Evaluation of the Effectiveness of Risk Minimisation Measures Targeting Physicians on Prescribing Practices of Thiocolchicoside Containing Medicinal Products for Systemic Use
Received: 30-Nov-2018 / Accepted Date: 18-Mar-2019 / Published Date: 28-Mar-2019 DOI: 10.4172/2161-1165.1000372
Abstract
Introduction: In 2014, Risk Minimisation Measures (RMMs) concerning thiocolchicoside for systemic use were implemented in all European countries marketing the product. A Direct Healthcare Professional Communication (DHPC) and Educational Materials (EMs), were distributed to health care professionals to increase awareness related to genotoxicity risk.
Objectives: The objective of this study was to measure the effectiveness of RMMs, by ascertaining the proportion of targeted physicians who understood and implemented the latest prescribing conditions and safety information about systemic thiocolchicoside provided in the DHPC and EMs.
Methods: A cross-sectional web-based survey of prescribers of systemic thiocolchicoside was conducted in France, Greece, Italy and Portugal. Sampling targets for responding physicians were determined based on country specific prescribing patterns. Frequency of correct responses was calculated for six knowledge questions and information related to recent patients’ prescriptions of TCC was analysed.
Results: Among 651 responding physicians, 68.6% remembered having received either the DHPC or the EM or both. Knowledge was the highest for contraindications to the use of systemic thiocolchicoside, related to patients’ age (85.0%), pregnancy (87.6%), and lactation (80.3%) and was lower (49.1%) regarding restriction of use in women of childbearing potential not using contraception. Overall, knowledge of the right indication for use of systemic thiocolchicoside was 63.2%, while knowledge of dose and duration of treatment was higher for the oral form (78.9%) than for the injectable form (55.4%). These results were confirmed by the analysis of recent patients’ prescriptions. Knowledge varied by physician specialty and country and was higher for physicians who reported receiving either the DHPC or the EM or both.
Conclusion: This study reveals geographical as well as across prescribers’ specialty contrasts in the knowledge of key messages of systemic TCC containing medicinal products associated RMMs. In addition, the results showed that when risk minimisation materials (DHPC and EM) receipt was acknowledged by physicians, it improved their knowledge and attitude towards appropriate systemic TCC prescribing to patients.
Keywords: Direct healthcare professional communication; Risk minimisation measures; Thiocolchicoside
Abbreviations
CHMP: Committee for Medicinal Products for Human Use; DHPC: Direct Healthcare Professional Communication; EM: Educational Materials; EMA: European Medicines Agency; EU: European Union; ENCePP: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance; GP: General Practitioner; HCP: Health Care Professional; PRAC: Pharmacovigilance and Risk Assessment Committee; RMM: Risk Minimisation Measures; SmPC: Summary of Product Characteristics; TCC: Thiocolchicoside
Introduction
Thiocolchicoside (TCC) is a semi-synthetic sulfurated colchicoside derivative with a muscle relaxant pharmacological activity [1]. It is currently nationally authorised in eight European Union (EU) Member States for oral and injectable use [2]. It was first launched in the 1950s.
In February 2013, the Italian Medicines Agency (AIFA) requested the European Medicines Agency (EMA) to assess concerns regarding genotoxic effect of a TCC metabolite highlighted in literature [2-6], and its impact on the benefit-risk balance of the medication. This effect was related to oral or injection intake, since topic forms do not produce substantial levels of concerned metabolite in the body. This evaluation was done under Article 31 pharmacovigilance referral (Directive 2001/83/EC). The European Commission (EC) decision was issued on 17 January 2014 [6] and had to be implemented by all Marketing Authorisation Holders (MAHs) in EU. It was recommended that the authorized uses for thiocolchicoside containing products for systemic use (oral or injection) should be restricted across the European Union. The restrictions concerned the maximum dose and number of days of treatment, and the contra-indication in pregnancy and lactation or in women of childbearing potential not using contraception, as well as in children and adolescents below 16 year old and, at last, for chronic (long-term) conditions. In addition, Risk Minimisation Measures (RMMs) had to be taken to ensure safe use of the products and address the risks of teratogenicity, embryo/foetotoxicity, spontaneous abortion, impaired male fertility and cancer. In addition to routine, additional RMM included dissemination of a Direct Healthcare Professional Communication (DHPC) and educational materials (Health Care Professional Guide, Patient Card).
After the outcome of the referral procedure [2] and finalization of routine and additional RMMs translation, country-specific dissemination, as well as the target groups of healthcare providers in each country was agreed upon with the respective national competent authorities. The national competent authority approval timelines varied between member states. The timing of dissemination varied by country, from February 2014 (Greece, Italy, Portugal) to April 2014 for DHPC and from September 2015 (Portugal) to April 2016 (France) for EM. Educational materials and DHPC were disseminated through various channels (post-mailed hard copies mainly, e-mail, fax) [7].
