Expanding access to medications for opioid use disorder in primary care: An examination of common implementation strategies
Received: 03-Sep-2020 / Accepted Date: 14-Oct-2020 / Published Date: 22-Oct-2020 DOI: 10.4172/2155-6105.1000407
Abstract
Objective: Buprenorphine, a medication for opioid use disorder, remains vastly underutilized despite its proven efficacy. This study sought to evaluate which strategies, within a system that employed a variety of concurrent strategies, effectively increased access to buprenorphine.
Methods: Over the course of 18 months, 25 federally qualified health centers were invited to participate in four commonly used implementation strategies. This study examines the impact of clinic attendance at strategy events on change in numbers of patients prescribed buprenorphine and numbers of buprenorphine-waivered providers by clinic.
Results: There was a nearly three-fold increase (2.84) in patients on buprenorphine and two-fold increase (1.90) in number of buprenorphine-waivered prescribers during the project period. Clinics attending at least half of the available didactic webinars and Project ECHO sessions were significantly more likely to increase both patients and providers than clinics attending fewer events.
Conclusions: In order to make informed decisions about how best to increase access to medications for opioid use disorder, systems and organizations need data on which implementation strategy options are most effective.
Keywords: Medications for opioid treatment (MOUD); Medications for addiction treatment (MAT); Opioid use disorders; Implementation strategies; Project ECHO; Expert coaching; Workforce training.
Introduction
Opioid related overdose mortality rates continue to rise [1]. The most effective treatments for opioid use disorders (OUD) include three FDA-approved medications: buprenorphine, methadone, and naltrexone [2,3]. However, access to medications for OUD (MOUD) remains limited due to inadequate numbers of buprenorphinewaivered providers to meet treatment need and limited prescribing to patients who could benefit from MOUD [4,5,6]. Through the 21st Century Cures Act, over $7.5 billion has been distributed to U.S. states and territories. These states have deployed a variety of implementation strategies with this funding to increase MOUD adoption and patient access. However, it has yet to be discovered which, if any, of these strategies are most effective.
Despite initiatives through federal, state, and philanthropic funding to increase both the providers authorized to and patients prescribed MOUD [7], barriers to expansion persist. Stigma toward OUD patients, low provider confidence in delivering specialty care, lack of institutional support, and reimbursement concerns continue to hinder primary care physicians from obtaining waivers [8,9]. Of those that have adopted MOUD, many do not treat to their provider caps; 30 patients in their first year, 100 patients in their second, and 275 patients subsequently [4,5,10]. Patients also themselves face barriers to treatment; perceived associations with “addiction,” beliefs around medicated treatment, and high out-of-pocket costs [11,12].
Federally Qualified Health Centers (FQHCs) have been identified for their potential to reach patients with OUD who do not seek services in specialty addiction programs [7,13-15]. This is due to both a strong existing primary care infrastructure and higher than average rates of OUD in the Medicaid populations they serve [13]. As such, fortyeight percent of FQHCs provide at least one of the three medications, significantly higher than the national average among non-FQHC primary care practices [16].
Despite these advances, there is little research on successful MOUD expansion efforts in primary care settings beyond specialty substance use treatment programs [17-26]. Many organizations employ multiple implementation strategies to address the above barriers, with the hopes that all or some may improve adoption of MOUD practice [7,27-31]. The cumulative effect of multiple strategies makes it difficult to determine which strategy(ies) directly improved outcomes. An accumulation of implementation science research supports tailoring chosen strategies to specified barriers; for greater precision and to accurately evaluate effects [30-35]. Without deconstructing which strategies effectively expand MOUD access, organizations may miss the mark on which reforms to focus on to address the opioid epidemic.
In this report, we examine the impact of four strategies: in-person workshops, Project ECHO, expert coaching, and didactic webinars on reach and adoption of MOUD among 25 FQHC clinics across the state of California. All clinics were eligible for and offered all strategies. Clinics participated in an array of strategies as they elected to do so. There was no randomization or matching in the design. This is common of many expansion projects; offering an array of strategies with the intent that some of them will prove effective. However, this study seeks to tease out which strategies were most effective on target outcomes, saving time and money for future endeavors.
