Research Article Open Access
Evidence of Neuronal Differentiation of Tendon Stromal Cells in Patients with Biceps Branchi Muscle Pain: A Histological and Immunohistochemical Study of 12 Patients
| Constantine Hadjileontis1* and George Kontakis2 | |
| 1Pathology Department of Serres General Hospital, Serres, Greece | |
| 2Department of Orthopaedic Surgery, Heraklion University Hospital, Crete, Greece | |
| Corresponding Author : | Constantine Hadjileontis Pathology Department of Serres General Hospital, Crete, Greece E-mail: conhad@hospser.gr |
| Received February 12, 2013; Accepted April 08, 2013; Published April 10, 2013 | |
| Citation: Hadjileontis C, Kontakis G (2013) Evidence of Neuronal Differentiation of Tendon Stromal Cells in Patients with Biceps Branchi Muscle Pain: A Histological and Immunohistochemical Study of 12 Patients. J Nov Physiother S2:007. doi:10.4172/2165-7025.S2-007 | |
| Copyright: © 2013 Hadjileontis C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
The tendon of the long head of biceps branchii muscles is a major source of pain in the shoulder region. Our objective was to detect histologically and characterize factors that may contribute to biceps tendon pain generation, and subsequently provide a possible explanation of pain origin. This would permit the investigation of targeted pharmaceutical treatments for long term post-operative restriction of pain. Bioptic samples were obtained from 11 patients operated for shoulder fractures and were harvested during shoulder reconstruction operations. As a control group we used biceps tendons from 10 forensic cases with no history of shoulder diseases. Histologic investigation included quantitative measurement of tendon degeneration, cellularity, neoangiogenesis, inflammation and metaplasia, as well as immunohistochemical detection of cells with neural differentiation within the tendon tissue proper (S-100 protein and neuropeptide Y). Studied lesions were significantly (Mann-Whitney and Wilcox on test) greater in the group of patients compared to the ones found in the control group (p<0,001), while these lesions were statistically proved (Pearson test) to relate with each other, resulting in the neural differentiation of tendon stromal cells, causing thus pain.
| Keywords |
| Long biceps tendon; Pain; Histologyimmunohistochemistry |
| Introduction |
| The long head of the biceps branchii is a gliding tendon, which allows the humeral head to move on the fixed tendon during motion at the shoulder joint. In order to investigate the actual cause of pain after shoulder fractures and injuries, we histologically examined the lesions present at the tendon, after sampling during surgery reconstruction. This would enable the clarification of the mechanisms involved in pain generation, and the detection of targeted pharmaceutical schemes, to achieve long term post-operative pain restriction [1,2]. |
| Until now very few studies concerning the histological lesions of biceps branchii tendons are released [3-5]. Results from these studies show that affected tendons exhibit marked histological changes, compared with normal tendons of both younger and aged individuals [3]. |
