Evaluation of the Hepatotoxic and Nephrotoxic Potential of Ethanolic Stem Back Extract of Xylopia Aethiopica (Annonaceae) in Male Rodents
Received: 15-Feb-2018 / Accepted Date: 20-Apr-2018 / Published Date: 25-Apr-2018 DOI: 10.4172/2476-2024.1000138
Abstract
Ethno pharmacological relevance: People in Nigeria and all over the world, use this plant preparations, for various therapeutic purposes, in folklore medicine. The aim of the study is to obtain the sub chronic nephrotoxic and hepatotoxic information on this plant.
Aim of study: Obtain nephrotoxic and hepatotoxic information regarding traditional use of plants.
Materials and methods: 32 male rats were grouped into 4 groups i.e. Group I (had no treatment), Group II (200 mg/kg body weight of extract), Group III (400 mg/kg body weight of extract) and Group IV (800 mg/kg body weight of extract) for a period of 28 days. On 14th and 28th day, three rats were sacrificed in each group and blood samples were obtained for kidney and hepatic function tests.
Result: It showed that there was a significant increase in weight, p<0.01 in Group III, but p<0.05. There was no significant effect on the kidney function parameters except for Urea at 200 and 400 mg/kg. Liver function tests, showed that ALP, AST and ALT were significantly reduced, p<0.001 compared to the control
Conclusion: It is possible therefore, to infer that the sub chronic toxicity study of X. aethiopica stem on liver and kidney has a toxic potential at high doses as it showed significant differences on some biomarkers of the kidney and liver functions. Moreover, the photomicrograph showed some slight histological alterations in both organs.
Introduction
Liver and kidney play central roles in transforming and clearing xenobiotics, which make them susceptible to toxicity from various chemical agents. When xenobiotics are administered in overdoses or sometimes within therapeutic ranges, they may injure the organs. Over a period of time, the chronic use of drugs may be associated with several toxicities. The liver and kidney are the major targets.
Hepatotoxicity implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease. Chemicals causing liver injury are called hepatotoxins [1]. Various drugs have been implicated in causing liver injury. It is the most common reason for a drug to be withdrawn from market. Hepatotoxicity and drug-induced liver injury also account for a substantial number of compound failures, highlighting the need for drug screening assays, like stem cell-derived hepatocyte-like cells, that are capable of detecting toxicity early in the drug development process. Chemicals often cause subclinical injury to liver, which manifests only as abnormal liver enzyme tests [2].
Nephrotoxicity is a kidney-specific feature in which excretion does not go smoothly owing to toxic chemicals or drugs [3]. Approximately 20% of nephrotoxicity is induced by drugs, but medication of the elderly increases the incidence of nephrotoxicity up to 66% as the average life span increases. Chemotherapy or anticancer medicine has been of limited use due to nephrotoxicity [4].
Nephrotoxicity occurs when kidney-specific detoxification and excretion do not work properly due to the damage or destruction of kidney function by exogenous or endogenous toxicants. Exposure to drugs often results in toxicity in kidney which represents the major control system maintaining homeostasis of body and thus is especially susceptible to xenobiotics [3].
Xylopia Aethiopica (Annonaceae), commonly called negro pepper, African pepper, Guinea pepper and spice tree, is an ever green aromatic tree growing up to 15-30 m high. It is a native to the low land rain forests and moist fringe forests in the savanna zones and coastal regions of Africa.
Folklore medicine claimed it to be useful as abortifacients, ecbolics as well as in the treatment of diarrhea, dysentery, stomach disorder, menstrual disorder, naso-pharyngeal infections, arthritis, rheumatism, infections, among others [5].
Interestingly, xenobiotics introduced to the body, will be metabolized by the liver and most of the time, excreted by the kidney. Therefore the liver and kidney are organs used in toxicity test, as any damage to these organs pose danger to life. These agents may readily be abused by its users, as it has been indicated in folklore management of several disorders. It is therefore important, to investigate the toxic potential of X. aethiopica.