As a function of participation in the strategies offered, we examine the change in: 1) the number of patients prescribed buprenorphine (reach); and 2) the number of waivered providers (adoption).
Methods
Participant Organizations and Sampling
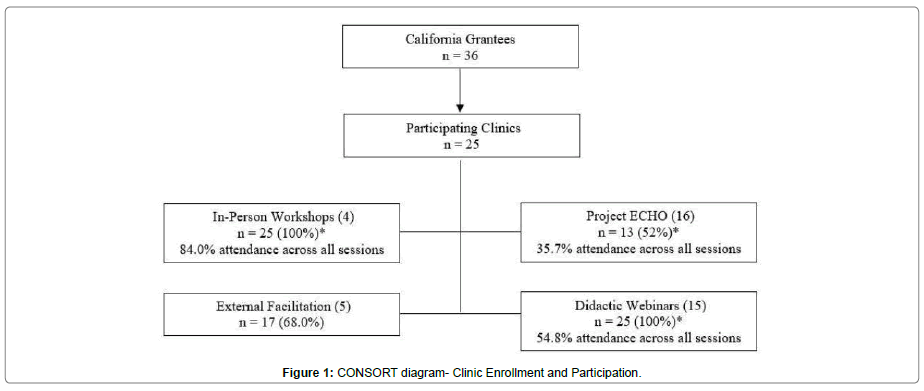
In early 2016, the Health Resources and Service Administration distributed grants to 36 California FQHCs to improve MOUD access. Twenty-five clinics chose to participate in additional grant-sponsored technical assistance programming over 18 months from September 2016 to February 2018. Clinics that participated in implementation strategies provided by this additional grant, comprise the final analytical sample (Figure 1).
Implementation Strategies: Four implementation strategies were made available to each of the participating clinics: 1) in-person workshops; 2) Project ECHO; 3) expert coaching; and
4) didactic webinars. The strategies included both in-person and remote learning options.
In-Person Workshops: Four full-day (6-hour) workshops were provided and held throughout California starting in October 2016 and continuing through December 2017. The content of the workshops included clinical aspects of medication prescribing and managing complex conditions, as well as process improvement tactics to support practice change in protocols and workflow. In-person workshops have shown moderate success in increasing adoption of evidence-based mental health care and reducing practice variation [36-39]. However, impact on MOUD prescribing has yet to be tested.
Project ECHO: Project ECHO uses teleconferenced didactic presentations and case-based discussions to expand specialty care in primary care settings [22]. A total of 13 hour-long sessions were held monthly from November 2016 to January 2017 and focused on topics relevant for healthcare providers. Research has shown Project ECHO can effectively train generalist physicians in pain management, treatment of substance use disorders, and reduce opioid overprescribing [17-19,21,22]. This study is among the first to specifically investigate MOUD prescribing [29].
Expert Coaching: Participating clinics were assigned to one of five expert coaches available for monthly, regularly scheduled phone consultations for MOUD expansion needs. While dosage varied, each clinic that opted into the strategy met at least once a month with their paired coach. They provided support on topics such as the mechanics of induction, overcoming the fear of prescribing, workflow, team-based care, and managing complex cases. Coaching models have shown success in integrating mental health care in primary care settings, reducing practice variation [23-26]. Several studies are currently underway to evaluate expert coaching effects on buprenorphine prescribing, but specific to emergency department settings [40,41].
Didactic Webinars: The monthly didactic webinars included interactive, real- time training tools on how to address MOUD in community-based settings. They occurred between the months of August 2016 and October 2017 for a total of 15 one- hour webinars. Topics included the hub and spoke treatment model, patient confidentiality, naloxone prescribing, and treating alcohol use disorder.
