Exploring the Pharmacological Activities of Hydrazone Derivatives: A Review
Received: 29-Dec-2017 / Accepted Date: 31-Dec-2017 / Published Date: 31-Dec-2017
Abstract
The development of new potent chemical architectures, possessing anti-inflammatory, analgesic, antibacterial, antifungal, antiviral, anti-hypertensive, antidepressant, anticancer, antiplatelet, antimalarial and anticonvulsant etc. biological activities remain the main focus thought of many researchers. Among others, hydrazone derivatives acquire azomethine -NHN=CH- group gain considerable attention due to its diverse biological activities. A wide variety of hydrazone derivatives have been synthesized and their biological activities also been evaluated from time to time. The goal of present review is to highlight the diverse potential pharmacological activities of hydrazone derivatives as observations for new drug development.
Keywords: Hydrazone derivatives; Azomethine; Pharmacological activities; Broad spectrum
Introduction
Hydrazone and its derivatives with azomethine -NHN=CH- group represents an important class of compounds with broad spectrum of pharmacological activities [1]. A variety of hydrazone derivatives have been synthesized with the potential pharmacological activities like anti-inflammatory, antibacterial, analgesic, antifungal, anti-hypertensive, antiplatelet, anticancer, antimalarial, antidepressant, anticonvulsant and antiviral etc [2]. In addition to their extended biological properties they also combine with other functional groups to provide pharmacologically active molecules. The main theme of this study is to explore the biological and pharmacological importance of hydarzone derivatives for future development of new drug entities.
Anti-inflammatory And Analgesic Activity
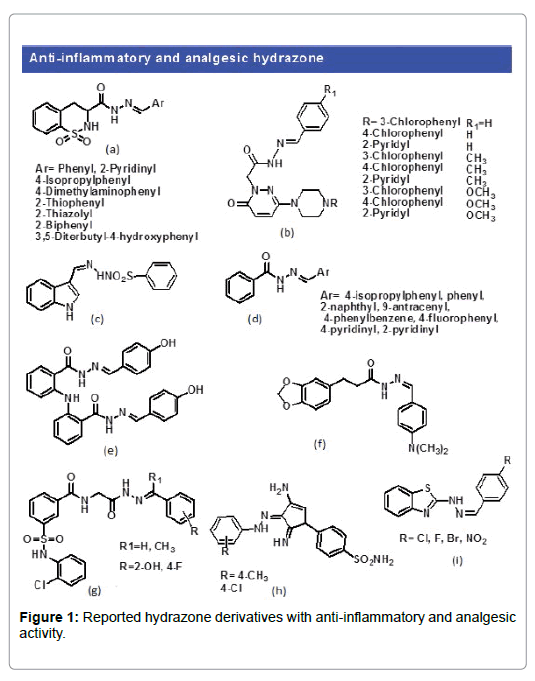
Inflammation refers to a commonly used broad term for a local response to cellular injury characterized by pain, capillary dilatation, odema, heat, redness and loss of function. To date, a number of hydrazone derivatives has been efficiently synthesized and evaluated for the analgesic and anti-inflammatory activities. It is important to mention that COX-I and COX-II have an important role in the inflammation. Structure modification of the parent bioactive molecule which is a common practice over the years may further enhance its biological importance. In this scenario, Barreiro et al. synthesized a series of novel hydrazone derivatives based on the structural modification of piroxicam (Figure 1a). The resulted compounds showed better anti-inflammatory and antinociceptive activity then the standard piroxicam drug [3]. A series of novel substituted hydrazone derivatives were also reported by Mehtap Gokce et al. that exhibit significant antiinflammatory activity in comparison to the standard indomethacin drug (Figure 1b) [4]. Additionally, Sondhi et al. synthesized a number of amidine and hydrazone derivatives and its evaluation showed promising anti-inflammatory and analgesic activities. (Figure 1c) [5]. Pyrazine N-acylhydrazone derivatives synthesized by Lima et al. [6] showed anti-inflammatory and analgesic activity (Figure 1d). Similarly, Eissa et al. reported anthranilic acid based hydrazone derivatives [7] with good exhibition of anti-inflammatory activity (Figure 1e). Moreover, Barreiro et al. developed a series of novel N-acylhydrazone from the natural safrole and were investigated for analgesic property. The compounds proved to have good analgesic activity (Figure 1f) [8]. Recently Korupolu et al. reported novel N-phenyl sulfonamide linked N-acylhydrazones [9]. The compounds are reported to have strong anti-inflammatory characteristic via inhibition of COX-2 enzyme (Figure 1g). Similarly Abdelgawad et al. demonstrated a series of new pyrazole bearing hydrazone derivatives and were tested for activity [10]. The novel hydrazone derivatives showed better anti-inflammatory property compared to the standard drug celecoxib (Figure 1h). Qin et al, synthesized a set of novel benzo[d]thiazole-hydrazones and investigated for biological activity [11]. Resulting new hydrazone derivatives displayed good anti-inflammatory character (Figure 1i).