Materials and Methods
Chemicals, reagents and equipment
Reagents: Ethanol, distilled water, chloroform.
Equipment’s: Mortar, pestle, Manual grinder, rotary evaporator, conical flask, filter paper, white handkerchief, funnel, 5ml syringes, stainless feeding needle.
Drugs
Plant collection and identification of plant material: The stem of X. aethiopica was obtained March, 14th 2017 from Ama Hausa at Ekeonunwa market Imo State and was identified at Federal Ministry of Environment Forestry Research Institute of Nigeria, Benin City Edo State.
Preparation of plant extract: The stem of the plant X. aethiopica was air dried for 48 h. Now it was broken down into smaller pieces with the aid of mortar, pestle and manual grinder. Further, 900 g of the pieces was macerated with ethanol (2000 ml) for 72 h with intermittent shaking in morning and evening. The extract was then filtered after 72 h and the filtrate concentrated using a rotary evaporator. The yield of the extract was found to be 25 g which was stored in refrigerator for further use.
Animal: Male albino rats (130-150 g) were used for this study. They were obtained from University of Nigeria Nsukka, Zoology Department and kept in the animal house, Department of Pharmacology and Toxicology, Madonna University, Elele Campus. They were acclimatized for 2 weeks prior to the experiment during which they were introduced to growers mash. The animals were housed in clean gauzed cages having free access to feed and water and maintained under standard conditions.
Acute toxicity test: This was carried out in 2 phases using the modified lorke’s method.
In phase 1, 9 rats which was divided into 3 groups containing 3 rats each was used. Each group of animals were administered different doses (10, 100 and 1000 mg/kg) of the plant extract. The animals were placed under observation for 24 h to monitor their behavior as well as to see if mortality would occur.
Phase 2 used 3 animals were divided into 3 groups of 1 animal each. The rats were administered higher doses of 1500, 2500 and 5000 mg/kg each of the plant extract and observed for 24 h for changes in behavior or mortality. The LD50 which is the square root of the highest dose with no mortality multiplied by the lowest dose with mortality could not be calculated since none of the doses killed the rat although some changes such as sluggish behavior, reduced appetite and thirst could be seen thus inferring that the plant extract has a wide therapeutic range [6].
Grouping of rats: The rats were grouped into 4 groups of 8 rats each. Group 1 was the control that took food and water only. Group 2 were treated with 200 mg/kg body weight of extract. Group 3 were treated with 400 mg/kg body weight of extract. Group 4 were treated 800 mg/kg body weight of extract.
Preparation and administration of drug suspension: The amount of test sample required for each rat was calculated based on body basis. The required quantity of extract was suspended in 5 ml of water and then administered orally to the rat using gavage technique by inserting stainless feeding needle into the esophagus, and delivering the drug directly into the stomach by means of syringe pressed steadily and slowly. This was done daily for 28 days.
Collection of samples: On the 14th day, blood samples were collected from three rats in each group into plain bottles for the liver function test and kidney function test to be carried out.
Sacrifice of rats: On the 28th day, the rats were sacrificed. First, chloroform was used to induce anesthesia and then blood was collected into plain bottles from three rats in each group for the liver function test and kidney function test to be carried out. The liver and two kidneys of these rats were also collected, weighed and put into plain bottles, and then formalin was added for preservation. This is for the histology to be carried out.
Analysis of the liver and kidney function test: The blood samples were sent to Salvation Laboratory Owerri where the liver function test and kidney function test were assayed. For the liver function test, these biomarkers were analyzed: Alanine Amino Transferase, Aspertate Amino Transferase, Alkaline Phosphatase, Total Bilirubin, Conjugated Bilirubin, Total Protein and Albumin. For renal function test, the following biomarkers were analyzed: urea, creatinine, potassium, chloride, bicarbonate, sodium.