Data Collection
Baseline and endpoint data on the primary and secondary outcomes were in clinic reports in September 2016 and December 2017. Data on the primary implementation outcomes which included the number of patients on addiction medications (reach) and number of waivered providers (adoption) were collected via these reports. Because data were aggregated at the clinic-level, the study was deemed exempt and approved by Stanford University’s institutional review boards.
Variables
Implementation Strategy Attendance: Attendance – a proxy for participation – is the primary independent variable.
Clinics were marked as attending if one staff member signedin for in-person events or logged-in for virtual events, which was tracked through Salesforce roll-up software. We measured attendance dichotomously; if a clinic attended more or less than half of the offered sessions for a given strategy type. However, this differed for expert coaching, which was categorized as binary since clinics either engaged with or did not engage with a coach. For in-person learning sessions, attendance across all possible sessions was high (84%), so this variable was categorized as attending all (4) or less than all the events.
Reach: Patients Prescribed Buprenorphine
A primary dependent variable for this study was reach, or the number of patients prescribed buprenorphine [42]. Clinic-level data was obtained from baseline and endpoint survey collection in September of 2016 and December 2017, respectively. Reach was operationalized as the change in number of patients between these time points.
Adoption: Number of Waivered Providers
The second outcome of interest was adoption or change in number of waivered providers over the study period. This was a continuous variable calculated as the difference in number of waivered providers from endpoint to baseline.
Data Analysis
This study is a naturalistic, non-randomized, retrospective analysis of baseline and endpoint aggregated data from 25 FQHCs in the state of California. Mann-Whitney unpaired tests (U-tests) tested for significant differences in outcomes between attendance groups by strategy. All tests were completed using R version 3.6.1.
Results
Table 1 illustrates clinic-level characteristics of enrolled clinics. Clinics had a median of 281 baseline patients receiving MOUD or addiction counseling for OUD and 2 waivered-providers. The clinics were predominantly urban (81%) and approximately equitably distributed between northern (12 clinics) and southern (13 clinics) California. The clinics employed a large number of employees (Mdn = 413) across many clinic sites (Mdn = 6).
| Clinic Characteristic and Attendance Group | Total (N=25) | |
|---|---|---|
| n/mediana | (%/range) | |
| Clinic-level change in | ||
| Patients prescribed buprenorphine | 25 | (-6, 110) |
| Waivered-providers | 2 | (0, 13) |
| Clinic Characteristics | ||
| Number of patients at Baseline | 2 | (0, 88) |
| Baseline waivered-providers | 2 | (0, 28) |
| Baseline eligible providers | 18.5 | (7, 70) |
| Baseline MATb participants | 28 | (0, 527) |
| Clinic sites | 6 | (1, 24) |
| Employees | 413 | (25, 3000) |
| Urbanicity | ||
| Rural | 4 | 16% |
| Urban | 21 | 84% |
| Clinic Attendance In-person workshops | ||
| All sessions (4) | 12 | 50% |
| Fewer sessions (<4) | 13 | 50% |
| Project ECHO | ||
| >= 9 sessions | 9 | 64% |
| < 9 sessions | 16 | 36% |
| External Facilitation | ||
| Engaged | 17 | 68% |
| Not Engaged | 8 | 32% |
| Didactic webinars | ||
| >= 9 sessions | 13 | 52% |
| < 9 sessions | 12 | 48% |
The CONSORT diagram in Figure 1 delineates program enrollment and attendance. Of the 36 California grantees, 25 participated in the final study. Overall, implementation activities were well attended, but attendance varied by implementation strategy (Figure 1). All clinics attended at least one in-person workshop (100%), with an overall attendance of 84% across four events. Project ECHO attendance was lower with 13 clinics attending at least one session (52.0%) and an overall attendance of 35.7% across all 16 events. Seventeen of the clinics utilized expert coaching (68.0%). All clinics attended at least one of the 15 didactic webinars (100%), with 54.8% attendance across all sessions. Across all available strategy types (in-person workshops, Project ECHO, expert coaching, and didactic webinars), seven clinics (28.0%) attended only two of the strategies, seven clinics (28.0%) attended three, and 11 clinics (44.0%) participated in all four activities.