Antimicrobial Activity
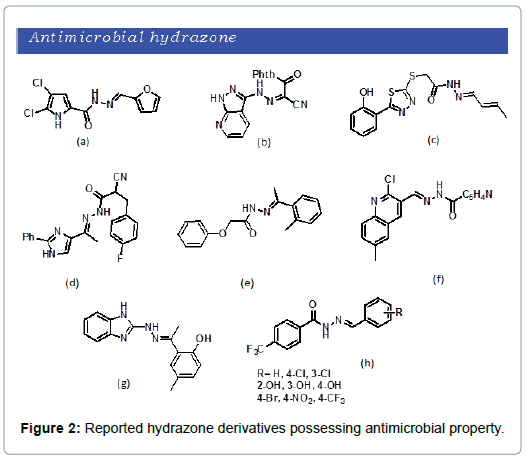
Rise in multi-drug resistance of bacterial infection in the last few decades cause increase risk of complications in antibiotic therapy [12] Therefore the development of new and effective antibiotics remains a challenging area for the researchers. Varity of hydrazone derivatives have been designed, synthesized and studied for antimicrobial characteristics. Chloropyrrole based aroyl hydrazone reported by Rane et al. (Figure 2a) Proven to have potential antimicrobial activity against gram negative and gram positive bacteria.[12] Additionally, Kalil et al. synthesized a series of novel hydrazone derivatives and reported to posse’s potent antimicrobial activity (Figure 2b) [13]. Also, Wang et al reported a set of 5-(2-hydroxyphenyl)-1,3,4-thiadiazol-2-yl-sulfanyl acetyl hydrazones that showed a potent antimicrobial activity (Figure 2c) [14]. Abdel‑Wahab et al. synthesized imidazole ring hydrazones that exhibit excellent antibacterial property (Figure 2d) [15]. Raja et al. developed 4-bromophenoxy acetic acid hydrazones and were investigated for antimicrobial activity (Figure 2e). The compounds were found to have good antibacterial property against gram positive, gram negative and mycobacterium [16]. Moreover, S. Kumar et al. demonstrated the synthesis of a series of 2-chloro-6-methylquinoline hydrazones and were investigated for pharmacological activity [17]. Most of the synthesized compounds were found to have maximum antibacterial activity (Figure 2f). Kale synthesized hydrazone Schiff’s base derivatives and were subjected to biological screening [18]. Hydrazone derivatives revealed antibacterial activity against E-Coli and S-aureus (Figure 2g). Recently M. Krátky et al. reported a series of 4-(trifluoromethyl)benzohydrazide derived hydrazones and subsequent investigation of antibacterial activity [19]. Most of the new hydrazones exhibit better antibacterial activity (Figure 2h).