Histology of the liver and kidney: The preserved organs were sent to Laboratory where the histology was carried out and interpretation of the result made.
Results and Discussion
According to Murray, a good knowledge is required regarding the toxicity, dosage, purity, suitable extraction solvent and adverse effect for the use of herbal drugs [7]. The phytocomponents of medicinal plants are fundamentals to the pharmacological effects elicited by these plants against pathogenic-infectious diseases. Phytochemicals as antioxidants play vital roles in human health [8]. X. aethiopica has been found to contain some phytochemicals which exhibit a wide range of biological effects as a consequence of their antioxidant properties.
It is deduced from study made by that the aqueous extract of X. aethiopica fruit is rich in phenolics and volatiles compounds, which act as strong natural antioxidants [9]. In that study, the aqueous extract normalized the toxic effect of panadol-induced hepatic and renal toxicity. Moreover, the results obtained confirmed the hepatoprotective effect of the methanolic extract of X. aethiopica.
Table 1 shows the qualitative result of the phytochemical constituents of the ethanolic extract of X. aethiopica stem. Alkaloids, saponins, reducing sugar, steroids, flavonoids and glycosides are present in the stem. The result of the study done by on the phytochemicals present in the petiole, seed, leaf, bark and root X. aethiopica reported that alkaloid, saponins and flavonoids are the more abundant bioactive compounds in X. aethiopica. An alkaloid is a type of plant derived organic compound, generally composed of oxygen, hydrogen, carbon and nitrogen. Although, some alkaloids are considered toxic but others are often used in medical therapies. Many alkaloids can be used for medical purposes [10].
| Screened Plant Parts | |||||
|---|---|---|---|---|---|
| Phytochemical constituents | Petiole | Seed | Leaf | Bark | Root |
| Alkaloids | + | + | + | + | - |
| Saponins | + | + | + | + | - |
| Tannins | + | - | + | - | - |
| Reducing Sugar | - | - | - | + | + |
| Phlobatannin | - | - | - | - | - |
| Anthraquinone | + | - | - | + | - |
| Steroids | + | + | + | - | - |
| Flavonoids | + | + | + | + | - |
| Glycosides | + | - | - | + | - |
| Key: +=Presence; -=absence of bioactive compounds. | |||||
Table 1: Phytochemicals present in the petiole, seed, leaf, bark and root X. aethiopica.
Flavonoids represent the most common and widely distributed of plant phenolics found in X. aethiopica [11]. Flavonoids prevent oxidative cell damage, have strong anti-cancer activity and protects against all stages of carcinogenesis. Saponins another phytochemical constituent of X. aethiopica have wide range of biological properties; maintenance of homeostasis, anti-inflammatory effect and anti- cancer actions. Saponins cause a reduction of blood cholesterol by preventing its re-absorption. They have antitumor and antimutagenic activities and can lower the risk of human cancers by preventing cancer cells from growing apart from their biocidal effects against pathogens [10].
Acute Toxicity Test
Tables 2.1 and 2.2 show the phase 1 and 2 of the acute toxicity test. There was no mortality; therefore the extract has a wide therapeutic range.
Phase One:
| Dose Administered | No of Death |
| 10 mg/kg | 0/3 |
| 100 mg/kg | 0/3 |
| 1000 mg/kg | 0/3 |
Table 2.1: The table below shows the acute toxicity test (LD50) result of phase 1 using the modified Lorkes method (Lorkes, 1983).
Phase Two:
| Dose Administered | No of Death |
| 1500 mg/kg | 0/1 |
| 2500 mg/kg | 0/1 |
| 5000 mg/kg | 0/1 |
Table 2.2: The table below shows the acute toxicity test (LD50) result of phase 2 using the modified Lorkes method.