MOUD Implementation Outcomes
Reach: Patients Prescribed Buprenorphine
Change in patients prescribed buprenorphine from baseline to endpoint are depicted in Figure 2a, by activity type. Reports demonstrated an increase in reach over time, from 626 patients to 1,776 patients prescribed buprenorphine over the study period, a nearly three-fold increase (2.84).
Adoption: Number of Waivered Providers
Change in the number of waivered providers from baseline to endpoint are depicted in Figure 2b. The overall number of providers saw a nearly two-fold increase (1.90), from 126 to 201 waivered providers over the study period.
Implementation Strategy Attendance and MOUD Outcomes
The group difference results between different attendance subgroups are presented in Table 2. The results indicated that the median change in number of patients prescribed buprenorphine was greater for clinics that attended half or more of the available didactic webinars (Mdn = 32.0) than for clinics that did not (Mdn = 14.0), W = 34.0, p=0.02. The change in buprenorphine-waivered providers was also higher for clinics that attended half or more of the available Project ECHO sessions (Mdn = 5.0) compared to clinics that attended fewer sessions (Mdn = 1.0), W = 24.0, p=0.02. There were no betweengroup differences in either outcome for in-person sessions or expert coaching. These results are also depicted in Figure 2a and 2b which represent change in clinic outcomes by strategy type. It should be noted that clinic attendance of these results did not control for dual clinic participation in more than one strategy type.
| Clinic Attendance | N | Median change in outcome (from baseline to endpoint) | |
|---|---|---|---|
| Patients prescribed buprenorphine | Waivered- providers | ||
| In-person workshops | |||
| All sessions (4) | 12 | 28.0 | 2.0 |
| Fewer sessions (<4) | 13 | 22.5 | 3.0 |
| Project ECHO | |||
| >= 9 sessions | 9 | 26.0 | 5.0* |
| < 9 sessions | 16 | 21.5 | 1.0* |
| External Facilitation | |||
| Engaged | 17 | 23.0 | 2.0 |
| Not Engaged | 8 | 27.0 | 3.5 |
| Didactic Webinars | |||
| >= 9 sessions | 13 | 32.0* | 3.5 |
| < 9 sessions | 12 | 14.0* | 1.0 |
Table 2: Clinic Attendance by Median Study Outcome.
Discussion
Summary of Findings
Overall, there was a three-fold increase in patients accessing MOUD and a two- fold increase in providers adopting MOUD. We also explored the impact of attendance at four implementation strategies on increased access to MOUD and waivered providers among 25 FQHC clinics. Clinics that attended more than half of the available didactic webinars experienced significantly greater increases in patients prescribed buprenorphine than clinics that attended less than half. Clinics that attended more than half of the offered Project ECHO sessions also experienced significantly higher growth in waiveredproviders compared to clinics with lower attendance. These results may be due to the nature of both strategies. Didactic webinars were available to all members of a clinic and target overall best-practices for addressing MOUD. Project ECHO as a model is specifically targeted to generalized providers, with the intent to expand their knowledge of specialty – in this case, addiction – service. This may explain the impact of this strategy on the outcome of interest.
These results provide mixed alignment with current research on MOUD expansion efforts. Clinic attendance at Project ECHO sessions did increase odds of waivered-provider growth, in alignment with recent Project ECHO research on increased specialty-practice adoption in primary care settings [21,43]. Didactic webinars were successful in increasing patients prescribed buprenoprhine, though other studies have found them to be less effective [13]. The in-person workshops and expert coaching strategies did not have significant effects on growth in the current study. This diverges from recent literature demonstrating increases in MOUD provider efficacy and other evidence-based practice adoption for in-person workshops and coaching, respectively [13,24,25,39].
