Research Article Open Access
Experimental Study on Factors Affecting the Recovery of Nickel from Spent Catalyst
Khalid MM and Athraa BA*
Chemical Engineering Department, Collage of Engineering, University of Nahrain, Iraq
- *Corresponding Author:
- Athraa BA
Chemical Engineering Department
/Collage of Engineering/University of Nahrain, Iraq
Tel: +964 770 716 0860
E-mail: athraaalshemary25@gmail.com
Received Date: December 07, 2016 Accepted Date: December 20, 2016 Published Date: January 30, 2017
Citation: Khalid MM, Athraa BA (2017) Experimental Study on Factors Affecting the Recovery of Nickel from Spent Catalyst. J Powder Metall Min 6: 146. doi:10.4172/2168-9806.1000146
Copyright: © 2016 Khalid MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
In this study, the recovery of nickel from spent NiMo/Al2O3 catalyst was investigated using 1 L stainless steel autoclave batch reactor. The effect of leaching process parameters such as temperature (40-80°C), time (1-4 h), pressure (1-3 bar), average particle size (69-550 μm) and agitation speed (400-600 min-1) on nickel extraction. The nickel percent in spent catalyst was found about 2.18%. The results obtained show that recovery of about 94% of nickel from spent catalyst was accomplished under the following conditions: temperature 80°C, pressure 1 bar, average particle size 200 μm, agitation speed 600 min-1, nitric acid concentration 40% and solid to liquid ratio1:50 after 4 h.
Keywords
Leaching; Spent catalyst; Nickel recovery
Introduction
Nickel is widely used as a catalyst in chemical and petrochemical industries. Due to their low cost competing replacements, these are the catalysts of choice in several industries. Such catalysts use silica and alumina as supports [1]. As these catalysts, is used, metals from the feedstock deposit on the internal surface of its pores and its external surface, eventually plugging the pores and decreasing the activity of the catalyst to such an extent it does not give the desired product quality [2]. If the regeneration of these catalysts is not economically feasible, they are discarded from petroleum processing and, at present, large dumps of spent catalysts are growing. Hence, the spent catalysts disposal poses an unavoidable environmental issue, which necessarily requires high capital investment, huge swaths of land and massive efforts [3]. In many countries, the hazardous nature of the spent catalysts is attracting the attention of environmental authorities and the refiners are suffering pressures from them for safe handling of spent catalysts. Several alternative methods such as reclamation of metals, disposal in landfills, utilisation as raw materials to produce other useful products and regeneration and reuse are available to the refiners to deal with the spent catalyst problem. The choice between these options depends on technical feasibility and economic considerations [4]. The recovery methods of nickel from spent catalysts can be classified as thermal treatment, chlorination, acid leaching, alkali leaching, bioleaching. The pyrometallurgy process was applied to the spent catalyst by Llanos et al. [5] who used alkali roasting, and Kim et al. [6] explored a low-temperature sulphuric acid baking and mild acid leaching for maximum dissolution of metal values from a spent Ni- MO/Al2O3 hydroprocessing catalyst. However, hydrometallurgical process can be regarded as the desired route for recovery of metals due to its economic operation, environmentally friendly and simplicity. Alkaline or acidic leaching was used by many researchers by using many reagents. Marcantonio [7] invented a process to extract valuable metals. In this study, the spent catalyst was roasted at (400-600°C) and then contacted with an aqueous solution of ammonium sulfate ((NH4)2SO2), ammonium carbonate ((NH4)2CO2) and hydrogen peroxide. The optimum leach conditions were 0.5 M (NH4)2SO4, 1 M (NH4)2CO3 and 80°C for three hours was obtained. Angelidis et al. applied a two-step of leaching alkali (sodium hydroxide) followed by acid (sulfuric acid) procedure and studied the selective recovery of metals, such as Mo, Co and Ni, from hydrodesulfurization (Mo- Ni/Al2O3–SiO2 and Mo-Co/Al2O3) catalysts [8]. Al-Mansi and Abdel Monem used sulfuric acid as a solvent to recovery of nickel from the spent catalyst (NiO/Al2O3) resulting from the steam reforming process [9]. It was found that 99% of nickel was extracted at 50% sulfuric acid concentration, particle size less than 500 μm, solid to liquid ratio (1:12) by weight for more than 800 rpm and 5 h at 100°C. Sahu et al. invented a process for nickel and alumina recovery by using 3 vol.