Research Article Open Access
Experimental and Kinetic Studies on Acid Red 88 Dye (AR88) Adsorption by Azolla filiculoides
| Davoud Balarak1 and Yousef Mahdavi2* | |
| 1Department of Environmental Health, Health Promotion Research Center, School of Public Health, Zahedan University of Medical Sciences, Zahedan, Iran | |
| 2MSc Student of Environmental Health engineering, Student Research Committee, Mazandaran University of Medical Sciences, Mazandaran, Iran | |
| Corresponding Author : | Yousef Mahdavi MSc Student of Environmental Health engineering Student Research Committee, Mazandaran University of Medical Sciences, Mazandaran, Iran Tel: 0098-9388517121 E-mail: mahdaviyusef@gmail.com |
| Received: August 08, 2015; Accepted: December 03, 2015; Published: January 01, 2016 | |
| Citation: Balarak D, Mahdavi Y (2016) Experimental and Kinetic Studies on Acid Red 88 Dye (AR88) Adsorption by Azolla filiculoides. Biochem Physiol 5:190. doi:10.4172/2168-9652.1000190 | |
| Copyright: © 2016 Balarak D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The use of Azolla filiculoides (AF) for the removal of Acid Red 88 dye (AR88) from aqueous solutions at different contact times, temperatures, pH, adsorbent doses and initial dye concentration was investigated. The extent of dye removal decreased with increasing adsorbent dosage and AR88 concentration, also increased with increasing contact time and temperature. The adsorption isotherms are described by means of the Langmuir, Freundlich, Tempkin and Sips isotherms. It was found that the Langmuir equation fit better than the other equation. Maximum adsorption capacity (qm) was calculated at different temperatures (20, 30, 40 and 50°C) 22.45, 23.95, 25.29 and 26.17 mg/g, respectively. The adsorption kinetics of Acid Red 88 could be described by the pseudo-second-order reaction model. The obtained results are: (1) high levels of color removal (>98%) were achieved with low contact times adsorbent/dye (less than 90 min contact); and (2) the whole Azolla filiculoides can be successfully used as adsorbent of AR88 in aqueous solutions. Azolla biomass, an inexpensive and easily available material, can be alternative for more costly adsorbents used for dye removal in wastewater treatment processes.
| Keywords |
| Adsorption; Isotherm; Acid Red 88; Azolla filiculoides; Kinetics |
| Introduction |
| Recently, the societies have been involved to the serious environmental issues due to development of industries and use of various chemical in industries [1-3]. The dyes have been introduced as a major group of pollutants. Although the dyes are applied in various industries; however, the textile industries are the main sectors in consumption of dyes [4-6]. Annually, a large amount of dyes are generated in the world. They are classified into several groups. The reactive dyes are one of these groups which are commonly applied in coloring of strings, wool and polyamide fibers. it has been expressed that 50% of reactive dyes is lost during the dying process [7]. The studies has been illustrated that there is a variety of unpleasant and detrimental consequences for human and environment associated with use of dyes [8-13]. Therefore, the dye removal from industrial effluents is high priority subject. Among the various techniques, the adsorption process has been showed substantial potential for dye removal from industrial effluents [14-16]. Currently, the activated carbon has become most popular adsorbent; but this is not economical adsorbent due to its high operation and regeneration costs [6,17,18]. Therefore, there is a strong demand to find a cost-effective alternative for dye removal from effluents. A number of materials such as Trametes versicolor, Cupuassu shell, Stoechospermum marginatum, pine tree leaves, Loofa egyptiaca and etc. were employed as adsorbent for dye removal [1,19-22]. Since the aquatic plant are capable to grow in contaminated water therefore they has been used in several study to remove the pollutants [23]. Azolla filiculoides is one of this plants which it is commonly found in ditches, ponds and slow moving streams. Its high growth rate is considered as main feature of Azolla [24,25]. Recently, the Azolla has been used in several studies to remove the pollutants [26-28]. Thus, the present study was conducted for following purposes: a) to investigate the potential of dried azolla for AR88 dye removal, b) to assess the effect of several variables including contact time, pH, temperature, initial AR88 dye concentration and adsorbent does on dye removal efficiency, c) to study the adsorption isotherm and kinetic. |