Joint marketing authorization holders, as a Consortium, were required to evaluate the effectiveness of the above described risk minimisation strategy based on two post-authorization safety studies [2]. A drug utilization study is being conducted in France and Italy [8] and complete a Health Care Professional survey which was conducted in France, Greece, Italy, Portugal [7]. The objective of this Health Care Professional (HCP) survey was to measure the effectiveness of the DHPC and EM, implemented as part of systemic TCC related RMMs, by ascertaining the proportion of targeted physicians who understood and implemented the latest prescribing conditions and safety information. Health Care Professional (HCP) survey results [7] are presented in the present article.
Materials and Methods
Study design
The study was an observational cross-sectional survey of physicians’ knowledge, understanding, and self-reported behaviour. It was conducted among a sample of physicians with recent experience with TCC in France, Italy, Greece, and Portugal. These countries were selected because they accounted for more than 96% of systemic TCC prescriptions in the EU, as agreed with the PRAC. The study specifically targeted General Practitioners (GPs), rheumatologists and orthopaedists/orthopaedic surgeons, based on the distribution of systemic TCC products prescriptions per specialty in national sales lists (Prescribing Insights, IQVIA, https://www.iqvia.com). In all countries, a random sample of potentially eligible physicians in each specialty of interest was contacted by e-mail from exhaustive national listings of practicing physicians by specialty (OneKey, IQVIA, https:// www.iqvia.com). The study aimed to recruit 120-150 physicians each in Greece and Portugal and 180-230 physicians each in France and Italy, aiming to assess at least 600 physicians’ completed questionnaires. These sample sizes, which were approved by the PRAC, were anticipated to be sufficient to support the planned analyses and to allow reasonable precision for the total sample and within each country to the extent possible. To participate, physicians needed to be a licensed and practicing rheumatologist, orthopaedist/orthopaedic surgeon or GPs (i.e., general medicine, family practice, internal medicine) and having been prescribing TCC to at least one patient in the previous 12 months. The study complied with all local regulatory and ethical requirements. All participants who completed the survey provided informed consent.
Survey deployment
A web–questionnaire comprising 18 items was developed using best practices for survey development [9]. The questionnaire was tested among physicians in each country before data collection to optimize the questionnaire items, wording, and response choices and to ensure consistency across cultures and languages. The questionnaire contained screener questions to confirm eligibility; an informed consent question; open-ended and closed-ended multiple-choice questions to measure knowledge of the prescribing conditions and safety information about systemic thiocolchicoside presented in the DHPC and EM; questions to characterize the physicians and their practices; and questions to investigate physician receipt and use of educational materials related to TCC. In addition, physicians were invited to provide information related to recent patient’s prescriptions of TCC. They could submit up to 5 prescriptions out of mind. This part included specific questions on patient demographic characteristics and details concerning the prescription related to study objectives. The questionnaires items are included in the Electronic Supplementary Material (ESM) accompanying this article. Invitations to complete the web-based survey were sent via e-mail to a randomly selected sample of physicians. Interested physicians logged into the study website by entering a unique identification number and password. Physicians were not able to modify answers to previous questions, which minimized their likelihood of searching for answers or changing responses based on content in subsequent questions. Respondents could only submit questionnaire when all questions were answered which prevented the occurrence of missing data. The physician survey was initiated in each country after a sufficient period to allow prescribers to have received the educational materials and use the information in their practice. Data collection ran from 01 February 2017 to 10 March 2017.
Data analysis
Data analyses were descriptive and focused on summarizing the frequencies and percentages of survey responses in eligible participants who provided informed consent. The percentages for individual questionnaire responses were based on the number of participants. Only completed questionnaires could be submitted by respondents, and no missing response was imputed. Descriptive responses were generated for all countries combined and stratified by country. Stratifications were also conducted by physician specialty, number of years of medical experience, receipt of educational materials, and number of patients prescribed during the previous 3 months.
To synthetize information gathered on the level of knowledge of surveyed prescribers, five indicators were calculated based on the responder’s success proportion for 5 items related to the main aspects of systemic TCC risk minimisation strategy. Criterion 1 was the proportion of physicians who prescribed systemic thiocolchicoside only as adjuvant treatment of painful muscle contractures associated with acute spinal pathology in adults and in adolescents from 16 years of age onwards; criterion 2 was the proportion of physicians who did not prescribe systemic thiocolchicoside for long-term treatment of chronic conditions; criterion 3 was the proportion of physicians who followed the recommendations regarding the doses and duration restriction; criterion 4 was the proportion of physicians who did not prescribe systemic thiocolchicoside during pregnancy and lactation; criterion 5 was the proportion of physicians who did not prescribe systemic thiocolchicoside in women of childbearing potential not using adequate contraception.