% of sulfuric acid with a small amounts of a persulphate salt additives (such as potassium, sodium and ammonium) [10]. It was concluded that as a leaching process is achieved with sulfuric acid alone, the recovery of nickel about 11.95% at 70°C and 4 ml/g after 6 h. High percent of nickel about 99.6% was extracted by using the additives and at 90°C, 1 g of persulphate salt, 4 ml/g liquid to solid ratio and after 1 h. Mulak et al. discussed a method of leaching by using oxalic acid solution with hydrogen peroxide addition [11]. The optimum conditions that give the high extraction of metals 65% Ni, 90% Mo, 33% Al and 94% V was obtained (3 M H2O2 and 0.5 M H2C2O4 solution at 50°C after 4 h). A combined acid-leaching was proposed by Lai et al. to recover valuable metals from spent hydrodesulfurization (HDS) catalyst [12]. An acid solution consisting of concentrated HCl/H2SO4/HNO3 with a volume ratio of 1:1:2 was used to leach the metals. The leaching yields of target metals (Ni, Mo and V) in the 1st stage of leaching reached 99, 90 and 99%, respectively under these conditions, solid to liquid ratio 40 g/l, 1 h leaching time, and 70°C temperature. Oza et al. investigated recovery of nickel from spent nickel catalysts using ultrasonication-assisted nitric acid leaching [13]. The effect on nickel recovery of temperature, solid to liquid (S:L) ratio, time of digestion, and nitric acid concentration were studied in detail and optimized for the ultrasonication route. High purity and faster recovery were accomplished for ultrasonicationassisted leaching than the chelation method (7 h) and conventional acid leaching (9 h). It was found that 95% recovery of nickel was produced at 90°C, S:L ratio 1:10 (g:ml) and 40% nitric acid concentration in 50 min. Mousa et al. investigated the recovery of nickel from the fly ash of the fired power station residue using nitric acid [14]. The influences of the acid concentration (10%, 20% and 30%), temperature (30-60°C) and time were studied. The recovery rates increase with the increase in temperature and nitric acid concentration, and at the first half of an hour, most of the nickel was recovered. 80% of nickel was recovered at 30% nitric acid solution and 60°C. Mousa et al. used ammonium hydroxide to recover nickel from the fly ash of heavy oil [15]. The effects of rotational speed (200-1000 rpm) and temperature (50- 80°C) were studied. It was found that the nickel recovery increased with increasing temperature till 70°C. The best rotational speed was obtained at 600 rpm where above 600 rpm the nickel recovery doesn’t increase further. The controlling steps were investigated, and it was found that the transfer through the film surrounding the particle (interphase) is the controlling step. Sheik et al. studied the influence of leaching parameters such as temperature (60-90°C), acid concentration (1.5-5 M), and average particle size (75-601 μm) on nickel recovery using nitric acid. Romas et al. used nitric acid leaching of a spent NiOAl 2O3 catalyst [16,17]. The results obtained show that at the following conditions: temperature 65°C, average particle size 75 μm, reaction time 120 min, stirring speed 600 min-1, nitric acid concentration 50% and solid/liquid ratio 1:3. About 85% extraction was achieved. In this study the effects of temperature, time, average particle size, pressure, and agitation speed on the recovery of nickel from spent catalyst by using nitric acid solution as a leaching agent were investigated. The optimum conditions that give the maximum percentage of nickel were obtained.