Table 3 shows that the weights of the albino rats increased with duration of the administration of the ethanolic extract of the stem of X. aethiopica. The results of the studies done by Obodo et al., suggest that dietary inclusion of crude X. aethiopica leaves influences body weight and the growth rate of Wistar rats [12].
| Before Administraton (g) | After 14 days of Administration (g) | After 28 days of Administration (g) | |
|---|---|---|---|
| Control | 110.00 ± 5.29 | 170.00 ± 5.03** | 148.67 ± 3.33 |
| 200 mg/kg | 130.00 ± 11.14 | 168.67 ± 23.70 | 172.00 ± 14.00 |
| 400 mg/kg | 138.67 ± 8.19 | 165.33 ± 10.35 | 198.00 ± 9.87** |
| 800 mg/kg | 160.00 ± 11.02 | 182.00 ± 8.33 | 204.33 ± 8.29* |
Table 3: Effect of ethanolic extract of X. aethiopica administered for 28 days on the weight of the rats.
Studies done on X. aethiopica fruits by Chike and Adienbo, Adefegha and Oboh, Woode et al., and Obodo et al., revealed that administration of the extract affected the body weight negatively [12-15]. This can be due to variation in the constituents of stem and fruit, as the studies stated above are on the fruits. This can also be affected by ethanolic content of the extract administered to the rats.
In Table 4, the Values represent relative organ weights using oneway ANOVA followed by Bonferroni post test.
| Control(g) | 200 mg/kg(g) | 400 mg/kg(g) | 800 mg/kg(g) | |
|---|---|---|---|---|
| Liver | 5.66 | 4.36 | 6.93 | 6.99 |
| Right Kidney | 0.36 | 0.36 | 0.39 | 0.64 |
| Left Kidney | 0.28 | 0.27 | 0.27 | 0.22 |
Table 4: Relative organ weight of the animals.
Table 5 shows the renal function study after 14 days of administration and there is no significant difference in the value of the urea, creatinine, potassium, chloride, bicarbonate and sodium of the animals treated with 200 mg/kg, 400 mg/kg and 800 mg/kg of ethanolic extract when compared with the control. It also shows the renal function study after 28 days of administration and there is a significant decrease (p<0.05) of urea at the doses of 200 mg/kg and 400 mg/kg when compared with the control.
| Marker Control | After 14 days of Administration (g) | After 28 days of Administration (g) | |||||
|---|---|---|---|---|---|---|---|
| 200 mg/kg | 400 mg/kg | 800 mg/kg | 200 mg/kg | 400 mg/kg | 800 mg/kg | ||
| Urea | 36.00 ± 3.06 | 31.00 ± 1.53 | 33.00 ± 5.57 | 41.00 ± 5.29 | 23.67 ± 2.60* | 22.00 ± 3.79* | 38.00 ± 2.52 |
| CRT | 0.77 ± 0.23 | 0.50 ± 0.10 | 0.60 ± 0.15 | 0.50 ± 0.15 | 0.80 ± 0.15 | 0.70 ± 0.12 | 1.00 ± 0.31 |
| K+ | 5.00 ± 0.58 | 6.30 ± 0.53 | 5.40 ± 0.81 | 5.60 ± 0.27 | 3.80 ± 0.38 | 4.10 ± 0.15 | 4.30 ± 0.10 |
| Cl‾ | 100.00 ± 4.93 | 103.00 ± 4.04 | 97.00 ± 4.16 | 101.00 ± 3.79 | 99.00 ± 7.23 | 97.00 ± 7.64 | 103.00 ± 2.52 |
| HCO3‾ | 27.00 ± 2.08 | 23.00 ± 2.08 | 26.00 ± 2.52 | 23.00 ± 1.53 | 25.00 ± 2.00 | 27.00 ± 1.16 | 24.00 ± 2.52 |
| Na+ | 141.00 ± 4.58 | 139.00 ± 3.46 | 143.00 ± 4.36 | 145.00 ± 3.61 | 135.00 ± 3.61 | 129.00 ± 2.65 | 137.00 ± 0.58 |
| KEY: CRT: Creatinine; K: Potassium; Cl: Chloride; HCO3‾: Bicarbonate; Na: Sodium. Values represent mean ± SEM (n=3) using one-way ANOVA followed by Bonferroni’s Multiple Comparison Tests. | |||||||
Table 5: Kidney function test result after 14 days and 28 days of administration.