Antifungal Activity
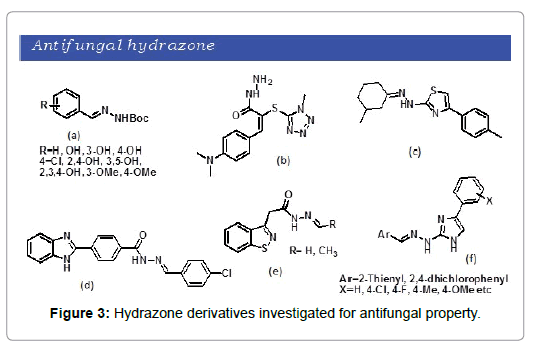
The increasing mortality rate in systemic fungal infection is attributed due to the high antifungal drug resistance. Such scenario creates the urge for developing safe and effective antifungal drugs for clinical use. A series of hydrazones were synthesized from the coupling of aldehydes with tert-butyl carbazate by Casanova et al. [20]. The resulting hydrazones were subjected to antifungal screening and promising activity was observed for most of the compounds (Figure 3a). Altintop et al. synthesized novel hydrazone derivatives through the addition-elimination reaction of 2-[(1-methyl-1H-tetrazol-5-yl)thio)] acetohydrazide and aromatic aldehydes/ketones [21]. Compounds were subsequently investigated and were observed to have significant antifungal potential (Figure 3b). A series of 2‑thiazolylhydrazones were developed by Chimenti et al. that exhibit potent antifungal activity against various Candida species (Figure 3c) [22]. Ozkay et al. synthesized benzimidazole based hydarzone derivatives and were subjected to antifungal and antibacterial studies and significant antifungal and antibacterial activity was observed [23]. Interestingly, one of the compound showed more activity then the standard antifungal ketaconazole (Figure 3d). Paola Vicini et al. reported the synthesis of a number of hydrazones of 1,2-benzisothiazole hydrazides [24]. The series of compound was tested against bacteria and yeast and were reported as good antibacterial agent as well as most of the hydrazone derivatives were active against yeast (Figure 3e). This year S. Kauthale et al. synthesized a set of thiazolyl hydrazones through one pot synthesis [25]. The resulting compounds were observed to possess moderate to good antifungal activity (Figure 3f).
Anticancer Activity
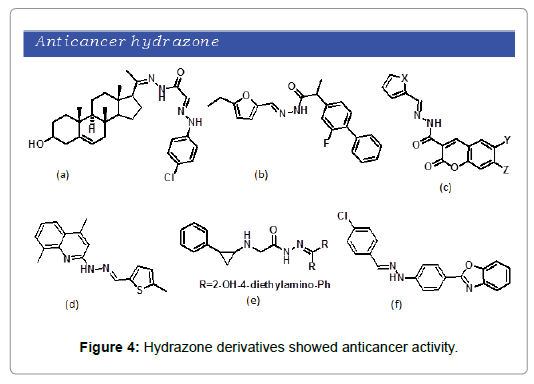
Cancer is a burning issue under investigation which refers to the uncontrolled and rapid abnormal cell division that can invade nearby tissue. In 2015 about 90.5 million people were cancer carrying and even its number is increasing every year. A number of hydarzone derivatives have been developed with anticancer properties. R. M. Mohareb et al. reported the synthesis of a number of hydrazide-hydrazone derivatives from ring D modification of pregnenolone [26]. These compounds were evaluated for anticancer activity and were reported highly effective against breast cancer, lung cancer and CNS cancer compared to the reference drug doxorubicin (Figure 4a). Similarly, Küçükgüzel et al. synthesized a series of hydrazide-hydrazone derivatives using Flurbiprofen as template [27]. The compounds were shown to have significant activity against leukemia cancer cell line and ovarian cancer cell lines (Figure 4b). Nasr et.al developed cumarine based hydrazide-hydrazone derivatives and bromocoumarins (Figure 4c) were reported to have good antitumor activity against pancreatic carcinoma and hepatic carcinoma [28]. Savini et al. synthesized a series of new 3- and 5-methylthiophene-2-carboxaldehyde a-(N)-heterocyclic hydrazones and were investigated for anticancer activity [29]. Compound N-(4,8-dimethyl-quinolin-2-yl)-N’-(5-methylthiophen- 2-ylmethylene)-hydrazine was found to be the most active antitumor agent (Figure 4d). Recently Sun et al. reported a set of phenylalanyl hydrazones that were evaluated for anticancer activity [30]. Novel hydrazons were found effective against gastric cancer (Figure 4e). Very recently a set of azole bearing hydrazone derivatives were reported by Labib et al. and were subjected to testing for anticancer activity [31]. The synthesized hydrazones were found effective against breast cancer and hepatic cancer cell lines (Figure 4f).