Experimental device
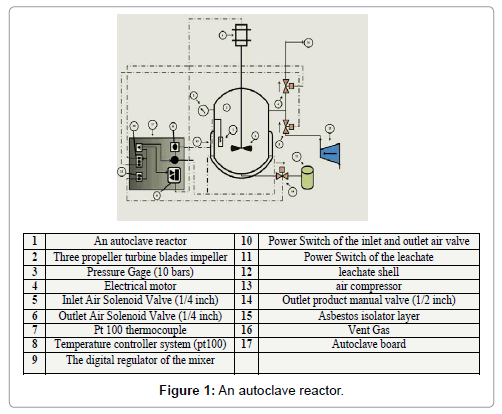
An autoclave stainless steel reactor, was used to process the experiments, its volume (1 liter). The inner diameter was 11.5, the thickness of the shell wall was 10 mm, and the height was 30 cm. This autoclave was provided by a 3-phase motor with Nmax equal to 1300 rpm, which able to agitate mixer that consists of stainless steel shaft and three turbine propeller blades impeller, the diameter of the impeller was 3 cm. The agitation speed was regulated by the digital regulator (50 digits) in the board, each degree in the board equals to almost 22 rpm. Six heaters were tied uniformly around the shell of the reactor (100 kW for each) and isolated by asbestos cloth layer the temperature of the reactor was controlled by controller. Pt 100 stainless steel thermocouple was put on the inside wall of the shell and connected to the regulating system on the board. Two solenoid valves (1/4 inch) were used to charge air to the reactor and discharge vent gases from it, which put on the upper side of the reactor wall. Manual stainless steel valve was planted on the bottom of the reactor to discharge the mixture of the product after leaching process was finished. Gage valve (10 bars) was connected to the wall of the shell to read the inside reactor pressure. All of the control options were put on the control board outside of laboratory hood. Figure 1 shows the details of the apparatus [18].
Materials and Method
The spent NiMo/Al2O3 catalysts used in this study were supplied by Midland oil Company refinery of Al-Dora which used these catalysts as a hydrogenation catalyst. The weight percent of nickel in spent catalyst was 2.18%. Solid sample of roasted spent catalyst particles were analyzed for nickel content by mean of Atomic Absorption Spectrometry at the analytical laboratory of Ibn Sina Company. This value was used in the calculation of nickel recovery for each experiment as shown next section. The spent catalyst was roasted at 500°C about 5 h to minimize the amount of oil and sulfur present on the catalyst; then the samples were ground and sieved to various particle sizes (69- 550) μm. The nitric acid was diluted to 40% solution and the solid to liquid ratio was 1/50 (10 gm of spent catalyst in 0.5 liters of nitric acid solution) this ratio remains constant at all experiments. The autoclave reactor was switched on, and the temperature was adjusted to (40- 80°C). The agitated regulator was set at the desired mixing speed (400- 800 min-1). The HNO3 solution was added to 10 gm of spent catalyst and inserted into the reactor. The air was compressed to the reactor to reach the desired pressure (1-3 bar). After the time you want (1-4 h) was reached the solenoid valve of discharging vent gas was switched on until 2 bar pressure was reached and then open the manual product valve then the solution was filtered to separate the unreacted nickel and impurities. Samples of solution from all experiments were analyzed for nickel using Atomic Absorption Spectrometry.
Results and Discussion
The dissolution of nickel oxide from the spent catalyst follows the following reaction [16,17].
NiO+2HNO3→Ni(NO3)+H2O (1)
Effect of reaction temperature
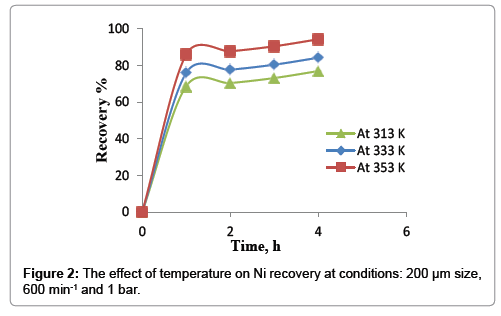
The effect of temperature on nickel recovery is shown in Figure 2 under the conditions of atmospheric pressure, average particle size of 200 μm and agitation speed of 600 min-1. The dissolution rate was low at lower temperature and increase with an increase in leaching temperature. At 40°C, 76.9% of nickel extraction was obtained in 4 hours while the percent increased to 86% by increasing the temperature to 80°C in the first hour. After, Ni extraction increase slowly to reach 94.3% in 4 hours. This increasing may be imputed to many reasons; firstly, is rising the temperature of leaching will increase the rate of reaction between the value mineral and the solvent (spent catalyst and nitric acid) according to Arrhenius equation Eq.(2) that relate the temperature and the reaction rate takes place at this temperature.