But there is no significant difference in the value of urea at the dose 800 mg/kg after 14 days of administration when compared with the control. It also shows that there is no significant difference in the value of the creatinine, potassium, chloride, bicarbonate and sodium of the animals treated with 200 mg/kg, 400 mg/kg and 800 mg/kg of ethanolic extract after 28 days of administration when compared with the control.
Table 6 shows the liver function study after 14 days of administration and there is a significant decrease (p<0.001) in the value of alanine amino transferase, aspartate amino transferase and alkaline phosphatase of the animals treated with 200 mg/kg, 400 mg/kg and 800 mg/kg of ethanolic extract when compared with the control. It also shows that there is no significant difference in the value of the total bilirubin, conjugated bilirubin, total protein and albumin of the animals treated with 200 mg/kg, 400 mg/kg and 800 mg/kg of ethanolic extract compared with the control.
| Marker Control | AFTER 14 DAYS OF ADMINISTRATION | AFTER 28 DAYS OF ADMINISTRATION | |||||
|---|---|---|---|---|---|---|---|
| 200 mg/kg | 400 mg/kg | 800 mg/kg | 200 mg/kg | 400 mg/kg | 800 mg/kg | ||
| ALT | 143.00 ± 6.25 | 29.00 ± 7.77*** | 25.00 ± 3.22*** | 18.00 ± 1.00*** | 89.00 ± 11.27*** | 87.00 ± 3.61*** | 131.00 ± 2.08 |
| AST | 127.00 ± 2.52 | 51.00 ± 2.31*** | 47.00 ± 0.51*** | 47.00 ± 1.53*** | 78.00 ± 7.81*** | 81.00 ± 3.79*** | 124.00 ± 1.73 |
| ALP | 139.00 ± 5.86 | 71.00 ± 8.08*** | 64.00 ± 3.22*** | 66.00 ± 14.50*** | 118.00 ± 3.06*** | 122.00 ± 1.73*** | 137.00 ± 3.61 |
| TB | 0.30 ± 0.06 | 0.30 ± 0.06 | 0.30 ± 0.06 | 0.40 ± 0.06 | 0.40 ± 0.06 | 0.30 ± 0.06 | 0.40 ± 0.06 |
| CB | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.20 ± 0.00 | 0.10 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 |
| TP | 7.70 ± 0.36 | 7.60 ± 0.27 | 7.20 ± 0.25 | 7.90 ± 0.15 | 7.40 ± 0.15 | 7.83 ± 0.23 | 7.80 ± 0.15 |
| ALB | 3.20 ± 0.21 | 3.40 ± 0.23 | 3.60 ± 0.10 | 3,20 ± 0.12 | 3.30 ± 0.15 | 3.40 ± 0.21 | 3.50 ± 0.12 |
| KEY- ALT: Alanine Amino Transferase; AST: Aspertate Amino Transferase; ALP: Alkaline Phosphatase; TB: Total Bilirubin; CB: Conjugated Bilirubin; TP: Total Protein; ALB: Albumin. Values represent mean ± SEM (n=3) using one-way ANOVA followed by Bonferroni’s Multiple Comparison Tests. | |||||||
Table 6: Liver function test results after 14 days and 28 days of administration.
Table 6 also shows the liver function study after 28 days of administration and there is a significant decrease (p<0.001) in the value of alanine amino transferase and aspartate amino transferase of the animals treated with 200 mg/kg and 400 mg/kg of ethanolic extract when compared with the control. It also shows that there is a significant decrease (p<0.001) in the value of alkaline phosphatase of the animals treated with 200 mg/kg of ethanolic extract and a significant decrease (p<0.01) in the value of alkaline phosphatase of the animals treated with 400 mg/kg of ethanolic extract after when compared with the control.