| Physiologically tendons show close and parallel arrangement of collagen fibers with slight waviness, that provide a deep pink-red staining result after treated with hematoxylin and eosin (H&E). These fibers include tenocytes with flattened and spindle shaped nuclei, often arranged in rows. These cells represent modified, inactive fibroblasts. No neural (sensor) cells are normally present within the tendon-fiber matrix (tendon tissue proper), while blood vessels and nerves are supplied from the surrounding tissues (paratendinous), branching up to a point into the periphery of tendon tissue proper, parallel to its collagen fibers [4]. |
| What’s new in this study is that all morphologic measurements were performed quantitatively and tendons were dissected in three parts (proximal, middle and distal) that were separately studied for each lesion. Furthermore, we tried to immunohistochemically detect cells with neural features and function within tendon tissue proper, that would explain pain manifestation. We also examined possible mechanisms that might stimulate and/or sustain the appearance of these cells, such as inflammation and neoangiogenesis. In order to achieve these goals we applied well-documented histological criteria for each lesion that are thoroughly substantiated in literature. |
| Materials and Methods |
| Tendons from all cases (n=12) were dissected in three parts and were oriented as proximal, middle and distal edge, according to their anatomical relation with the affected joint. Biopsies were then fixed in formalin buffer 4% for 24 h in order to proceed to the histological and immunohistochemical evaluation. The lesions studied included tendon cellularity and vascularity, the presence and density of inflammation (both histologically and immunohistochemically by using common leukocyte antigen), the degree and type of tendon degeneration, as well as the expression of S-100 protein and neuropeptide Y in the affected tissues. For the angiogenic evaluation we applied Burkhardt’s histological criteria [1] for neo-angiogenesis. The immunohistochemical marker used for staining endothelial cells was CD34. Morphological evaluation was performed after applying the routine stain of H&E, whereas for immunohistochemistry we used the Vision Biosystem Bond max staining machine, prior to applying the immunohistochemical markers (Table 1). This staining machine includes steps to dewax, pretreat and stain slides, using a primary antibody that attaches to the tissue antigens. A polymer-conjugated chain with attached secondary antibodies and peroxidase is then applied and attaches to the primary antibody. DAB (Di amino benzadine) is then added and acts as a substrate for the peroxidase. Endogenous peroxidase in tissue is blocked prior to the addition of antibodies to prevent non-specific reactions. |
| All lesions were evaluated quantitatively through a millimetric greed at 400 X magnification by two different observers, and were expressed as number of cells (or micro-vessels) per millimetric square (mm2) of tissue cut surface [6]. |
| Degenerative lesions |
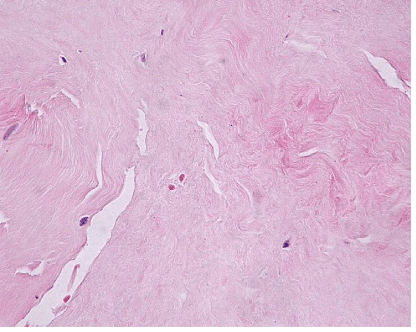
| Degenerative lesions were studied in H&E histochemical stains and included structural anomalies (loss of normal pattern of tendon fiber arrangement), staining anomalies (loss of collagen stainability exhibited with pale collagen fiber staining, hyalinization and/or basophilic degeneration and stromal homogenopoieses), as well as areas of cellular morphological changes in the form of rounded cells with pyknotic nuclei. These lesions were grated as mild, moderate and severe. |