To be able to extend the survey results to the overall target population, weighted results adjusted for sampling approach were used throughout when interpreting the study findings and drawing generalisations. Analyses were first performed on raw data per specialty and country, and then weighted by country and within each country by specialty [8]. An additional adjustment was performed on the information related to recent patient’s prescriptions of TCC to apply a similar weight to physicians whatever the number of prescriptions they submitted.
Possible selection biases were assessed by comparing the distributions of available characteristics (e.g. country, age, gender, type of practice and specialty) between respondent and non-respondent physicians (‘Online Resource 2’ in the ESM accompanying this article). All analyses were performed using SAS software, Version 9.4 (SAS Institute, Inc., Cary, NC, USA, 2002–2012).
Results
Demographics and clinical practice characteristics
Of the 23,832 physicians invited to participate, 651 physicians completed the survey. Data-collection efforts were stopped once the country-specific targets were met. Table 1 presents the sample characteristics. The percentage of physicians contacted who completed the survey ranged from 1.2% in France to 7.0% in Greece (Table 2).
| Question | No. of physicians (%) | ||||
|---|---|---|---|---|---|
| France (n=200) | Greece (n=124) | Italy (n=203) | Portugal (n=124) | Overall (n=651) | |
| Specialty | |||||
| General Practitioners | 105(52.5%) | 62 (50.0%) | 93 (45.8%) | 82 (66.1%) | 342 (52.5%) |
| Rheumatologists | 51 (25.5%) | 31 (25.0%) | 42 (20.7%) | 21 (16.9%) | 145 (22.3%) |
| Orthopedists/orthopedic surgeons | 44 (22.0%) | 31 (25.0%) | 68 (33.5%) | 21 (16.9%) | 164 (25.2%) |
| Physicians age, years | |||||
| ≤ 30 years old | 4 (2.0%) | 1 (0.8%) | 2 (1.0%) | 11 (8.9%) | 18 (2.8%) |
| 31-39 years old | 43 (21.5%) | 15 (12.1%) | 21 (10.3%) | 37 (29.8%) | 116 (17.8%) |
| 40-49 years old | 44 (22.0%) | 67 (54.0%) | 25 (12.3%) | 24 (19.4%) | 160 (24.6%) |
| 50-59 years old | 82 (41.0%) | 28 (22.6%) | 79 (38.9%) | 28 (22.6%) | 217 (33.3%) |
| ≥ 60 years old | 27 (13.5%) | 13 (10.5%) | 76 (37.4%) | 24 (19.4%) | 140 (21.5%) |
| Years in practice | |||||
| 1-5 years | 19 (9.5%) | 18 (14.5%) | 11 (5.4%) | 31 (25.0%) | 79 (12.2%) |
| 6-10 years | 22 (11.1%) | 35 (28.2%) | 16 (7.9%) | 20 (16.1%) | 93 (14.3%) |
| >10 years | 158 (79.4%) | 71 (57.3%) | 176 (86.7%) | 73 (58.9%) | 478 (73.5%) |
| Practice Type | |||||
| Office based | 109 (54.5%) | 77 (62.1%) | 86 (42.4%) | 50 (40.3%) | 322 (49.5%) |
| Hospital based | 60 (30.0%) | 17 (13.7%) | 61 (30.0%) | 27 (21.8%) | 165 (25.3%) |
| Both, office and hospital | 31 (15.5%) | 30 (24.2%) | 56 (27.6%) | 47 (37.9%) | 164 (25.2%) |
| Number of prescriptions of systemic TCC* | |||||
| Mean (SD) | 95.4 (140.72) | 102.4 (173.77) | 99.7 (140.23) | 139.9 (184.49) | 106.6 (156.84) |
| Median | 50 | 50 | 50 | 85 | 50 |
| Q1-Q3 | (20.0-100.0) | (20.0-100.0) | (30.0-100.0) | (30.0-165.0) | (25.0-115.0) |
| Range | (1.0-1000.0) | (2.0-1000.0) | (1.0-1000.0) | (4.0-1000.0) | (1.0-1000.0) |
*Number of prescriptions(not patients) of systemic thiocolchicoside containing medicinal products written within the last 12 months
Table 1: Physician demographics and prescribing and practice characteristics.
| Country | Completed | Invited | Completion (%) |
|---|---|---|---|
| France | 200 | 16,614 | 1.20% |
| Greece | 124 | 1,776 | 7% |
| Italy | 203 | 3,192 | 6.40% |
| Portugal | 124 | 2,250 | 5.50% |
| Overall | 651 | 23,832 | 2.70% |
Table 2: Summary of completion percentage per country.
Following results are presented as weighted results.