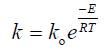 (2)
(2)
Secondly, is increasing in diffusion coefficient with the temperature increasing. Another reason that an increasing in a leaching temperature causes an increase of kinetic energy of particles (molecules’ speed) and collide very frequently causing an increase of collision frequency of the molecules which leads to accelerating the reaction.
Effect of pressure
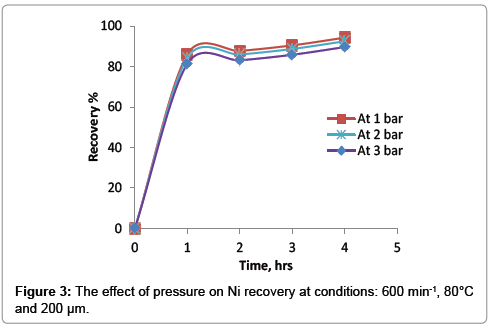
The effect of pressure on the recovery of nickel was shown in Figure 3. It was found that there is an adverse effect of the pressure on the leaching recovery. As the pressure increase the percentage of nickel will slightly decrease. At optimum conditions, the recovery of nickel is 94.3% at atmospheric pressure and decreases to 89.6%. while the pressure increases to 3 bar.
Effect of average particle size
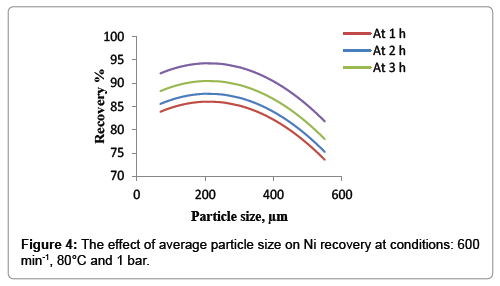
In order to study the effect of average particle size on the nickel recovery a set of experiments were conducted, Figure 4 was illustrated at the conditions of other variables of 80°C temperature, 600 min-1 agitation speed and atmospheric pressure. The results show that the recovery percent of nickel increases with reduction of the average particle size. This behavior continues until the mean particle size reaches 250 μm because the reduction of average particle size increases the interfacial area between the liquid and solid; therefore the smaller is the distance that the solute must diffuse within the solid and the higher is the transfer rate of the reactant and products reaction. From 250 μm to 180 μm it was observed that no significant effect of average particle size on the nickel recovery and it remains almost constant. At very fine particles (lower than 180 μm) it was found that the nickel dissolution rate was decreased very slightly with the decreasing of average particle size, these phenomena may be according to impede the circulation of liquid with the presence of the fine particles and thereby lead to reduced mixing speed and thus the recovery of nickel will decrease [19]. The optimum average particle size of 200 μm was observed in this study.
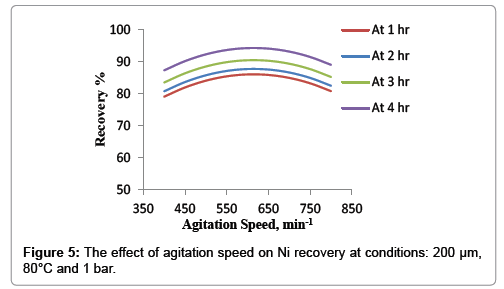
Effect of agitation speed
Figure 5 shows the effect of agitation speed at the conditions of temperature 80°C, average particle size 200 μm and atmospheric pressure. It was concluded that the figure could be divided into two regions. The first one begins from the lowest agitation speed to the 600 min-1. It was observed slightly increasing of nickel recovery with agitation speed from 400 min-1 to 600 min-1 which only 7% of recovery was achieved. The second region was detected after 600 min-1, in this region the agitation speed did not significantly influence the leaching rate. This indicates that the external diffusion of HNO3 between the surface of particles and the fluid is fast, and hence does not control the dissolution of nickel within the investigated range of agitation speed this agree with Ramos and Mousa et al. study [18].
Conclusions
From the results of this investigation the following conclusions can be drawn:
• The optimum leaching conditions were obtained as temperature 80°C, pressure 1 bar, average particle size 200 μm and agitation speed 600 min-1 after 4 h which give the percentage recovery about 94%.