| Cellularity was also studied in H&E histochemical stains and was measured only within the surface of tendon tissue proper. Cellularity was expressed as the number of cells found per Millimetric Square of tendon surface, as measured through the millimetric grid. |
| Neoangiogenesis |
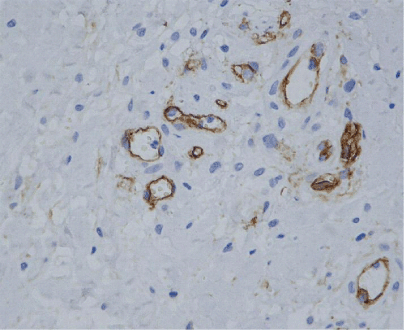
| Neoangiogenesis was measured within tendon tissue proper and only in areas having no obvious connection with the pre-existing vascular net, calculating the angiogenic index as the result of the number of micro-vessels (mvs) found through the millimetric grid, per mm2 of tissue surface. For the angiogenic evaluation we applied Burkhardt’s histological criteria for neo-angiogenesis [7]. |
| In order to detect cells with signs of neural differentiation we applied both histological and immunohistochemical criteria. Histological criteria included the presence of cells with more cytoplasm and larger nuclei, with or without spindle shape configuration. In all cases immunohistochemistry was performed, using S-100 protein and neuropeptide Y (N-Y), in order to substantiate the presence of neural differentiation. Measurement of positive cells was performed only when all the above histological criteria were fulfilled, the staining pattern of S-100 protein was both cytoplasmic and nuclear, and when cells positive for N-Y expressed a micro-granular cytoplasmic reaction. Furthermore S-100 protein revealed the presence of fine cytoplasmic projections similar to those of dentritic and neural cells. |
| Inflammation |
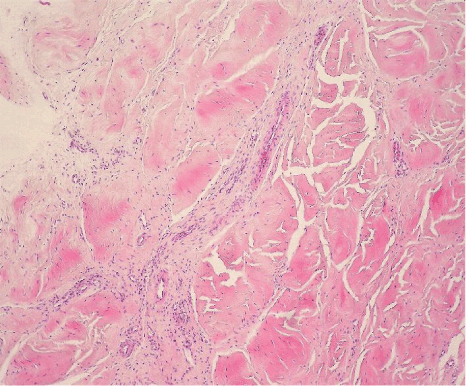
| Inflammation was expressed as the number of inflammatory cells found per mm2 of tendon surface, by evaluating the immunohistochemical stain for the common leukocyte antigen (LCA). |
| The presence of metaplasia was expressed as cells per mm2. The cells counted showed chondrocytic features, loss of their spindleshaped cellular form and appeared with rounded nuclei. These cells were gathered in small groups (up to three cells) in chondrocytic spaces and also expressed S-100 protein, but not N-Y. |
| No clinical data were available at the time of microscopical study. After all laboratory results were obtained, they were correlated with the clinical findings. All patients had pain symptoms and bioptic material was sampled during surgery in a time interval ranging from one to three weeks from injury [8]. |
| All procedures of the present study were carried out in accordance with the ethical standards of the Greek Orthopedic Society on human experimentation and with the Helsinki Declaration of World Medical Association. |
| For statistical evaluation we applied the Mann-Whitney and Wilcox on test for lesions present separately in every tendon part and whole the tendon, in correlation with the control group, while all lesions were compared with each other using the Pearson correlation, in order to detect the statistically significant correlations between variables. Every probability level of <0.05 was considered significant. All p-values are two sided and confidence intervals refer to 95% boundaries. |
| Results |
| Both the inter- and intra-observer variability in the angiogenic estimation of all the above cases did not exceed 5%. |
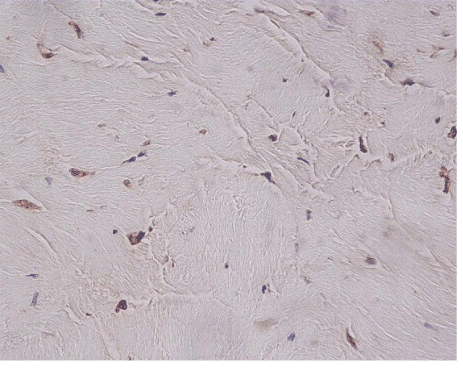