Knowledge of prescribing conditions and safety information warnings of TCC
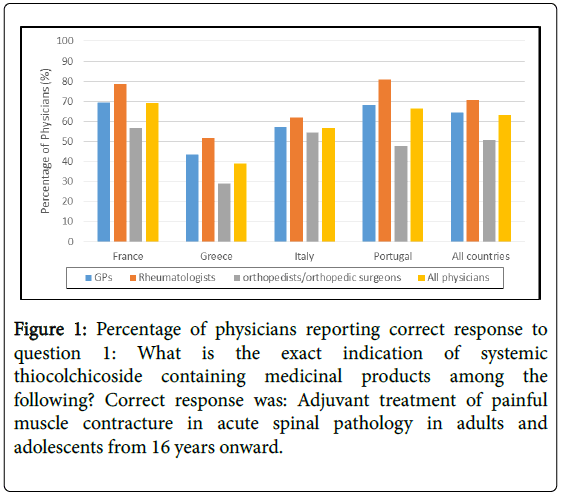
Knowledge of indication and contraindications: In total, 63.2% of responding physicians had the appropriate understanding that the indication for systemic TCC prescription should be as adjuvant treatment of painful muscle contracture in acute spinal pathology in adults and adolescents from 16 years and older (Figure 1). The other mainly cited indication (24.2%) was acute painful muscle contracture conditions. A minority (6.5%) of physicians responded that the indication was long term treatment of chronic conditions.
Figure 1: Percentage of physicians reporting correct response to question 1: What is the exact indication of systemic thiocolchicoside containing medicinal products among the following? Correct response was: Adjuvant treatment of painful muscle contracture in acute spinal pathology in adults and adolescents from 16 years onward.
In all countries, orthopaedists/orthopaedic surgeons were the less aware of the right indication (50.7% overall), while rheumatologists were the best informed (70.8% overall). There were differences between countries with about 39.0% of physicians knowing the right indication in Greece, while there were up to 69.2% in France.
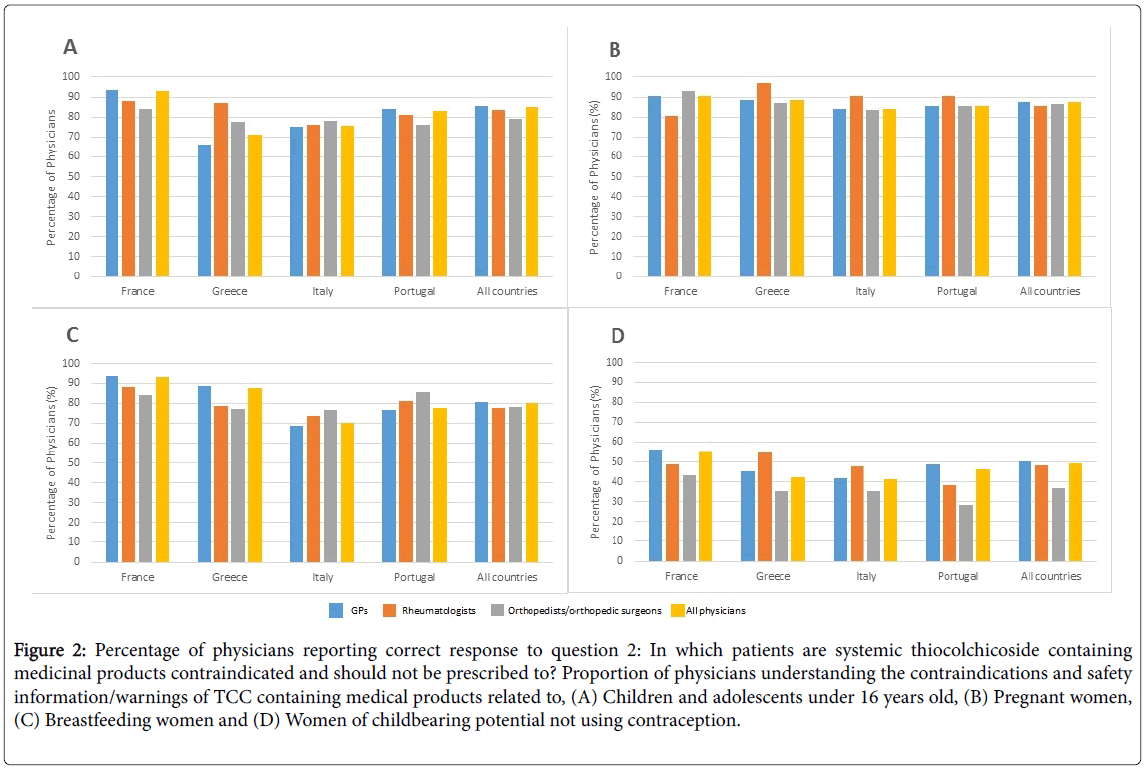
Overall, more than 85% of physicians reported that the use of systemic TCC containing medicinal products was contraindicated for pregnant women or patients under 16 years old, and over 80% for breastfeeding women (Figure 2). These rates were consistent among specialties and countries. However, half (49.1%) of physicians responded that TCC should not be prescribed to female of childbearing potential not using contraception. Among all physicians, orthopaedists/orthopaedic surgeons were the least aware of the latter contraindication (from 71.4% in Portugal to 56.8% in France) (Figure 2D).
