Short Communication Open Access
Experience with Aerosolized Imipenem in Patients with Cystic Fibrosis and Achromobacter xylosoxidans
Maria Celeste Marcos*, Emma Vazquez Espinosa, Layla Diab Caceres, Tamara Alonso Perez, Ana Martínez Meca, Carolina Cisneros Serrano and Rosa M Giron Moreno
Department of Pulmonology, La Princesa Institute for Health Research, Hospital Universitario de La Princesa, Madrid, Spain
- *Corresponding Author:
- María Celeste Marcos
Pulmonology Department, La Princesa Institute for Health Research
Hospital Universitario de La Princesa, c/Diego de León 62, 28006, Madrid, Spain
Tel: +34 91 5202277
E-mail: cele141082@hotmail.com
Received date: September 10, 2017; Accepted date: September 21, 2017; Published date: September 25, 2017
Citation: Marcos MC, Espinosa EV, Cáceres LD, Pérez TA, Meca AM, et al. (2017) Experience with Aerosolized Imipenem in Patients with Cystic Fibrosis and Achromobacter xylosoxidans. J Infect Dis Ther 5:335. doi: 10.4172/2332-0877.1000335
Copyright: © 2017 Marcos MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Achromobacter xylosoxidans is a gram negative non-fermentative rod that infects adult cystic fibrosis patients with moderate to severe lung compromise. Its clinical implications are still to be determined. The aim of this study was to analyze the impact of inhaled imipenem on forced expired volume in 1 sec (FEV1) and number of exacerbations in adult cystic fibrosis patients. Patients colonized by A. xylosoxidans with more than two intravenous antibiotic cycles and/or ≥ 5% FEV1 impairment during the last year were included.
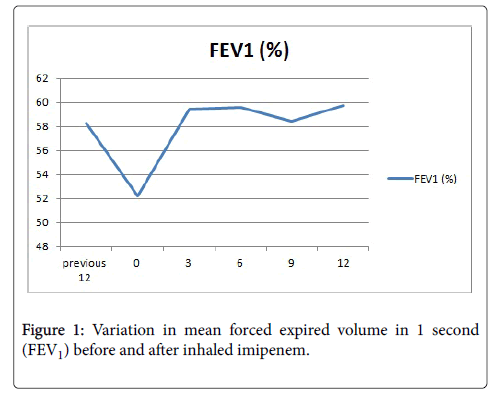
We included six patients with a mean age of 24.8 years. At the beginning of treatment FEV1 was 1.18 l (52%) with a mean of 3.4 exacerbations in the previous 12 months and 2.24 (p=0.04) the following 12 months. There was a significant difference between the initial FEV1 value and three months after treatment (p=0.005).
Keywords
Cystic fibrosis; Achromobacter xylosoxidans; Inhaled antibiotic; Forced expiration volume in 1 sec (FEV1); Exacerbation; Imipenem
Introduction
Achromobacter xylosoxidans is a gram-negative non-fermentative rod that is increasingly cultured in sputum from adult cystic fibrosis (CF) patients with moderate to severe lung compromise [1,2]. Its clinical implications are still to be determined [3] and therefore there is limited evidence on specific treatment. Chronic colonization occurs when the same potentially pathogenic microorganism is detected in three or more consecutive cultures separated by at least one month during a period of six months without concomitant antibiotic treatment [4]. While some patients may be asymptomatically colonized with A. xylosoxidans for prolonged periods of time, others experience rapid deterioration of lung function following infection [5]. In our Department the mean annual decline of lung function in chronically colonized patients was 2.49% and the most active antibiotics were piperacillin/tazobactam and imipenem [6].
Nebulized antibiotic therapy is an interesting therapeutic option for A. xylosoxidans given the high concentration of the drug obtained at the site of infection, minimizing adverse effects and possible drug interactions [7]. The aim of this study was to analyze the impact of imipenem as inhaled therapy on forced expired volume in 1 sec (FEV1) and number of exacerbations for adult cystic fibrosis (CF) patients.