It is also shown here, that there is no significant difference in the value of the total bilirubin, conjugated bilirubin, total protein and albumin of the animals treated with 200 mg/kg and 400 mg/kg of ethanolic extract after 28 days of administration when compared with the control. It also reveal that there is no significant difference in the value of the alanine amino transferase, aspartate amino transferase, alkaline phosphatase, total bilirubin, conjugated bilirubin, total protein and albumin of the animals treated with 800 mg/kg of ethanolic extract when compared with the control.
According to Nadia et al., the aqueous fruit extract normalized the toxic effect of panadol-induced renal toxicity thus inferring that there is no harm in ingesting the fruit [9]. Comparing this study with results of Tables 5 and 6, a little variation is observed and this can be due to difference in the plant part studied. It can also be due to difference in extraction solvent. The results obtained confirmed the hepatoprotective effect of the methanolic leaf extract of X. aethiopica. In the research work, done by it was reported that the aqueous fruit extract normalized the toxic effect of panadol-induced hepatic toxicity [9]. These infer that there is no harm in ingesting the fruit and leaf. Comparing this study with results of Tables 5 and 6, a little variation is observed and this can be due to difference in the constituent of plant part studied. It can also be due to difference in extraction solvent.
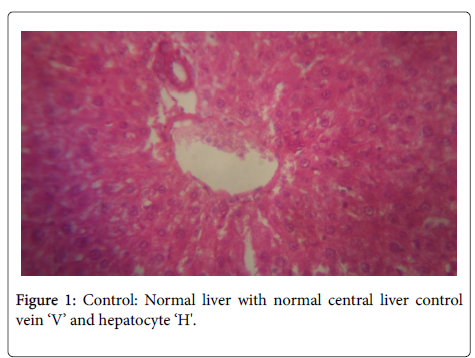
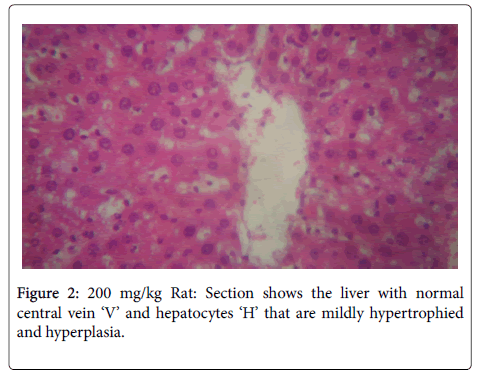
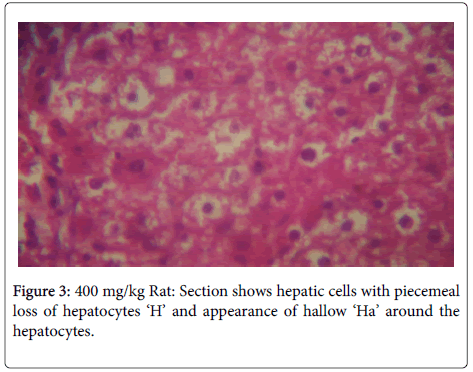
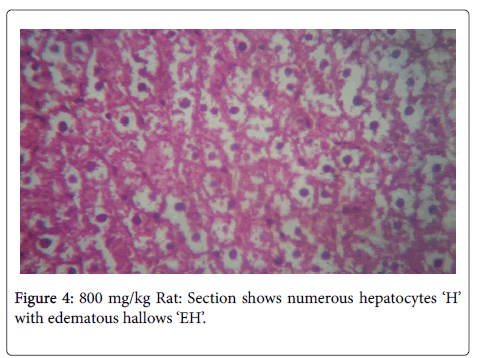
In the histological study of the liver shown in Figures 1-4 for the different doses, morphological changes were observed. The deviations from normal, in Figures 3-5 seemed to have been proportional to the increase in dose. It is in accordance with the study of Chris-Ozoko et al., where they opined that the histological findings examination of liver revealed a mild to acute hepatitis in treated rats [16].