Antidepressant Activity
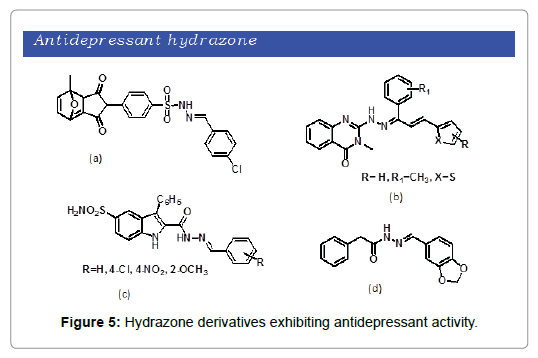
According to WHO (world health organization), depression is a mental disorder characterized by persistent sadness and a loss of interest in daily activities. Depression affected 300 million people around the globe and is considered as a leading cause of suicide. A number of hydrazone derivatives are reported to have antidepressant activity. de Souza et al. synthesized a set of sulphonamides and sulphonyl hydrazones of maleimide [32]. Upon evaluation, the compounds were found to have promising antidepressant activity. Interestingly, sulphonyl-hydrazone was found to be the most active compound than imipramine (Figure 5a). A series of hydrazone derivatives were developed and investigated for antidepressant property by Gokhan‑Kelekci et al. [33]. Most of the compounds showed MAO inhibition activity (Figure 5b). Ergec et al. demonstrated a series of ten arylidenehydrazides and were tested for antidepressant activity [34]. The resulting compounds showed good antidepressant property (Figure 5c). Pandeya reported the synthesis of a series of phenylacetic acid hydrazones [35]. All of the compounds exhibit promising antidepressant activity when compared with tranylcypromine (Figure 5d).
Anticonvulsant Activity
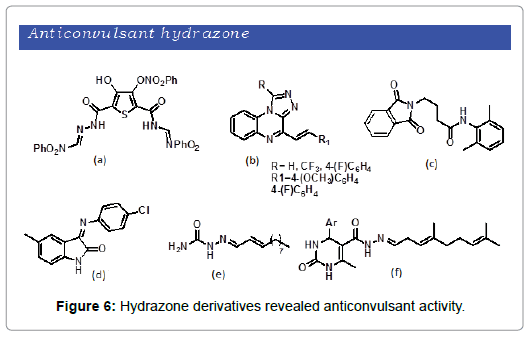
According to epilepsy foundation, epilepsy is a chronic disorder characterized by recurrent unpredictable and unprovoked seizures. It is the fourth most common neurological disorder affecting people of all ages. Verity of hydrazone derivates are reported possessing anticonvulsant activity. Kulandasamy et al. showed a series of new hydrazones and bishydrazones and were screened for anticonvulsant activity [36]. The compounds showed significant antiepileptic activity (Figure 6a). Wagle et al. demonstrated the synthesis and anticonvulsant investigation of hydrazone derivatives and found that the compounds exhibit potential anticonvulsant activity with respect to reference diazepam drug (Figure 6b) [37]. Ragavendran et al. prepared two sets of phthalimide-GABA anilides/hydrazones and were subsequently tested for neurotoxic and anticonvulsant activities [38]. The compounds possess anticonvulsant property. Notably, the most active compound was found to be the 4-(1,3-dioxo-1,3-dihydro-2H-isoindol- 2-yl)-N-(2,6-dimethylphenyl)butanamide (Figure 6c). Sridhar et al. synthesized and screened hydrazone, Schiff and Mannich bases for anticonvulsant activity [39]. The compounds showed significant anticonvulsant property but the most active compound observed was 3-(4-Chlorophenylimino)-5-methyl-1,3-dihydro-indol-2-one (Figure 6d). Dimmock et al. synthesized a series of oxamoylhydrazones, acetylhydrazones, and semicarbazones [40]. Upon their pharmacological investigation it was observed that acetylhydrazones, and semicarbazones (Figure 6e) afford promising anticonvulsant activity as compared to the oxamoylhydrazones. Similarly in recent year Sanchit et al. reported the synthesis of pyrimidine hydrazones Schiff bases [41]. The newly synthesized hydrazone derivatives exhibit good anticonvulsant activity (Figure 6f).