Limitations
As a naturalistic and non-randomized study, the lack of a comparator limits causal interpretations of implementation strategies and their impact on the MOUD reach and adoption. For example, clinics already motivated to expand MOUD may have attended more implementation strategy sessions rather than these strategies causing MOUD expansion. Data on reach and adoption were reported by the clinics in a standardized approach, but were not objectively verified by administrative, health record, or claims data. Although participation in the strategies was carefully tracked for any clinic member attendance, neither how many staff nor staff member role (e.g., physician, behavioral health clinician, clinic manager) were examined. This is a significant limitation because the constellation of implementation strategies was offered to the FQHCs as a team-based approach to MOUD care. Team-based approaches to MOUD that involve the prescriber, a nurse or care manager and a behavioral health clinician are well-documented [44]. The present study did not evaluate the degree to which all clinic members participated in the strategies. Another limitation is combination and sequencing of the strategies may have produced synergistic or interactive effects. A few early didactic webinar sessions early in the project may have been sufficient to learn the mechanics of prescribing MOUD. But as more complex patients presented, and more complicated clinical decisions were being made, the importance of case-based learning (Project ECHO) and developing care pathways (expert coaching) may have been increasingly valuable. The expert coaching program was also based on models of facilitation, but with deviations [23,24]. A more direct implementation of this model may have produced increased effects. Lastly, because this was a sample of FQHCs only, the findings may not be generalizable to other types of primary care practices. Further research should expand this investigation to other primary care settings and with more robust, randomized-control design.
Implications and Future Research
Our study is among the first to examine the influence of commonly used implementation strategies in a MOUD reach and expansion endeavor. In an 18-month period, significant increases in MOUD reach (more patients) and MOUD adoption (more prescribers) were major and positive outcomes. There is no benchmark for expected increases in reach or adoption at present. The increases reported here can be framed as positive implementation outcomes. The unclear association between specific implementation strategies (i.e. the interventions of implementation) and implementation outcomes is also important. This points to a need for more rigorously designed, controlled studies on strategies for MOUD expansion. Endeavors to scale up evidence-based addiction treatments such as MOUD could benefit from the selection and matching of strategies to specific contextual barriers and needs rather than a la carte approaches [31-34]. Systems and organizational decision-makers often express a desire for tailored and efficiency in approaches, but lack the validated research to guide choices about which strategies to use [27,32,33]. National, state, regional, and local endeavors may address the opioid epidemic with greater efficiency by adapting implementation strategies to public health outcomes. In adding specificity in their strategy selection, they may move the needle on reduced overdose mortality and the benefits of effective treatments.
Acknowledgments
This study would not have been possible without the staff, clinicians, and patients of the clinics involved. We are grateful to Susannah Brower a former employee of the Center for Care Innovations, Dr. Kelly Pfeifer from the California Healthcare Foundation, Dr. Jean Marsh from the California Society for Addiction Medicine, and the Weitzman Institute for Project ECHO support. The authors would like to specifically thank the staff of Center for Behavioral Health Services and Implementation Research for manuscript review and editing feedback.
References
- Wilson N, Kariisa M, Seth P, Smith H, Davis NL (2019) Drug and Opioid Involved Overdose Deaths – United States, 2017 – 2018. MMWR. Morbidity and Mortality Weekly Report. 69(11):290-297.
- Mattick RP, Kimber J, Breen C, Davoli M (2004) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. (3):CD002207.
- Volkow ND, Frieden TR, Hyde PS, Cha SS (2014). Medication-assisted therapies-- tackling the opioid-overdose epidemic. N Engl J Med. 370(22):2063â€2066.
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E (2015). National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health. 105(8):e55â€e63.
- Knudsen HK (2015). The Supply of Physicians Waivered to Prescribe Buprenorphine for Opioid Use Disorders in the United States: A State-Level Analysis. J Stud Alcohol Drugs. 76(4):644â€654.
- Rosenblatt RA, Andrilla CH, Catlin M, Larson EH (2015). Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 13(1):23â€26.