| The most common lesion found within tendon tissue proper was degeneration (Figure 1), while all other lesions were mostly prominent in paratendinous tissues and concerned the presence of inflammation (Figure 2), as well as increased cellularity and neoangiogenesis. In 6 cases, however, lesions expanded within tendon tissue proper and coexisted with degenerative and metaplastic lesions. Degenerative lesions included structural anomalies, loss of collagen stainability (pale collagen fiber staining), as well as areas of stromal homogenopoesies in the form of hyalinization and/or basophilic degeneration (Figure 3). Metaplastic changes were present in three of the cases and coexisted with degenerative lesions of severe type. The increased density of both cellularity and newly formed vessels (neoangiogenesis) observed, ranged from mild to moderate in all cases (4-80 c/mm2, 2-80 mvs/mm2). |
| Newly formed vessels were found along the pre-existing vascular net, exhibiting a linear distribution from the periphery of the tendon in to tendon tissue proper. Nevertheless, neoangiogenesis was also observed in 3 cases, showing a nodular pattern of development within the tendon, in areas having no connection with the pre-existing vascular net (Figure 4). |
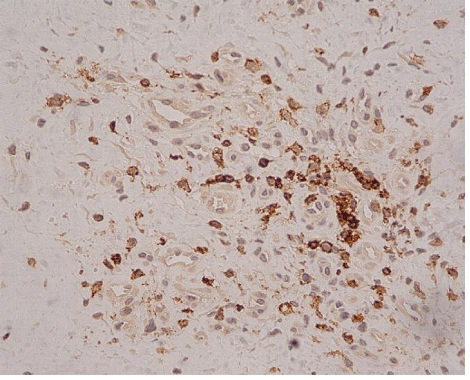
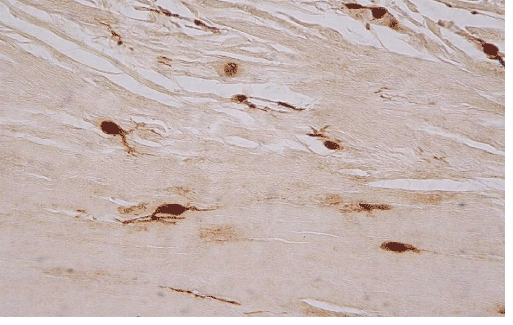
| In ten of the eleven cases we also found an increased h immunohistochemical expression of S-100 protein by stromal cells, showing a neural appearance. This included the presence of cytoplasmic expansions similar to those of dentritic and neural cells, as well as larger nuclei with or without spindle shape configuration (Figure 5). Nine of the ten cases expressing S-100 protein also expressed neuropeptide Y (Figure 6). The immunohistochemical reaction of positive cells was demonstrated by a dense, diffuse, cytoplasmic and nuclear staining pattern for S-100 protein, while neuropeptide Y expressed a micro-granular cytoplasmic reaction. |
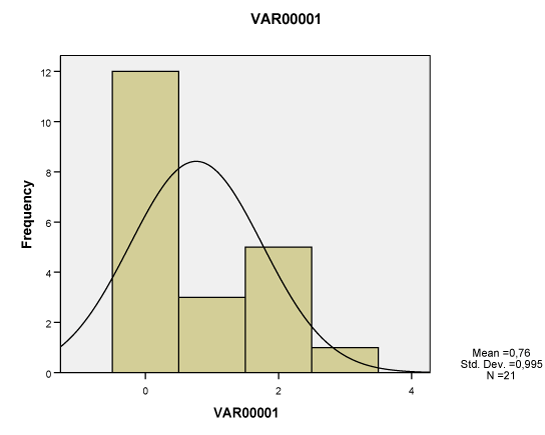
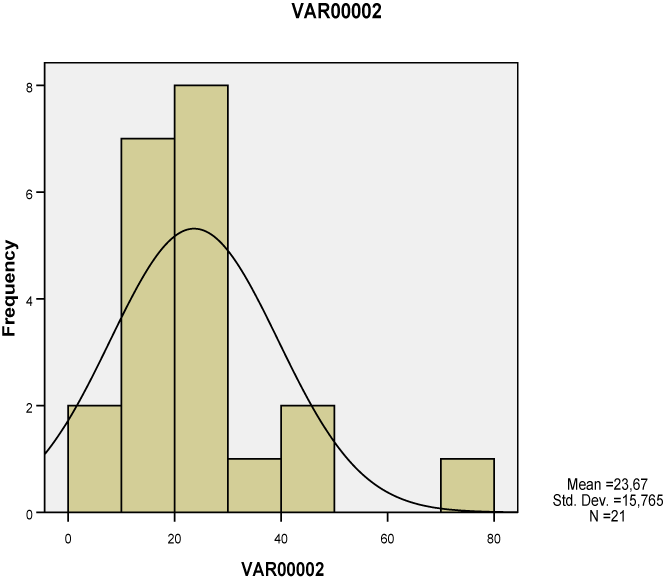
| All of the seven lesions were significantly greater in the study group compared to the ones of the control group, as studied both separately for the proximal, middle and distal tendon segment (p<0.01-0.001), as well as in whole the tendon (p<0.05-0.001). None of these lesions however corresponded to Gauss’s distribution, neither when studied separately for tendon segments (Charts 1-7) nor when measured in whole the tendon (Table 2). Nevertheless, Pearson’s test revealed a statistically significant correlation between neoangiogenesis and S-100 protein expression, between S-100 protein and N-Y expression, as well as between study/control group, degeneration, S-100 protein and N-Y expression (Table 3). |
| Discussion |
| This study is according to our knowledge the first one to provide quantitative methodology for assessing histopathological lesions of branchii muscle tendons from active individuals. Furthermore, we are the first to examine these tendons separately for each of the three parts (proximal, middle and distal) that have different neural supply and the first ones to attempt to correlate the morphological lesions in order to provide a possible explanation of the mechanisms involved in pain generation at the region. |