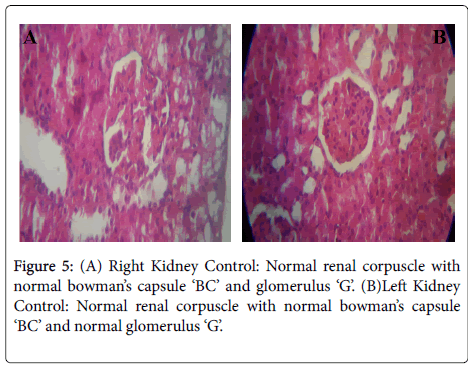
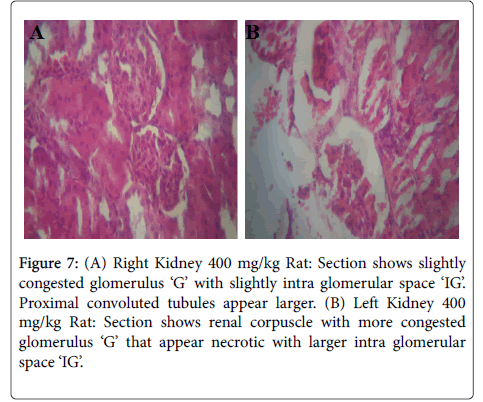
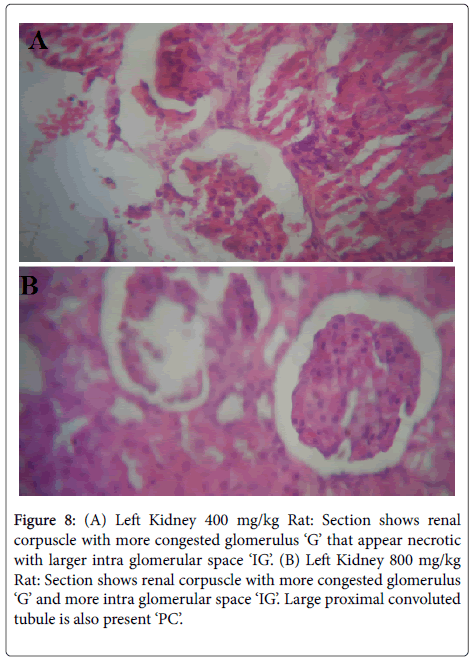
Figures 5-8 were histological results of the Kidney. The photomicrograph revealed that renal corpuscle had more congested glomerulus ‘G’ that appear necrotic with larger intra glomerular space in the treated groups, in a dose dependent manner. This may suggest risks of interstitial nephritis in the treated rats.
Figure 7: (A) Right Kidney 400 mg/kg Rat: Section shows slightly congested glomerulus ‘G’ with slightly intra glomerular space ‘IG’. Proximal convoluted tubules appear larger. (B) Left Kidney 400 mg/kg Rat: Section shows renal corpuscle with more congested glomerulus ‘G’ that appear necrotic with larger intra glomerular space ‘IG’.
Figure 8: (A) Left Kidney 400 mg/kg Rat: Section shows renal corpuscle with more congested glomerulus ‘G’ that appear necrotic with larger intra glomerular space ‘IG’. (B) Left Kidney 800 mg/kg Rat: Section shows renal corpuscle with more congested glomerulus ‘G’ and more intra glomerular space ‘IG’. Large proximal convoluted tubule is also present ‘PC’.
Conclusion
It is possible therefore, to infer that the sub chronic toxicity study of X. aethiopica stem on liver and kidney has a toxic potential at high doses as it showed significant differences on some biomarkers of the kidney and liver functions. Moreover, the photomicrograph showed some slight histological alterations in both organs. Further studies maybe recommended ascertaining what chronic effects; the agent may pose to these vital organs and the mechanism by which these potential damages are mediated.