Figure 2: Percentage of physicians reporting correct response to question 2: In which patients are systemic thiocolchicoside containing medicinal products contraindicated and should not be prescribed to? Proportion of physicians understanding the contraindications and safety information/warnings of TCC containing medical products related to, (A) Children and adolescents under 16 years old, (B) Pregnant women, (C) Breastfeeding women and (D) Women of childbearing potential not using contraception.
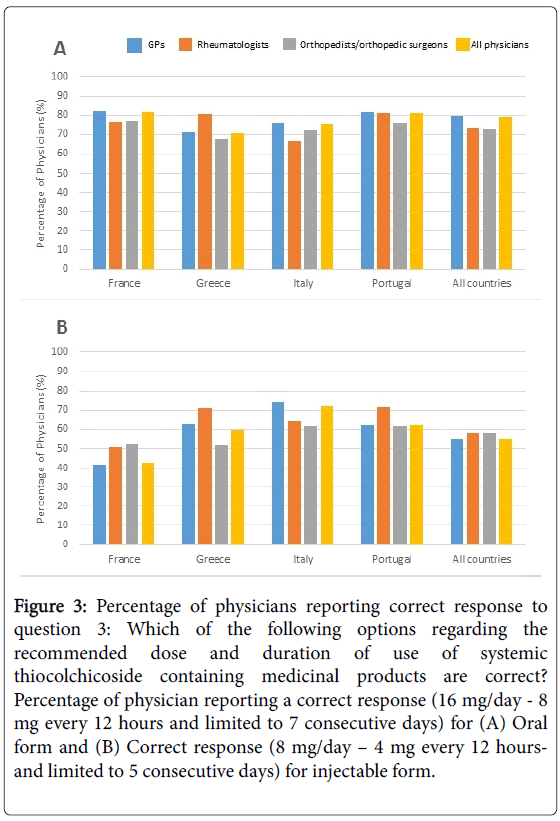
Knowledge of dose and treatment duration: For the oral form, overall, 78.9% of physicians gave the appropriate response (16 mg/day - 8 mg every 12 hours and limited to 7 consecutive days) (Figure 3A). A smaller dosage (8 mg/day) with no restriction on treatment duration was perceived as the recommended one by 13.4% of physicians (20.4% of orthopaedists/orthopaedic surgeons). In all countries, GPs were the most aware of the right dose and duration (79.7%). For the injectable form, overall, 55.4% of physicians gave the appropriate response (8 mg/day – 4 mg every 12 hours and limited to 5 consecutive days) (Figure 3B). A higher dosage and duration (16 mg/day - 8 mg every 12 hours and limited to 7 consecutive days) was perceived as the recommended dose by 21.0% of physicians. Finally, 18.2% of physicians acknowledged they did not know the exact dose and duration of treatment with injectable form of systemic TCC containing medicinal products.
Figure 3: Percentage of physicians reporting correct response to question 3: Which of the following options regarding the recommended dose and duration of use of systemic thiocolchicoside containing medicinal products are correct? Percentage of physician reporting a correct response (16 mg/day - 8 mg every 12 hours and limited to 7 consecutive days) for (A) Oral form and (B) Correct response (8 mg/day – 4 mg every 12 hoursand limited to 5 consecutive days) for injectable form.
Awareness of and attitude towards the latest prescribing conditions and safety information warnings of TCC
Overall, 68.6% of physicians reported receiving either or both the DHPC and the EM related to systemic thiocolchicoside containing medicinal products. Greece reported the lowest proportion (51.1%) and Italy the highest (72.3%). In the overall sample, the largest percentage of physicians who reported receiving either or both the DHPC and the EM was among rheumatologists (range, 58.1% (Greece) to 85.7% (Portugal)), as compared to GPs (range, 48.4% (Greece) to 74.2% (Italy)) and orthopedists/surgeons (range, 38.6% (France) to 61.9% (Portugal)). In addition, a larger proportion of physicians reported having received DHPC (66.0%) as compared to the EM (27.1%).
Overall, a larger proportion of GPs and rheumatologists (both 67.9%) were aware of recent changes in package leaflet compared to orthopedists/orthopedic surgeons (53.3%).