Antihypertensive Activity
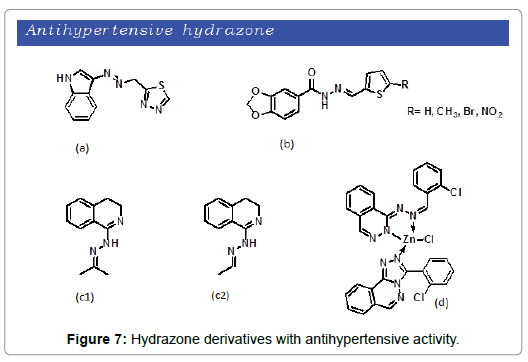
According to the WHO, hypertension also known as elevated blood pressure, is a condition in which the blood vessels have a persistent high pressure. In majority of the cases the cause is nonspecific/unknown. Long term hypertension is considered one of the major risk factor for cardiovascular diseases. To overcome this rapidly increasing health problem, a number of researchers have paid attention to developed verity of hydrazone derivatives for the management of hypertension. Leal et al. reported the synthesis of safrole based N-acylhydrazones [42]. The compounds were evaluated and found to have antihypertensive characteristic (Figure 7a). As vasodilatation is one of the factors that reduces elevated blood pressure, Silva designed and synthesized new acylhydrazone derivatives and subjected to vasodilator screening [43] The new compounds showed vasodilator effect by directly acting on smooth muscle endothelium (Figure 7b). Diana et al. synthesis a series of 1-substituted 3,4-dihydroisoquinolines [44]. The resulted compounds were tested and were declared that 1-hydrazino homologue and the corresponding acetaldehyde and acetone hydrazones show significant antihypertensive activity (Figures 7c1 and 7c2). Bakale demonstrated the synthesis of ligand zn(II) complex of 2-chlorobenzaldehyde, hydralazine and hydrazine [45] The complex was evaluated for antihypertensive activity and was found to exhibit promising activity through in situ generating 1,2,4-triazole moiety (Figure 7d).
Anti-platelet Activity
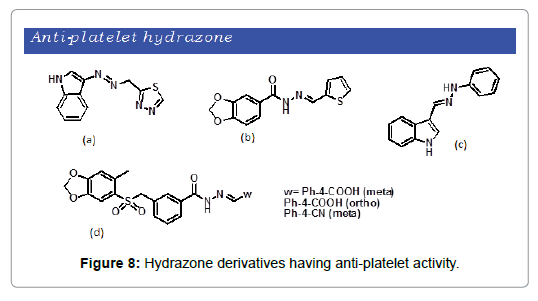
Platelet aggregation has a pivotal role in blood clotting and thrombosis. Thrombosis is a leading cause of cardiovascular diseases that are considered as the major cause of natural death [46]. A number of hydrazone derivatives has been synthesized and evaluated for antiplatelet property. A series of Indole moiety bearing hydrazones derivatives were reported by Mashayekhi et al. that showed antiplatelet aggregation character (Figure 8a) [47] Brito et al. demonstrated the synthesis and antiplatelet activity of a set of thienylacylhydrazone derivatives [48] The most active compound reported was ((2-thienylidene)3,4-methylenedioxybenzoylhydrazine (Figure 8b). A series of new hydrazones consisting of three families of arylsulfonate-acylhydrazone derivatives were synthesized through structural modification of known thrombin inhibitors by Lima et al. [49] All of the three classes of compounds indicated antiplatelet aggregation activity (Figure 8c). Mohebbi et al. has demonstrated the synthesis of a range of indole-based aryl(aroyl)hydrazone derivatives [50]. Among the resulting compounds, Phenylhydrazone derivatives exhibit significant antiplatelet activity (Figure 8d).