- Watson DP, Andraka-Christou B, Clarke T, Wiegandt J (2020) Introduction to the special issue on innovative interventions and approaches to expand medication assisted treatment: Seizing research opportunities made available by the opioid STR program. J Subst Abuse Treat. 108:1â€3.
- Huhn AS, Dunn KE (2017) Why aren't physicians prescribing more buprenorphine?. J Subst Abuse Treat. 78:1â€7.
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA (2014) Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med. 12(2):128â€133.
- Schuckit MA (2016). Treatment of Opioid-Use Disorders. N Engl J Med. 375(16):1596â€1597.
- Hewell VM, Vasquez AR, Rivkin ID (2017) Systemic and individual factors in the buprenorphine treatment-seeking process: a qualitative study. Subst Abuse Treat Prev Policy. 12(1):3.
- Mackey K, Veazie S, Anderson J, Bourne D, Peterson K (2019) Evidence Brief: Barriers and Facilitators to Use of Medications for Opioid Use Disorder. Washington (DC): Department of Veterans Affairs (US).
- Caton L, Shen H, Miele G, Darfler K, Sandoval J, et al (2020) Opening the black box: the impact of four common implementation strategies on expansion of medications for opioid use disorder. Imp Sci Res Prac. Accepted.
- Rosenbaum S, Tolbert J, Sharac J, Shin P, Gunsalus R, et al (2020) Community Health Centers: Growing Importance in a Changing Health Care System. Kaiser Family Foundation. Accessed May 15, 2020.
- Arfken CL, Johanson CE, Di Menza S, Schuster CR (2010). Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J Subst Abuse Treat. 39(2):96â€104.
- Orgera K, Tolbert J (2019) The Opioid Epidemic and Medicaid’s Role Facilitating Access to Treatment.
- Carey KJ, Huang W, Linas BP, Tsui JI (2016) Hepatitis C Virus Testing and Treatment Among Persons Receiving Buprenorphine in an Office-Based Program for Opioid Use Disorders. J Subst Abuse Treat. 66:54â€59.
- Katzman JG, Comerci GD, Landen M, Loring L, Jenkusky SM, et al (2014) Rules and values: a coordinated regulatory and educational approach to the public health crises of chronic pain and addiction. Am J Public Health. 104(8):1356â€1362.
- Katzman JG (2020). ECHO Telementoring for Pain, Palliative Care, and Opioid Management: Progress, Challenges, and Future Goals. Pain Med. 21(2):220â€225.
- Boudreau DM, Lapham G, Johnson EA, Bobb JF, Matthews AG, et al (2020). Documented opioid use disorder and its treatment in primary care patients across six U.S. health systems. J Subst Abuse Treat. 112S:41â€48.
- Komaromy M, Duhigg D, Metcalf A, et al (2016). Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 37(1):20â€24.
- Ritchie MJ, Parker LE, Edlund CN, Kirchner JE (2017). Using implementation facilitation to foster clinical practice quality and adherence to evidence in challenged settings: a qualitative study. BMC Health Serv Res. 17(1):294.
- Kirchner JE, Ritchie MJ, Pitcock JA, Parker LE, Curran GM, et al. (2014) Outcomes of a partnered facilitation strategy to implement primary care-mental health. J Gen Intern Med. 29 Suppl 4(Suppl 4):904â€912.
- Stetler CB, Legro MW, Rycroft-Malone J, Bowman C, Curran G, et al (2006). Role of "external facilitation" in implementation of research findings: a qualitative evaluation of facilitation experiences in the Veterans Health Administration. Implement Sci. 1:23.
- Dogherty EJ, Harrison M, Graham I, Keeping-Burke L (2014). Examining the use of facilitation within guideline dissemination and implementation studies in nursing. Int J Evid Based Healthc. 12(2): 105â€127.
- Powell BJ, Proctor EK, Glass JE (2014). A Systematic Review of Strategies for Implementing Empirically Supported Mental Health Interventions. Res Soc Work Pract. 24(2):192â€212.