Finally, physicians ranked the DHPC as their most recent source for safety information regarding systemic thiocolchicoside containing medicinal products. National authorities’ website, medical/ pharmaceutical representatives and SmPC were reported to be their most recent source for safety information concerning systemic TCC by 28.7%, 25.9% and 25.4% of physicians respectively. Ranking varied by specialties and countries, with SmPC being the first source of safety information for rheumatologists in the overall sample and in Greece (all specialties).
Criteria of success
Among physicians who reported to remember receiving both or either DHPC and EM (68.6% of physicians), 57.6% answered appropriately to at least 4 over the 5 criteria, while this was 32% for those who reported receiving none of the RMM materials. Similarly, among physicians who acknowledged receipt of both or either DHPC and EM, 28.8% answered appropriately to the five criteria, while this number dropped to 17.9% for those who did not recall receiving any of the RMM materials.
Specifically, among the physicians having acknowledged the receipt of DHPC and/or EM, 66% prescribed systemic TCC only as adjuvant treatment of painful muscle contractures associated with acute spinal pathology in adults and in adolescents from 16 years onwards (Criterion 1), 93% did not prescribe systemic TCC for long-term treatment of chronic conditions (Criterion 2), 63.4% followed the recommendations regarding the doses and duration restriction (Criterion 3), 83.2% did not prescribe systemic TCC during pregnancy and lactation (Criterion 4) and 56.9% did not prescribe systemic TCC in women of childbearing potential not using adequate contraception (Criterion 5).
The corresponding proportion in each success criterion (Criteria 1 to 5) for those who reported not receiving neither the DHPC nor the EM, were 56.6%, 94.4%, 51.5%, 69.8% and 31.9% respectively.
Information about recent prescriptions
In this survey, all participating physicians completed at least one systemic TCC prescription within the last 12 months. In total, 3,205 prescriptions of systemic TCC containing medicinal products to patients were collected from the 651 participating physicians.
Among patients prescribed systemic TCC containing medicinal products, 2.2% were labelled as being less than 16 years old, 35% were women of whom 32.6% were of childbearing potential.
Overall, physicians made more prescriptions of oral TCC (65.3%) than injectable TCC (32.4%). However, these proportions varied widely according to the country: a majority of physicians reported prescribing oral TCC in France (92.9%) and Portugal (60.8%), while intra-muscular TCC was more prescribed in Italy (68.9%) and in Greece (51.4%). For oral form, a median daily dose of 16 mg/day with a median duration of prescription of 6.0 days was reported. For injectable form, a median daily dose of 8 mg/day with a median duration of prescription of 5.0 days was reported.
A large majority (72.8%) of physicians reported that they prescribed systemic TCC containing medicinal products with the indication of adjuvant treatment of painful muscle contractures in acute spinal pathology in adults and in adolescents from 16 years onwards.
When prescribing to women of childbearing potential, only a minority of physicians reported not asking to the patient if she was using an effective method of contraception (11% of prescriptions) and if she was pregnant (6.8% of prescriptions). In less than 1% of prescriptions to women of childbearing potential, the physicians reported that the female patient was planning to be pregnant or breastfeeding and for 6.8% of prescriptions, they prescribed systemic TCC containing medicinal products to women not using effective methods of contraception.
Discussion
This study sought to measure the effectiveness systemic TCC associated risk minimisation strategy, by ascertaining the proportion of targeted physicians who understood and implemented the latest prescribing conditions and safety information.
In the overall sample, knowledge regarding the indicated use of systemic TCC as adjuvant treatment of painful muscle contracture in acute spinal pathology was 63.2%, while knowledge of dose and duration of treatment was higher for the oral form (78.9%) than for the injectable form (55.4%). The majority of physicians were aware of contraindications to the use of systemic TCC, in children under 16 years old (85.0%), pregnancy (87.6%), and lactation (80.3%). In contrast, less than half (49.1%) of them were aware of the restriction of use regarding women of childbearing potential not using contraception. Results from the analysis of recent prescriptions highlighted different habits between countries: a majority of physicians reported prescribing oral TCC in France (92.9%) and Portugal (60.8%), while a majority reported prescribing intra-muscular TCC in Italy (68.9%) and in Greece (51.4%).
The percentage of physicians who reported receiving either or both the DHPC or/and the EM was 68.6%. Physicians receipt of DHPC and EM varied according to specialties and countries. This could reflect inherent differences in physician behaviour or different intensities of educational efforts and have been reported in recently published studies [10,11]. In all countries, the distribution process was conducted by post-mailed hard copies mainly, with additional e-mail and fax in France for the DHPC. This was contracted out to certified vendors, which all have guaranteed large and up to date distribution lists. In all 4 countries, both hospital and office-based practitioners were targeted. The time from distribution to the start of data collection ranged from approximately 34 months (Italy, Greece, Portugal) in France to 36 months in France for DHPC and from approximately 14 months in France to 17 months in Portugal for EM.