Conclusion
In summary hydrazone and its derivatives represent an exciting group of compounds with broad spectrum of pharmacological and biological activities. A verity of hydrazone derivatives have been designed synthesized and screened for its biological activities. The present review aim on exploring pharmacological potentials of hydrazone derivatives. In the current scenario of demand and search for potent chemical architectures, appropriate designing, synthesis, SAR and docking studies of novel hydrazone derivatives can lead to the development of new drugs molecules with diverse therapeutic activities.
References
- Verma G, Marella A, Shaquiquzzaman M, Akhtar M, Ali MR, et al. (2014) A review exploring biological activities of hydrazones. J Pharm Bioallied Sci 6: 69-80.
- Rollas S, Kucukguzel SG (2007) Biological activities of hydrazone derivatives. Molecules 12: 1910-1939.
- de Miranda AS, Junior W, da Silva Y, Alexandre-Moreira M, Castro R, et al. (2012) Design, synthesis, antinociceptive and anti-inflammatory activities of novel piroxicam analogues. Molecules 17: 14126-14145.
- Gokce M, Utku S, Kupeli E (2009) Synthesis and analgesic and anti-inflammatory activities 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(p-substituted/nonsubstituted benzal)hydrazone derivatives. Euro J Med Chem 44: 3760-3764.
- Sondhi SM, Dinodia M, Kumar A (2006) Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg Med Chem 14: 4657-4663.
- da Silva YK, Augusto CV, de Castro BM, de Albuquerque MGM, de Queiroz AC, et al. (2010) Synthesis and pharmacological evaluation of pyrazine N-acylhydrazone derivatives designed as novel analgesic and anti-inflammatory drug candidates. Bioorg Med Chem 18: 5007-5015.
- S. G. Mohamed Eissa AA, Khataibeh MH (2012) Chem Pharm Bull 60: 290-300.
- Lima PC, Lima LM, da Silva KC, Leda PH, de Miranda AL, et al. (2000) Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur J Med Chem 35: 187-200.
- Gorantla V, Gundla R, Jadav SS, Anugu SR, Chimakurthy J, et al. (2017) Molecular hybrid design, synthesis and biological evaluation of N-phenyl sulfonamide linked N-acyl hydrazone derivatives functioning as COX-2 inhibitors: new anti-inflammatory, anti-oxidant and anti-bacterial agents. New J Chem 41: 13516-13532.
- Abdelgawad MA, Labib MB, Abdel-Latif M (2017) Pyrazole-hydrazone derivatives as anti-inflammatory agents: Design, synthesis, biological evaluation, COX-1,2/5-LOX inhibition and docking study. Bioorg Chem74: 212-220.
- Wang SM, Zha GF, Rakesh KP, Darshini N, Shubhavathi T, et al. (2017) Synthesis of benzo[d]thiazole-hydrazone analogues: molecular docking and SAR studies of potential H+/K+ ATPase inhibitors and anti-inflammatory agents. Med Chem Comm 8: 1173-1189.
- Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009) Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Euro J Med Chem 44: 4362-4366.
- Khalil AM, Berghot MA, Gouda MA (2009) Synthesis and antibacterial activity of some new heterocycles incorporating phthalazine. Euro J Med Chem 44: 4448-4454.