Methods
Cystic fibrosis patients chronically colonized by A. xylosoxidans with more than two intravenous antibiotic cycles and/or ≥ 5% FEV1 impairment during the last year were included in the study. Decline in lung function was assessed using the variable annual percentage loss of FEV1. Informed consent for compassionate use was obtained from all individual participants. Before the treatment a bronchodilator was administered and chest physical therapy was carried-out [7]. An amount of 500 mg of imipenem powder (for infusion) was reconstituted with 5 ml of saline solution 0.9% and administered using eFlow-rapid (PARI) ® nebulizer. First dose was supervised by a nurse at the hospital. At home, the patient measured peak flow the following two weeks and maintained telephone contact with nurse and Unit physician.
The results were analyzed using SPSS version 19 (SPSS Inc). In the descriptive analysis, the quantitative variables were expressed as mean (SD) and the qualitative variables as percentages. Mean FEV1 percentage was registered the previous twelve months and the following three, six, nine and twelve months. T Student test was used to assess differences between start and end of treatment. Statistical significant p value considered was p<0.001.
Results
Six patients were included, four of them males, with a mean age of 24.8 years. One female patient with severe lung compromise presented an episode of bronchospasm after aerosolized imipenem and was excluded. FEV1 was 1.18 1 (52.2 ± 10.1%) at the beginning of treatment and 1.34 l (59.4 ± 11.71%) after three months. The mean of exacerbations in the previous 12 months before imipenem was 3.4 and five patients required one or more courses of intravenous antibiotics. The following 12 months the mean was 2.2 (p=0.04) and only three patients required one intravenous antibiotic cycle.
There was a significant difference between the initial FEV1 value and three months after treatment (p=0.005) (Figure 1).
Discussion
Our study calls attention to a potential new therapy [8] for A. xylosoxidans chronic infection which might have a negative impact on the clinical course of disease. The present observations indicate that aerosolized imipenem may have a clinically relevant influence on CF patients’ condition, as FEV1 values changed significantly after treatment, and there was also a reduction in the number of exacerbations [9].
Inhaled antibiotic therapy for chronic infection was first used in CF patients in 1940 [10]. The numerous parenteral antibiotics studied for inhaled use include aminoglycosides, fosfomycin, glycopeptides, betalactams, quinolones and polymyxin. Peculiarities of their formulation such as composition, acidity and hypertonicity led to airway reaction with cough, wheezing or chest pain [11,12]. Administration of inhaled formulations has protected lung function and reduced toxicity. In Spain five of them have been approved: colistimethate sodium, tobramycin nebulizer solution, tobramycin dry powder, colistimethate dry powder and inhaled aztreonam lysine [13]. Furthermore many trials with specifically designed inhaled formulations are currently underway: levofloxacin (Aeroquin, Mpex Pharmaceuticals), liposomal amikacin (Arikace, Insmed Incorporated), liposomal ciprofloxacin (Pulmaquin™, Aradigm Corporation) and dry powder ciprofloxacin (Bayer Healthcare AG). There is also a phase II study with vancomycin inhalation powder (Aerovanc®) for patients with CF and Staphylococcus aureus methicillin resistant infection [13].
Imipenem belongs to the carbapenem family and is very active against aerobic gram-positive microorganisms including A. xylosoxidans. There are limited references about its use for aerosolized therapy. There is only one reference of meropenem being used together with tobramycin in a patient with cystic fibrosis cepacia syndrome [8].
A threat of chronic use of imipenem is the possibility of selecting carbapenems resistant strains of Pseudomonas aeruginosa or enterobacteriaceae (mutants with impaired permeability or carbapenemases producing strains) [14]. These bacteria together with A. xylosoxidans could chronically colonize these patients.
In our experience is necessary to shake the nebulizer chamber to obtain proper results because of its high osmolarity. We used nebulizer systems that have been developed and marketed to nebulize specific antibiotics, optimizing delivery to the airway. The PARI eFlow in-line nebulizer system is a vibrating mesh nebulizer with a stainless-steel vibrating plate placed on the inspiratory arm of the ventilator [15].