Interestingly, a higher percentage of physicians remembered having received the DHPC (66.0%) as compared with the EM (27.1%). Therefore, the second step of distribution, dedicated to the Ems (HCP brochure, patient information card complemented by SmPC) was less noticed by physicians than the first (DHPC). Previous work has shown that the most preferred senders of safety information were national competent authorities and professional bodies [12]. DHPCs are letters predominantly distributed by pharmaceutical companies following content approval by the national competent authorities. The layout of the document often clearly states that the information is provided under the control of national health authorities. In contrast, even though these documents go through a similar content approval process by the national competent authorities, EM come usually as a sole communication of the MAH (joint MAHs in our case). This may explain the higher awareness towards the DHPC.
Concerning RMM effectiveness, physicians who reported receiving either or both DHPC and the EM, scored higher in most of questions compared to those who reported having received neither the DHPC nor the EM. The most striking difference was observed regarding the contraindication related to the use of systemic thiocolchicoside during pregnancy or lactation; correct responses increased from 69.8% (physicians reporting not having received either DHPC or EMs) to 83.2% (physicians reporting having received DHPC and/or EMs). Similarly, regarding the contraindication related to the use of systemic thiocolchicoside in women of childbearing potential not using adequate contraception, correct responses increased from 31.9% (physicians reporting not having received DHPC or EMs) to 56.9% respectively (physicians reporting having received DHPC and/or EMs). In addition, it should be noted that risk minimisation tools were well perceived by HCPs, since DHPC was stated as the preferred source of safety information regarding systemic thiocolchicoside containing medicinal products by a majority (42.1%) of surveyed prescribers.
There are several conceptual frameworks or models published as guides to assess the effectiveness of risk-minimization measures [13-18]. These models recommend a comprehensive approach not limited to a single element of the risk minimisation strategy, and capable of generating different lines of evidence at relevant intervals to help regulators to promptly assess decision-relevant information. In that regard, the PRAC agreed that assessment of the effectiveness of systemic TCC RMMs in place could benefit from both the questionnaire-based survey on clinical knowledge presented here and a drug utilization study (ongoing) because of the complementarity of the information that would be provided. Thus, this survey was designed as an evaluation of physicians’ knowledge of key safety information for systemic thiocolchicoside and their receipt of educational materials. The companion drug utilisation study will evaluate prescriber behaviour in the context of systemic thiocolchicoside prescription [8].
This study is characterized by several strengths. The questionnaire was tested among prescribers for its clarity, comprehensibility, consistency and the appropriateness of medical terms, according to ENCePP guidelines [9]. It was also checked whether there were questions which would suggest a specific answer for any reason, for example social desirability. The targeted enrolment in each country was achieved, in terms of country and physicians’ specialties, ensuring a diverse and representative sample of prescribers surveyed. The access to the web questionnaire interface was strictly limited to those invited, with a single possibility to participate and with a traceable system. Thus, stakeholder bias or unverified respondents were not applicable.
Some limitations inherent to cross-sectional physician surveys must also be considered. Thus, a recall bias could be cited concerning physicians’ answer about the TCC related RMMs and their recent prescriptions of TCC. Although the study aimed to select a diverse and representative sample of prescribers, the study participants may not necessarily represent all relevant prescribers, especially in France, where completion rate was low. Moreover, it is possible that participants who completed the questionnaire differed from nonparticipants in characteristics that were not measured in the questionnaire. Physician response rates for surveys historically have been somewhat low, and low response rates may increase the likelihood that participating physicians are not representative of all prescribing physicians. In this study, the completion percentages reflected early responses and truncation of the survey once target numbers had been met. Thus, the resulting estimates of physician understanding about systemic thiocolchicoside may be biased. Finally, there was heterogeneity of some results by country; it is unknown how generalizable the results are to other countries not surveyed.
Conclusion
In summary, this study reveals geographical and across prescribers’ specialty contrasts in the knowledge of key messages of systemic TCC containing medicinal products associated RMMs. In addition, the results showed that when risk minimisation materials (DHPC and EM) receipt was acknowledged by physicians, it improved their knowledge and attitude towards appropriate systemic TCC prescribing to patients.
Acknowledgement
The authors thank the members of the consortium who participated in this study and the marketing authorisation holders funding this study: Acarpia services farmaceuticos Lda, Alter laboratoire, Angelini, Angenerico SpA, Arrow Generiques, Biogaran, Cristers, Daiichi Sankyo, Doc Generici, Dompe Farmaceutici SpA, EG labo, EG SpA, Epifarma Srl, Farmaceutici Caber SpA, Generis Farmaceutica, Korangi, Laboratorio Farmaceutico CT Srl, MDM, Mylan, Sandoz, Sanofi Aventis Groupe, SF Group Srl, SPA, Teofarma Srl, Union Health Srl. We thank Intissar Bourahla and Geoffrey Bizouard from IQVIA for project supervision and statistical analysis respectively. All procedures performed in this study involving human participants were in accordance with the ethical standards of the applicable institutional review board and ethics committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
References
- European Medicines Agency (2014) Scientific conclusions and grounds for variation to the terms of the marketing authorisations. Committee for Medicinal Products for Human Use (CHMP).