- Abdel-Wahab BF, Awad GEA, Badria FA (2011) Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Euro J Med Chem 46: 1505-1511.
- Raja AS, Agarwal AK, Mahajan N, Pandeya SN, Ananthan S (2010) Antibacterial and antitubercular activities of some diphenyl hydrazones and semicarbazones. Indian J Chem 49B: 1384-1388.
- Bawa S, Kumar S, Drabu S, Kumar R (2009) Synthesis and antimicrobial activity of 2-chloro-6-methylquinoline hydrazone derivatives. J Pharm Bioallied Sci 1: 27-31.
- Kale VT, Burghate AS, Wadhal SA (2016) Synthesis and antibacterial activity of n-heterocyclic substituted hydrazone schiff’s bases. Indo Am J Pharm Res 6: 6404-6410.
- Kratky M, Bosze S, Baranyai Z, Stolarikova J, Vinsova J (2017) Synthesis and biological evolution of hydrazones derived from 4-(trifluoromethyl)benzohydrazide. Bioorg Med Chem Lett 27: 5185-5189.
- Casanova BB, Muniz MN, de Oliveira T, de Oliveira LF, Machado MM, et al. (2015) Synthesis and biological evaluation of hydrazone derivatives as antifungal agents. Molecules 20: 9229-9241.
- Altintop MD, Ozdemir A, Turan-Zitouni G, Ilgin S, Atli O, et al. (2012) Synthesis and biological evaluation of some hydrazone derivatives as new anticandidal and anticancer agents. Eur J Med Chem 58: 299-307.
- Chimenti F, Bizzarri B, Maccioni E, Secci D, Bolasco A, et al. (2007) Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg Med Chem Lett 17: 4635-4640.
- Ozkay Y, Tunali Y, Karaca H, Isikdag I (2010) Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur J med chem 45: 3293-3298.
- Vicini PFZ, Cozzini P, Doytchinova I, Zani F (2002) Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 37: 553-564.
- Kauthale S, Tekale S, Damale M, Sangshetti J, Pawar R (2017) Synthesis, antioxidant, antifungal, molecular docking and ADMET studies of some thiazolyl hydrazones. Bioorg Med Chem Lett 27: 3891-3896.
- Mohareb RM, Al-Omran F (2012) Reaction of pregnenolone with cyanoacetylhydrazine: novel synthesis of hydrazide-hydrazone, pyrazole, pyridine, thiazole, thiophene derivatives and their cytotoxicity evaluations. Steroids 77: 1551-1559.
- Aydin S, Basu NK, Arora P, Basu A, Talele TT, et al. (2013) Microwave assisted synthesis of some novel flurbiprofen hydrazidehydrazones as anti-HCV NS5B and anticancer agents. Marmara Pharm J 1: 26-34.
- Nasr T, Bondock S, Youns M (2014) Anticancer activity of new coumarin substituted hydrazide–hydrazone derivatives. Eur J Med Chem 76: 539-548.
- Savini L, Chiasserini L, Travagli V, Pellerano C, Novellino E, et al. (2004) New alpha-(N)-heterocyclichydrazones: evaluation of anticancer, anti-HIV and antimicrobial activity. Eur J Med Chem 39: 113-122.
- Sun K, Peng JD, Suo FZ, Zhang T, Fu YD, et al. (2017) Discovery of tranylcypromine analogs with an acylhydrazone substituent as LSD1 inactivators: Design, synthesis and their biological evaluation. Bioorg Med Chem Lett 27: 5036-5039.
- Labib MB, Philoppes JN, Lamie PF, Ahmed ER (2017) Azole-hydrazone derivatives: Design, synthesis, in vitro biological evaluation, dual EGFR/HER2 inhibitory activity, cell cycle analysis and molecular docking study as anticancer agents. Bioorganic chemistry 76: 67-80.
- de Oliveira KN, Costa P, Santin JR, Mazzambani L, Burger C, et al. (2011) Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg Med Chem19: 4295-4306.