An unanswered question about antibiotics is whether continuous treatment such as the one used in this study is a better option for severely ill patients than currently used on-off cycles. It is hypothesized that aerosolized antibiotics could improve clinical outcomes based on the principle that antibiotics decrease bacterial density in the airways and therefore reduce inflammation and lung damage [15]. In addition to this, according to Wang et al. early treatment with inhaled antibiotics for A. xylosoxidans may prevent or postpone chronic infection and, as a result, clinical deterioration in patients with cystic fibrosis [16]. Their results are in line with experience with Pseudomonas aeruginosa, where randomized trials have shown efficacy of inhaled antibiotics in the absence of concurrent systemic therapy. Further studies should focus on treatment outcomes different from FEV1 and number of exacerbations such as survival, bacterial eradication, quality of life, decrease of sputum bacterial load and decrease of local inflammation to assess efficacy of aerosolized imipenem [15].
A limitation of the current study was the small size and heterogeneity of the sample, with patients of different ages and degree of severity. Another limitation was dosage of imipenem powder for infusion. Due to the lack of prior research on aerosolized imipenem for Achromobacter xylosoxidans, dosage was decided on an empirical basis, by using the same dose as for parenteral use. As mentioned before, there are no studies to endorse its security or to determine the percentage of lung deposition of the nominal dose [13].
Aerosolized imipenem can be a therapeutic option for CF patients colonized with A. xylosoxidans with bad evolution. Further studies should be done focusing on dosage and also in treatment outcomes to assess the efficacy of aerosolized imipenem. Given the fact that this is not a specific aerosol formulation, a supervised first inhalation at the hospital and a close monitoring of patients are important.
References
- De Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M (2007) Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 6: 75-78.
- Ridderberg W, Bendstrup KE, Olesen HV, Jensen-Fangel S, Norskov-Lauritsen N (2011) Marked increase in incidence of Achromobacter xylosoxidans infections caused by sporadic acquisition from the environment. J Cyst Fibros 10: 466-469.
- Hansen CR, Pressler T, Nielsen KG, Jensen PØ, Bjarnsholt T, et al. (2010) Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J Cyst Fibros 9: 51-58.
- Cantón R, Cobos N, De Gracia J, Baquero F, Honorato J, et al. (2005) Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect 11: 690-703.
- Hansen C, Pressler T, HoibyN, Gormsen M (2006) Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 5: 245-251.
- Llorca Otero L, Girón Moreno R, Buendía Moreno B, Valenzuela C, Guiu Martínez A, et al. (2016) Achromobacter xylosoxidans infection in an adult cystic fibrosis unit in Madrid. Enferm Infecc Microbiol Clin 34: 184-187.
- Solé A, Girón RM (2015) Inhaled medication and inhalation devices for lung disease. Rev Esp Quimioter 1: 19-24.
- Girón RM, Oriol S, Cabanillas JJ (2017) Formulaciones endovenosas empleadas por vía inhalada. Nuevas indicaciones de los antibióticos inhalados.
- Marcos MC, Alonso Pérez T, López Riolobos C, García Castillo E, Domingo García D, et al. (2015) Experiencia con imipenem en nebulización en pacientes con fibrosis quística y Achromobacter. Arch Bronconeumol 51: 239.
- Di Sant´Agnese PA, Andersen DH (1946) Celiac síndrome. IV. Chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the páncreas. Am J Dis Child 72: 17-61.
- Kuhn RJ (2001) Formulation of aerosolized therapeutics. Chest 120: 94S–98S.
- Vazquez Espinosa E, Marcos C, Alonso T, Giron RM, Gomez-Punter RM, et al. (2016) Tobramycin inhalation powder (TOBI Podhaler) for the treatment of lung infection in patients with cystic fibrosis. Expert Rev Anti Infect Ther 14: 9-17.
- Máiz Carro L, Nieto Royo R, Sueiro Bendito A (2014) Presente y futuro de la antibioterapia inhalada. Monogr Arch Bronconeumol 1: 103–108.
- Meletis G (2016) Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis 3: 15-21.
- Restrepo MI, Keyt MD, Reyes LF (2015) Aerosolized antibiotics. Respir Care 60: 762-771.
- Wang M, Ridderberg W, Hansen CR, Høiby N, Jensen-Fangel S, et al. (2016) Early treatment with inhaled antibiotics postpones next occurrence of Achromobacter in cystic fibrosis. J Cyst Fibros 12: 638-643.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 3488
- [From(publication date):
October-2017 - Apr 18, 2025] - Breakdown by view type
- HTML page views : 2656
- PDF downloads : 832