| Materials and Methods |
| Azolla filiculoides was collected from Anzali wetland, Iran. The biomass was oven-dried at 50°C for 12 h, then ground and screened through a sieve with 110 meshes to obtain particle sizes less than 0.135 mm. The crushed particles were then treated with 0.1 M HCl for 5 h, then washing with distilled water and then kept for shaded dry. The resultant biomass was subsequently used in sorption experiments [29]. |
| The morphological features and surface characteristics of A. filiculoides before and after use were examined using an environmental scanning electron microscopy (ESEM) instrument (Philips XL30). The specific surface area of adsorbent was determined by the BET method using the Gemini 2357 of micrometrics Co. |
| Acid Red 88 (EC number=216-760-3; chemical formula: C20H13N2NaO4S; molecular weight=400.39 g/mol; maximum wavelength=503 nm) were obtained from Merck Co. and used without further purification. All AR88 solutions used in this study were prepared by weighing and dissolving the required amounts AR88 in distilled water. The chemical structures of AR88 are presented in Figure 1. |
| Batch adsorption studies |
| Batch adsorption experiments were performed using 100 ml glass bottles with addition of 0.4 mg purified AF and 50 ml of AR88 solution of increased initial concentrations (C0) from 25 to 200 mg/L. The glass bottles were sealed and placed within a temperature control box to maintain water temperature. The pH of the samples was adjusted by adding 0.1 M HCl or 0.1 M NaOH to each 200 ml of the prepared solution to pH 7. The pH of solutions was measured with a pH meter. In the experiments on the effect of temperature, the temperature was held at 20, 30, 40 and 50 and the pH was fixed at 7. At the end of the equilibrium period, the suspensions were separated for later analysis of the dye concentration. The amount of AR88 adsorption at equilibrium qe (mg/g) was calculated from the following equation [30]: |
| Where C0 and Ce (mg/L) are the liquid-phase concentrations of dye at initial and equilibrium, respectively, V (L) the volume of the solution and W (g) is the mass of adsorbent used. The concentration of AR88 after and before adsorption was determined using a spectrophotometer (kmax = 503 nm). |
| The dye removal percentage can be calculated as follows [31]: |
| Results and Discussion |
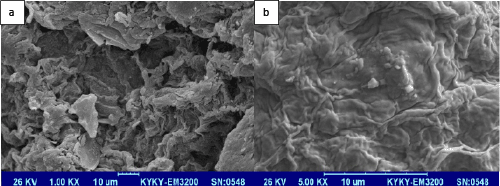
| The Dried Azolla filiculoides was also examined before and after use using environmental scanning electron microscopy. Figure 2 (a) clearly shows the pore textural structure of dried AB before use. However, as shown in Figure 2 (b), clear pore textural structure is not observed on the surface of dried AB after use which could be due to either agglomeration on the surface or the incursion of AR88 into the pores of dried AB. |
| Effect of contact time |
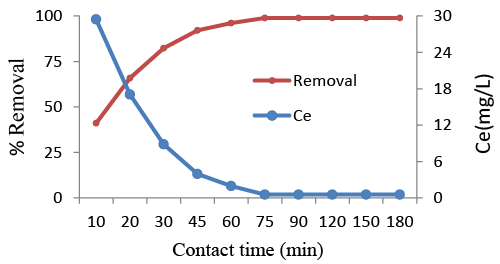
| The adsorption of AR88 onto Azolla was studied as a function of contact time in order to decide whether the equilibrium was reached. For this, 50 mg L−1 of AR88 solutions at pH 3 were contacted with 4 g L−1 of Azolla suspensions. The samples were taken at different periods of time and analyzed for their AR88 concentration (Figure 3). Dye adsorption onto Azolla filiculoides increased from 42.1% to 98.9% when the contact time was increased from 10 to 75 min. The AR88 adsorption rate is high at the beginning of the experiment because initially the adsorption sites are more available and AR88 ions are easily adsorbed on these sites [32,33]. The equilibrium can be reached within 75 min, and thus, further adsorption experiments were carried out for a contact time of 75 min. |
| Effect of pH |
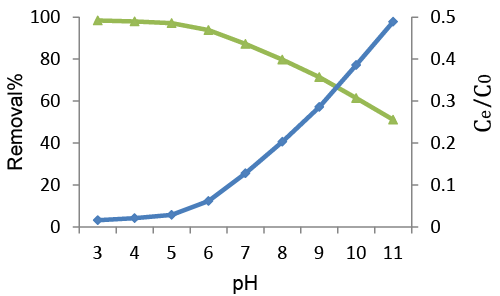
| pH is an important factor in controlling the removal of AR88 dye onto AF adsorbent. The removal of AR88 dye was studied at a temperature of 27 ± 2 °C and 50 mg/L (Figure 4). Concentration by varying the pH from 3.0 to 11.0, the solution was equilibrated for 75 min. The result indicates that AF biomass adsorbent showed good removal capacity in acidic medium than in basic medium. The percentage removal of AR88 dye of adsorption on AF biomass adsorbents progressively decreased on the pH of the solution increased from 3.00 to 11.0. At higher pH, the percentage removal was found to decrease because the surface area of the adsorbent was more protonated and competitive adsorption occurred between H+ and free AR88 ions and their OH- towards the fixation sites [29]. Therefore, H+ ions react with anionic functional groups on the surface of the adsorbent and results in restriction of the number of binding sites favorable for the removal of AR88. However, a favorable increase in percentage removal for AF biomass adsorbent was observed below pH 3. |