| The presence of cells with morphological features of neural differentiation within tendon tissue proper in our cases was substantiated immunohistochemically, by applying S-100 protein and N-Y antigens. S-100 protein is an immunohistochemical marker expressed by glial cells of central and peripheral nervous system and by tumors originating from neural cells. This expression however is not specific for cells with neural differentiation, since it has also been detected in chondrocytes, melanocytes, adipocytes, smooth and skeletal muscles, as well as in fibroblasts [5]. N-Y on the other hand is a specific marker that detects neuron cells, since it binds neurotransmitters, high molecular weight proteins, that are released from neurons during neuronal function (cell signaling). N-Y is expressed by neurons of both central and peripheral nervous system, including the hypothalamus and pituitary gland [9]. The combination of these two markers positive expression, may safely characterize cells with neural differentiation. |
| The fact that the expression of S-100 protein and N-Y was statistically correlated in our cases, gives further proof of the differentiation of the cells that expressed them [10]. Another mostly interesting statistical correlation that came up was the one relating the findings from the study and the control group, the presence of degeneration and the expression of S-100 protein and N-Y. This could be interpreted as that the forms of degeneration found in ruptured tendons give rise to the evolution of cells with neural differentiation, whereas such cells are normally absent (control group). On the other hand, no such correlation was found for metaplastic cells that were also expressing S-100 protein but not N-Y. Additionally, the statistical correlation found between neoangiogenesis and S-100 protein expression, might give a clue in understanding the mechanisms involved in the presence of these cells within tendon tissue proper. The latest could mean that cells with neural differentiation induce neoangiogenesis or vice versa. The newly formed vessels on the other hand support and sustain the presence of neural cells. The fact however that inflammation was not found to have any significant statistical correlation, neither with the presence of degenerative lesions and angiogenesis nor with the expression of S-100 protein and N-Y, excludes the possibility that neoangiogenesis is due to angiogenic amines produced by inflammatory cells. Such amines are most-likely to be produced by the cells with neural differentiation [6], since degenerated collagen fibers are not known to produce this effect. |
| Conclusions |
| The above findings enhance the hypothesis that pain is due to neuronal differentiation of tendon stromal cells, that are normally absent in normal tendons. The histological findings supporting this hypothesis include both the increased number of cells with morphological features of neural differentiation within tendon tissue proper, as well as their immunohistochemical profile. The positive reaction for S-100 protein confirms that the morphological changes observed point towards neural differentiation, while the N-Y expression in some of those neural cells demonstrates that they were functionally active. |
| The evolution of this differentiation is supported by neoangiogenic stimulation in the area, while inflammation seems to be of minor importance both in the cause of pain and the angiogenic promotion. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
 |
 |
 |
 |
| Chart 1 | Chart 2 | Chart 3 | Chart 4 |
 |
 |
 |
| Chart 5 | Chart 6 | Chart 7 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 13217
- [From(publication date):
specialissue-2013 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 8714
- PDF downloads : 4503