- European Medicines Agency (2014) Thiocolchicoside containing medicinal products Article-31 referral assessment report. Committee for Medicinal Products for Human Use (CHMP).
- Parry JM, Jenkins GJ, Haddad F, Bourner R, Parry EM (2000) In vitro and in vivo extrapolations of genotoxin exposures: consideration of factors which influence dose-response thresholds. Mutat Res 464: 53-63.
- Parry JM, Al-Obaidly A, Al-Walhaib M, Kayani M, Nabeel T, et al. (2002) Spontaneous and induced aneuplloidy considerations which may influence chromosome malsegregation. Mutat Res 504: 119-129.
- Parry EM, Parry JM, Corso C, Doherty A, Haddad F, et al. (2002) Detection and characterization of mechanisms of action of aneugznic chemical. Mutagenesis 17: 509-521.
- Kirsch-Volders M, Vanhauwaert A, De Boeck M, Decordier I (2002) Importance of detecting numerical versus structural chromosome aberrations. Mutat Res 504: 137-148.
- IMS Health (2018) Evaluation of the Effectiveness of Risk Minimisation Measures: A Joint PASS Survey among Health Care Professionals to Assess their Knowledge and Attitudes on Prescribing Conditions of Thiocolchicoside containing Medicinal Products for Systemic Use in France, Greece, Italy and Portugal. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance.
- IMS Health (2018) Drug Utilization Study of Thiocolchicoside (TCC) containing medicinal products for systemic use in France and Italy: an electronic medical records database study. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance.
- European Medical Agency (2018) ENCePP Guide on Methodological Standards in Pharmacoepidemiology. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance.
- Brody RS, Liss CL, Wray H, Iovin R, Michaylira C, et al. (2016) Effectiveness of a risk-minimization activity involving physician education on metabolic monitoring of patients receiving quetiapine: results from two postauthorization safety studies. Int Clin Psychopharmacol 31: 34-41.
- Davis KH, Asiimwe A, Zografos LJ, McSorley DJ, Andrews EB (2017) Evaluation of Risk-Minimization Activities for Cyproterone Acetate 2 mg/Ethinylestradiol 35 µg: A Cross-Sectional Physician Survey. Pharmaceut Med 31: 339-351.
- de Vries ST, van der Sar MJM, Cupelli A, Baldelli I, Coleman AM, et al. (2017) Communication on Safety of Medicines in Europe: Current Practices and General Practitioners' Awareness and Preferences. Drug Saf 40: 729-742.
- Banerjee AK, Zomerdijk IM, Wooder S, Ingate S, Mayall SJ (2014) Post-approval evaluation of effectiveness of risk minimisation: methods, challenges and interpretation. Drug Saf 37: 33-42.
- CIOMS (2014) Evaluating effectiveness of risk minimisation. In: Practical approaches to risk minimisation for medicinal products: report of CIOMS Working Group IX. Geneva: Council for International Organizations of Medical Sciences.
- Kesselheim AS, Campbell EG, Schneeweiss S, Rausch P, Lappin BM, et al. (2015) Methodological approaches to evaluate the impact of FDA drug safety communications. Drug Saf 38: 565-575.
- Prieto L, Spooner A, Hidalgo-Simon A, Rubino A, Kurz X, et al. (2012) Evaluation of the effectiveness of risk minimization measures. Pharmacoepidemiol Drug Saf 21: 896-899.
- Mayall SJ, Banerjee AS (2014) Therapeutic risk management of medicines. Cambridge: Woodhead Publishing.
- Smith MY, Morrato E (2014) Advancing the field of pharmaceutical risk minimization through application of implementation science best practices. Drug Saf 37: 569-580.
Citation: Jouaville LS, Ehrhardt C, Kürzinger ML, de Voogd H, Toussi M (2019) Evaluation of the Effectiveness of Risk Minimisation Measures Targeting Physicians on Prescribing Practices of Thiocolchicoside Containing Medicinal Products for Systemic Use. Epidemiology (Sunnyvale) 9: 372. DOI: 10.4172/2161-1165.1000372
Copyright: © 2019 Jouaville LS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4075
- [From(publication date): 0-2019 - Apr 17, 2025]
- Breakdown by view type
- HTML page views: 3194
- PDF downloads: 881