| Effect of adsorbent dosage |
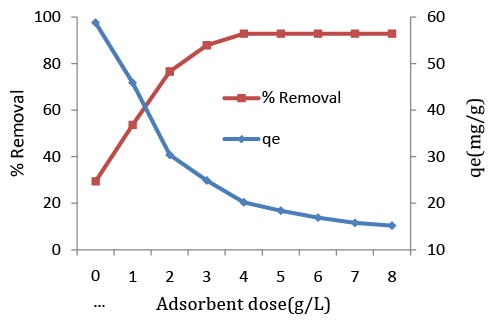
| Adsorbent dosage is an important parameter because this factor determines the capacity of an adsorbent for a given initial concentration of the adsorbate. The effect of adsorbent dosage (adsorbent prepared in different batch) was studied on AR88 removal by keeping all other experimental conditions constant (Figure 5). The results show that as the adsorbent concentration increased, the percentage of adsorption also increased, but the amount adsorbed per unit mass of the adsorbent decreased considerably. The decrease in unit adsorption with increasing dose of adsorbent is basically due to adsorption sites remaining unsaturated during the adsorption reaction [34,35]. |
| Effect of initial dye concentration |
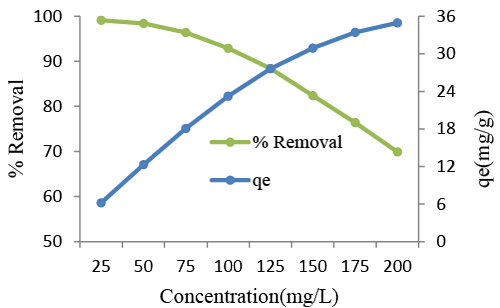
| The equilibrium adsorption capacity of the adsorbent for AR88 increased with increasing initial dye concentration, as is also shown in Figure 6. The dye adsorption capacity is highly concentration dependent. The increase in loading capacity of the adsorbent with relation to dye ions is probably due to a high driving force for mass transfer [36,37]. In fact, the more concentrated the solution, the better the adsorption. At 27 °C, when the initial dye concentration was increased from 25 to 200 mg/L, the loading capacity of dried adsorbent increased from 6.19 to 34.95 mg of AR88 per gram of Azolla biomass. |
| Adsorption isotherms |
| The adsorption isotherms provide information about the distribution of dye molecules within the adsorbent at equilibrium. In general, adsorption isotherm studies are carried out in order to correlate the adsorption capacity and the residual concentration of the adsorbate, present in the aqueous solution. Several isotherm equations are available in literature; four of them were selected for this study: Langmuir, Freundlich, Tempkin and Sips equations. |
| The Langmuir equation [38]: |
| The Freundlich equation [39]: |
| The Tempkin equation [40]: |
| The Sips equation [41]: |
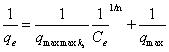 |
| KL is the Langmuir constant (L mg−1) related to the affinity of binding sites and the free energy of sorption; Qmax (mg g−1) represent the maximum adsorption capacity when the surface is fully covered with dye molecules that assists in the comparison of adsorption performance; qe is the dye concentration at equilibrium onto the adsorbent (mg g−1); Ce is the dye concentration at equilibrium isolation (mg L−1). Kf and n are the Freundlich constants, A and B are the Tempkin constant, T is the absolute temperature in Kelvin R is the universal gas constant. The Ks (1/mg) and qmax (mg/g) are the Sips equilibrium constant and maximum adsorption capacity values. The essential Characteristic of the isotherm could be expressed by a dimensionless constant called equilibrium parameter RL, Defined by as follows [42]: |
| The value of RL indicates the type of the isotherm; which is unfavorable (RL>1), linear (RL=1), favorable (0<RL<1) or irreversible (RL=0). |
| Table 1 lists the parameters of the isotherm models along with the regression coefficients (R2). The R2 values in Table 1 reveal that the Langmuir isotherm fits the experimental results better than other model, implying that the adsorption of AR88 onto AF biomass follows the mechanism of monolayer adsorption (chemisorption) on a homogenous surface. Although the Langmuir and Sips equations have similar correlation coefficients, the Langmuir is a simpler equation and therefore easier to use. Consequently, it was selected to predict qe values in the present work. |
| The effect of temperature on the adsorption rate of AR88 on Azolla biomass was investigated at four different temperatures (20, 30, 40 and 50 °C) using initial concentration of 100 mg/L (Table 1). The major effect of temperature is influence by the diffusion rate of adsorbate molecules and internal pores of the adsorbent particle. The increase in temperature from 20 to 50 °C increased the Azolla biomass monolayer adsorption capacities from 22.45 to 26.17 mg/g (Table 1). This phenomenon indicates that the adsorption process is endothermic in nature. This may be due to the mobility of molecules which increases generally with a rise in temperature, thereby facilitating the formation of surface monolayers. |