- Gokhan-Kelekci N, Koyunoglu S, Yabanoglu S, Yelekci K, Ozgen O, et al. (2009) New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg Med Chem 17: 675-689.
- Nedime Ergeq NSG, Demirdamar R (1998) Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur J Med Chem 33: 143-148.
- Pandeya SN, Manjula H, Singh PN (2000) Antidepressant activity of some phenylaceticacid hydrazones and 2-chlorophenyl semicarbazones. Indian J Physiol Pharmacol 44: 509-510.
- Kulandasamy R, Adhikari AV, Stables JP (2009) A new class of anticonvulsants possessing 6Hz activity: 3,4-Dialkyloxy thiophene bishydrazones. Eur J Med Chem 44: 4376-4384.
- Wagle S, Adhikari AV, Kumari NS (2009) Synthesis of some new 4-styryltetrazolo[1,5-a]quinoxaline and 1-substituted-4-styryl[1,2,4]triazolo[4,3-a]quinoxaline derivatives as potent anticonvulsants. Eur J Med Chem 44: 1135-1143.
- Ragavendran JV, Sriram D, Patel SK, Reddy IV, Bharathwajan N, et al. (2007) Design and synthesis of anticonvulsants from a combined phthalimide–GABA–anilide and hydrazone pharmacophore. Eur J Med Chem 42: 146-151.
- Sridhar SK, Pamdeya SN, Stables JP, Ramesh A (2002) Anticonvulsant activity of hydrazones, schiff and mannich bases of isatin derivatives. Eur J Pharm Sci 16: 129-132.
- Jonathan R. Dimmock, Vashishta SV, Stables JP (2000) Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur J Med Chem 35: 241-248.
- Srivastav Sanchit, Kanishk L, Pandeya SN (2017) World J Pharm Res 5: 2101-2108.
- Leal CM, Pereira SL, Kummerle AE, Leal DM, Tesch R, et al. (2012) Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur J Med Chem  55: 49-57.
- Silva AG, Zapata-Sudo G, Kummerle AE, Fraga CA, Barreiro EJ, et al. (2005) Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg Med Chem 13: 3431-3437.
- Bakale RP, Naik GN, Mangannavar CV, Muchchandi IS, Shcherbakov IN, et al. (2014) Mixed ligand complex via zinc(II)-mediated in situ oxidative heterocyclization of hydrochloride salt of 2-chlorobenzaldehyde hydralazine hydrazone as potential of antihypertensive agent. Eur J Med Chem 73: 38-45.
- Narang R, Narasimhan B, Sharma S (2012) A review on biological activities and chemical synthesis of hydrazide derivatives. Current Med Chem 19: 569-612.
- Mashayekhi V, Tehrani HMEK, Amidi S, Koberfard F (2013) Synthesis of novel indole hydrazone derivatives and evaluation of their antiplatelet aggregation activity. Chem Pharm Bull (Tokyo) 61: 144-150.
- Brito FC, Kummerle AE, Lugnier C, Fraga CA, Barreiro EJ, et al. (2010) Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 638: 5-12.
- Lima LM, Frattani FS, Dos Santos JL, Castro HC, Fraga CA, et al. (2008) Synthesis and anti-platelet activity of novel arylsulfonate–acylhydrazone derivatives, designed as antithrombotic candidates. Eur J Med Chem 43: 348-356.
- Tehrani KHME, Mashayekhi V, Hashemid M, Kobarfard F, Gharebaghid F, et al. (2015) Synthesis, Antiplatelet Activity and Cytotoxicity Assessment of Indole-Based Hydrazone Derivatives. Iran J Pharm Res 14: 1077-1086.
Citation: Hussain I, Ali A (2017) Exploring the Pharmacological Activities of Hydrazone Derivatives: A Review. J Phytochemistry Biochem 1: 104
Copyright: © 2017 Hussain I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 7218
- [From(publication date): 0-2017 - Apr 28, 2025]
- Breakdown by view type
- HTML page views: 5805
- PDF downloads: 1413