• The rate of nickel recovery was increased with increasing the temperature up to 80°C and the pressure give the adverse effect on the nickel recovery which decreases with the pressure increasing.
• The nickel percentage recovery increases with the reduction of average particle size until 250 μm. Then the reduction of average particle size doesn’t affect the nickel recovery.
References
- Sahu KK, Pandey BD, Chand P (2004) Process for recovery of nickel from spent catalyst. Council of Scientific and Industrial Research. US Patent 6,733,564.
- Hubred GL (1985) Leaching nickel, cobalt, molybdenum, tungsten, and vanadium from spent hydroprocessing catalysts. Chevron Research Company. US Patent 4,514,368.
- Henry E (1965) Method for the recovery of metal values from a spent hydrocarbon conversion catalyst. Sinclair Research Inc. US Patent 3,180,706.
- Marafi M, Stanislaus A (2003) Options and processes for spent catalyst handling and utilization. Journal of hazardous materials 101: 123-132
- Llanos ZR, Provoost GF, Deering WG, Debaene FJ (1997) Integrated process for the recovery of metals and fused alumina from spent catalysts. Gulf Chemical & Metallurgical Corporation. US Patent 5,702,500.
- Kim HI, Park KH, Mishra D (2009) Influence of sulfuric acid baking on leaching of spent Ni–Mo/Al2O3 hydro-processing catalyst. Hydrometallurgy 98: 192-195.
- Marcantonio PJ (1990) Leaching cobalt, molybdenum, nickel, and vanadium from spent hydroprocessing catalysts. Chevron Research Company . US Patent 4,927,794
- Angelidis TN, Tourasanidis E, Marinou E, Stalidis GA (1995) Selective dissolution of critical metals from diesel and naptha spent hydrodesulphurization catalysts. Resources, conservation and recycling 13: 269-282.
- Al-Mansi NM and Monem NA (2002) Recovery of nickel oxide from spent catalyst. Waste management, 22: 85-90
- Sahu KK, Agarwal A, Pandey BD (2005) Nickel recovery from spent nickel catalyst. Waste management & research 23: 148-154
- Mulak W, Szymczycha A, Lesniewicz A, Zyrnicki W (2006) Preliminary results of metals leaching from a spent hydrodesulphurization (HDS) catalyst. Physicochemical Problems of Mineral Processing 40: 69-76.
- Lai YC, Lee WJ, Huang KL, Wu CM (2008) Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process. Journal of hazardous materials 154: 588-594
- Oza R, Shah N, Patel S (2011) Recovery of nickel from spent catalysts using ultrasonicationâ┬?┬Éassisted leaching. Journal of Chemical Technology and Biotechnology 86: 1276-1281.
- Mousa K, Fouad A, May E (2011) Nickel recovery from residue of heavy oil using nitric acid. Journal of petroleum research and studies pp. 87-96.
- Mousa K, Ali I, Hadi H (2012) Extraction of nickel from fly ash of heavy oil using ammonium hydroxide. Al-Qadisiya Journal for Engineering Sciences 5: 38-43.
- Sheik AR, Ghosh MK, Sanjay K, Subbaiah T, Mishra BK (2013) Dissolution kinetics of nickel from spent catalyst in nitric acid medium. Journal of the Taiwan Institute of Chemical Engineers 44: 34-39.
- Ramos-Cano J, González-Zamarripa G, Carrillo-Pedroza FR, de Jesús Soria-Aguilar M, Hurtado-Macías A, et al. (2016) Kinetics and statistical analysis of nickel leaching from spent catalyst in nitric acid solution. International Journal of Mineral Processing 148: 41-47.
- Mousa KM, Dawood MM (2012) Experimental, modelling and kinetic study for the recovery of molybdenum from spent (Ni-Mo/Al2O3) catalyst of hydrotreating process. In Engineering Sciences (FNCES), First National Conference for IEEE, pp. 1-3
- Levenspiel O (1999) Chemical Reaction engineering (3rdedn) John-Wiley and Sons Inc. New York 566-585.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 4513
- [From(publication date):
April-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 3543
- PDF downloads : 970