| Maximum adsorption capacity (qm) was calculated at different temperatures (20, 30, 40 and 50 °C) 22.45, 23.95, 25.29 and 26.17 mg/g, respectively which this value is greater than obtained qe for Lemna (9.8 mg/g) and Canola (11.2 mg/g), which it probably is due to presence of greater specific surface area in studied adsorbent than the Canola and Lemna [9,38]. |
| Adsorption kinetics |
| In order to examine the mechanism and rate-controlling step in the overall adsorption process, three kinetic models, pseudo-firstorder, pseudo-second-order and intra-particle diffusion, are adopted to investigate the adsorption process. |
| The pseudo-first-order equation can be expressed as the following equation [43]: |
| Where qe and qt (mg/g) are the AR88 adsorption capacity at equilibrium and at time t (min), respectively, and k1 (min-1) is the rate constant of the pseudo-first-order. |
| The parameters k1 and qe could be calculated from the slope and intercept of the plots of log (qe-qt) versus t and are given in Table 2. The values of the correlation coefficient R2 obtained at all the studied concentrations are low, in the range 0.689–0.784. Furthermore, the experimental values of qe, exp (mg/g) are far from the calculated qe, cal (mg/g). This suggests that the pseudo-first-order kinetic model is not suitable to describe the adsorption process. |
| On the other hand, the pseudo-second-order kinetic model can be expressed in linear form as follows [44]: |
| Where k2 (g/mg min) is the rate constant of the pseudo-secondorder. The kinetic parameters of AR88 under different conditions were calculated and are given in Table 2. It is seen that the pseudo-second order model well represented the experimental data (R2>0.995). This suggests that adsorption AR88 onto Azolla biomass predominantly follows the pseudo-second-order kinetic model. Besides, the calculated data (qe, cal) agree well with the experimental data (qe, exp). The parameters are listed in Table 2. The same conclusion was found in the literature on the adsorption of AR88 by lemna minor [29] . |
| The two models above cannot identify the diffusion mechanism during the adsorption process, so the experimental data are tested by the intra-particle diffusion model, which can be expressed by following equation [45]: |
| Where qt (mg/g) is the amount of AR88 adsorbed at time t (min), k (mg/g min1/2) is the intra-particle diffusion rate constant and C is the intercept. |
| The adsorption of a solute from solution by porous adsorbents is essentially related to three consecutive steps. The first step is the external surface adsorption or the instantaneous adsorption. The second step is the gradual adsorption stage where intra-particle diffusion is rate-limiting. The third step is the final equilibrium stage where intra-particle diffusion started to slow down due to the extremely low adsorbate concentrations left in the solutions. One or more of these three steps control the adsorption rate. The parameters are listed in Table 2 and show a double straight line nature. Initially, it is postulated that AR88 is transported to the external surface of Azolla biomass through film diffusion within a very short time. The first linear part could be due to the entry of AR88 molecules into the Azolla biomass particle by intra-particle diffusion. The second linear part represents the final equilibrium stage. As given in Table 2, it is obvious that values of k increase from 1.65 to 6.94 mg/g min1/2 when the initial AR88 concentrations increase from 25 to 200 mg/L. |
| Conclusion |
| The efficiency of AR88 dye removal from aqueous solution by Azolla biomass as a natural biosorbent was evaluated. The effect of parameters such as contact time, pH, AR88 concentration, adsorbent doses and temperature were analyzed. In general, the best, efficiency of AR88 removal by Azolla biomass was obtained at pH acidic, dose adsorbent of 4 g/L, initial concentration of AR88 of 25 and 50 mg/L and contact time 75 min. The adsorption isotherms are described by means of the Langmuir, Freundlich, Tempkin and Sips isotherms. It was found that the Langmuir equation fit better than the other equation. The rate of adsorption was found to conform to pseudo-secondorder kinetics with a good correlation. The increase in temperature increased the Azolla biomass monolayer adsorption capacities. Finally Azolla biomass, an inexpensive and easily available material, can be an alternative for more costly adsorbents used for dye removal in wastewater treatment processes. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 10902
- [From(publication date):
March-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10100
- PDF downloads : 802