Acknowledgement
Sincere thanks to Members of the department of Pharmacology, technical and academic staff, also the department of Pharmacognosy, Faculty of Pharmacy Madonna University, Nigeria.
References
- Friedman SE, Grendell JH,McQuaid KR (2003) In: Robert Burakoff, Richard Blumberg, Norton Greenberger (eds.) Current diagnosis andtreatment in gastroenterology. McGraw-Hill, Newyork, United States.
- Greenhough S, Hay DC (2012). Stem Cell-Based Toxicity Screening: Recent Advances in Hepatocyte Generation. Pharm Med 26: 85-89.
- Sun YK, Aree M (2012) Drug-Induced Nephrotoxicity and Its Biomarkers. Biomol Ther 20: 268-272.
- Nagai J, Takano M (2010) Molecular-targeted approaches to reduce renal accumulation of nephrotoxic drugs. Expert Opin Drug Metab Toxicol 6: 1125-1138.
- Erhirhie OE (2014) Xylopia Aethiopica: A Review of its Ethnomedicinal, Chemical and Pharmacological Properties. Am J PharmTech Res 4: 21-37.
- Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54: 275-287.
- Murray A (1998) Dietary reference intake for antioxidant nutrients. J Am Diet Assoc 100: 637-640.
- Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, et al. (2008) Phytochemical Screening and Antioxidant Activities of some selected Medicinal Plants used for Malaria Therapy in Southwestern Nigeria. Trop J Pharm Res 7: 1019-1024.
- Nadia NA, Hatil NE, Manal MR, Kadry ZG, Abdel R (2013) Volatile compounds protect against Panadol-induced hepatic and renal toxicity in male rats. World Appl Sci J 27: 10-22.
- Aguoru CU, Pilla C, Olasan JO (2016) Phytochemical Screening Xylopia Aethiopica with emphasis on its medicinally active principles. J Med Plants Res 10: 306-309.
- Keita B, Sidibe L, Figneredo G, Chalchat JC (2003) Chemical composition of essential oil of Xylopia Aethiopica (Duna) A. Rich from Mali. J Essent Oil Res 15: 267-269.
- Obodo BN, Iweka FK, Obhakan JO, Oyadonghan GP, Agbo GE (2013) The effect of Xylopia Aethiopica on body weight and growth performance. Int J Herbs Pharm Res 2: 14-19.
- Chike CP, Adienbo OM (2010) The effect aqueous extract of Xylopia Aethiopica on some biochemical profile of male guinea pigs. Afr J Appl Zool Environ Biol 12: 72-75.
- Adefegha SA, Oboh G (2012) Effect of diets supplemented with Ethopian pepper, Xylopia Aethiopica (Dun) A.Rich (Annonaceae) and Ashanti pepper, Piper guineense Schumach et Thom (Piperaceae) on some biochemical parameters in normal rats. Asian Pac J Trop Biomed 12: 558-566.
- Woode E, Ameyaw EO, Boakye-Gyasi E, Abotsi WK (2012) Analgesic effects of an ethanol extract of the fruits of Xylopia Aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. J Pharm Bioallied Sci 4: 291-301.
- Chris-Ozoko LE, Ekunolina V, Winiki C (2015) Histomorphological effect of Xylopia Aethiopica on the liver and kidney of albino wistar rats. Sch Acad J Biosci 3: 150-154.
Citation: Ehigiator BE, Ezeani DN (2018) Evaluation of the Hepatotoxic and Nephrotoxic Potential of Ethanolic Stem Back Extract of Xylopia Aethiopica (Annonaceae) in Male Rodents. Diagn Pathol Open 3: 138. DOI: 10.4172/2476-2024.1000138
Copyright: © 2018 Ehigiator BE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4546
- [From(publication date): 0-2018 - Nov 27, 2024]
- Breakdown by view type
- HTML page views: 3822
- PDF downloads: 724