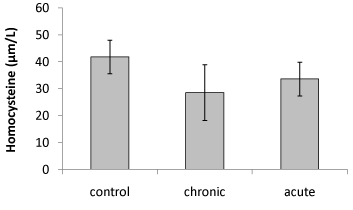
| Our data showed that brain Hcy concentration significantly (P<0.05) decreased on the 15th day of embryonic stage of chicken in acute and chronic groups relative to control group (Figure 1). Figure 2 shows that exposure to acute and chronic ethanol had no significant effect on Hcy concentration on the first day of hatch. |
| Brain Hcy concentration on the 15th day of embryonic stage and the first day of hatch had not significant difference in acute and chronic exposure to ethanol. |
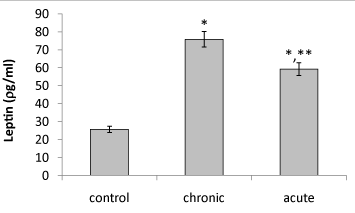
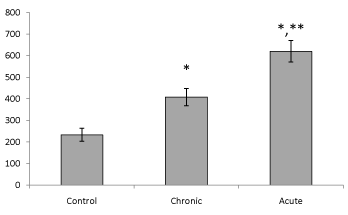
| Present data showed that following acute and chronic exposure to ethanol, brain leptin significantly (P<0.05) increased on the 15th day of embryonic stage of chick and on the first day of chick hatching (Figures 3 and 4). Also, serum leptin significantly (P<0.05) increased following acute and chronic exposure to ethanol on the first day of chick hatching (Figure 5). |
| Brain leptin concentration on the 15th day of embryonic stage and the first day of hatch, significantly lower in acute than chronic exposure to ethanol. But serum leptin concentration on the first day of hatch higher in acute than chronic exposure to ethanol. |
| Discussion |
| Leptin and Hcy as atherosclerotic and intima media thickness factors have great roles in almost all disease physiopathology [15]. Ethanol intake in different amounts can affect both of these pathogen factors. In some studies plasma Hcy was indicated as the blood alcoholization factor [16]. However, in other studies there was an inverse relation between Hcy and ethanol [17,18]. |
| In the present investigation ethanol in acute and chronic exposure causes a significant decrease in chick brain Hcy on 15th day of embryonic stage. It should be noted that an adequate amount of vitamin B groups and folate in egg help Hcy removal pathway and prevents brain Hcy level elevation in embryos. For instance, chicken egg naturally contains 22 μg of folate [12]. Furthermore, it is supposed that hepatic enzymes and methylation- transsulfuration pathway are activated by ethanol, causing more Hcy removal. Also, during fertile eggs incubation period, embryos do not have methionine intake as a precursor of Hcy. Moreover, Lakashman et al. [19] found that ethanol causes an increase in Paraoxanase 1(PON1) by upregulation of liver PON1 expression. PON is known as an antioxidant enzyme which can detoxify Hcy metabolite [19]. Also, ethanol in acute and chronic exposure amounts causes a significant rise in S-adenosyl methionine and that activates methylation pathway in the hepatocytes, converting Hcy to methionine [11] . |
| On the other hand, we understood that acute and chronic exposure had no effect on 1 day chick brain Hcy. That could be a result of folate and vitamin B group’s shortage in the last embryonic days. Sakuta et al. [18] in showed a different relation between ethanol and plasma Hcy (elevating, decreasing, unchanged). In this study they pointed out that in hypofolatemia condition, the relation between ethanol and Hcy would be positive. Lack of folate causes methylation cycle impairment. In addition, Belich et al. [20] showed that, ethanol gradually decreases liver ability in folate maintenance and storage [20]. |
| Bree et al in 2001 also explained an inverse relation between folate and Hcy level [21]. From a 20% increase in folate level, a 3.2% decrease in Hcy was seen. This relation is more prominent in alcoholics (5.4%). Folate has a great role in methyl donation in remethylation cycle. In the final days, the lack of folate would be one reason for brain Hcy accumulation. |
| However, Shinohara et al. [22] indicated that ethanol has different affects in different species, for instance, mice ethanol showed hyperhomocysteinemia, whereas rats showed no changes in Hcy level [22]. |
| In the present study acute and chronic ethanol exposure lead to an increase in the concentration of leptin on 15 day chick brain embryo, and acute and chronic ethanol exposure lead to an increase in the concentration of leptin in both the brain and serum on the first day of hatching in chick. It is supposed that the leptin elevation was due to the ethanol effects on weight gain and adiposity. Short and long chain leptin receptors were seen on the 5th and 17th day of the developmental stage in liver, adipose tissue and yolk sac [13]. Leptin regulates energy balance and expenditure in embryonic period. Donahue et al. [10] also confirmed the positive relation between adiposity and leptin level [10]. Balasubramanian et al. [23] pointed out the influential effects of ethanol on cytokine synthesis in hepatocyts [23]. Cytokines, particularly TNFα, are one of the most important stimulating factors for leptin elevation. In addition, ethanol causes a hypothalamic resistance to leptin and the level of this polypeptide begins to rise. Moreover, Donahue et al showed that ethanol as a high energy molecule can have an effect on glucose and insulin plasma level. Insulin resistance increases leptin level in plasma and brain [10]. In the present study on the first day of hatch brain leptin was higher in chronic than acute exposure to ethanol, but serum leptin was higher in acute than chronic exposure to ethanol. It seems that the enzyme in synthesis and metabolism of leptin are different in brain and serum, so the effect of acute and chronic ethanol in brain and serum leptin concentration was different. Patterson-Buckendahl et al. [24] reported that chronic ethanol had less effect than acute ethanol on serum leptin [24]. |
| Conclusion |
| In the present study: |
| 1. Acute and chronic exposure to ethanol leads to decline of brain Hcy concentration on the 15th day of embryonic stage and the first day of hatch. There is no difference between acute and chronic exposure to ethanol. |
| 2. Acute and chronic exposure to ethanol leads to increase of brain Leptin concentration on the 15th day of embryonic stage and the first day of hatch and serum leptin on the first day of hatch. There is no difference between acute and chronic exposure to ethanol. Brain leptin was higher in chronic than acute exposure to ethanol on the 15th day of embryonic stage and the first day of hatch, but serum leptin was higher in acute than chronic exposure to ethanol on the first day of hatch. |
| According to our results it seems that cardiovascular disorder that caused by alcohol consumption, may be due to change in Hcy and leptin. |
| Acknowledgment |
| We want to thank Datis Teb Company and Dr Saadat’s laboratory for their excellent technical support. |
| Conflict of Interest |
| All the authors can confirm that there is no financial or other relationship that would cause a conflict of interest. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13413
- [From(publication date):
May-2013 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 9027
- PDF downloads : 4386