- Powell BJ, Haley AD, Patel SV, et al. (2020) Improving the implementation and sustainment of evidence-based practices in community mental health organizations: a study protocol for a matched-pair cluster randomized pilot study of the Collaborative Organizational Approach to Selecting and Tailoring Implementation Strategies (COAST-IS). Implement Sci Commun. 1:9.
- Holmes CM, Keyser-Marcus L, Dave B, Mishra V (2020) Project ECHO and Opioid Education: a Systematic Review. Curr Treat Options Psych. 7:9–22.
- Proctor EK, Powell BJ, McMillen JC (2013) Implementation strategies: recommendations for specifying and reporting. Implement Sci. 8:139.
- Bosch M, Van Der Weijden T, Wensing M, Grol R (2007) Tailoring quality improvement interventions to identified barriers: a multiple case analysis. J Eval Clin Prac. 13(2):161-168.
- Krause J, Van Lieshout J, Klomp R, Huntink E, Aakhus E, et al (2014) Identifying determinants of care for tailoring implementation in chronic diseases: an evaluation of different methods. Implement Sci. 9:102.
- Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, et al (2017) Methods to Improve the Selection and Tailoring of Implementation Strategies. J Behav Health Serv Res. 44(2):177â€194.
- Squires JE, Sullivan K, Eccles MP, Worswick J, Grimshaw JM (2014). Are multifaceted interventions more effective than single-component interventions in changing health-care professionals' behaviours? An overview of systematic reviews. Implement Sci. 9:152.
- Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, et al. (2015) Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. (4):CD005470.
- Institute for Healthcare Improvement (IHI). The Breakthrough Series: IHI's Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston, MA: 2003.
- Becker DR, Drake RE, Bond GR, Nawaz S, Haslett WR, et al (2011) Best practices: A national mental health learning collaborative on supported employment. Psychiatr Serv. 62(7):704â€706.
- Nadeem E, Olin SS, Hill LC, Hoagwood KE, Horwitz SM (2014) A literature review of learning collaboratives in mental health care: used but untested. Psychiatr Serv. 65(9):1088â€1099.
- Nordstrom BR, Saunders EC, McLeman B, Meier A, Xie H, et al (2016) Using a Learning Collaborative Strategy With Office-based Practices to Increase Access and Improve Quality of Care for Patients With Opioid Use Disorders. J Addict Med. 10(2):117†123.
- D'Onofrio G, Edelman EJ, Hawk KF, Pantalon MV, Chawarski MC, et al. (2019) Implementation facilitation to promote emergency department-initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness-implementation study (Project ED HEALTH). Implement Sci. 14(1):48.
- Croff R, Hoffman K, Alanis-Hirsch K, Ford J, McCarty D, et al. (2019) Overcoming Barriers to Adopting and Implementing Pharmacotherapy: the Medication Research Partnership. J Behav Health Serv Res. 46(2):330â€339.
- Glasgow RE, Vogt TM, Boles SM (1999) Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 89(9):1322â€1327.
- Tofighi B, Isaacs N, Byrnes-Enoch H, et al (2019). Expanding treatment for opioid use disorder in publicly funded primary care clinics: Exploratory evaluation of the NYC health + hospitals buprenorphine ECHO program. J Subst Abuse Treat. 106:1â€3.
- LaBelle CT, Han SC, Bergeron A, Samet JH (2016) Office-Based Opioid Treatment with Buprenorphine (OBOT-B): Statewide Implementation of the Massachusetts Collaborative Care Model in Community Health Centers. J Subst Abuse Treat. 60:6†13.
Citation: Caton L, Shen H, Assefa MT, Fisher T, McGovern MP (2020) Expanding access to medications for opioid use disorder in primary care: An examination of common implementation strategies. J Addict Res Ther 11:407. DOI: 10.4172/2155-6105.1000407
Copyright: © 2020 Caton L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2072
- [From(publication date): 0-2020 - Dec 03, 2024]
- Breakdown by view type
- HTML page views: 1459
- PDF downloads: 613