Research Article Open Access
Evaluation of Stearylamine-Modified Liposomes for the Oral Vaccine Adjuvant
| Division of Veterinary Science, Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Izumisano, Japan | ||
| Corresponding Author : | Watarai S Division of Veterinary Science Graduate School of Life and Environmental Sciences Osaka Prefecture University, 1-58 Rinkuohraikita Izumisano, Osaka 598-8531, Japan Tel: +81-72-463-5720 Fax: +81-72-463-5720 E-mail: swatarai@vet.osakafu-u.ac.jp |
|
| Received March 25, 2014; Accepted April 27, 2014; Published May 02, 2014 | ||
| Citation: Watarai S and Sasaki Y (2014) Evaluation of Stearylamine-Modified Liposomes for the Oral Vaccine Adjuvant. J Infect Dis Ther 2:141. doi:10.4172/2332-0877.1000141 | ||
| Copyright: © 2014 Watarai S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
The usefulness of stearylamine (SA)-modified liposomes for the oral vaccine adjuvant in the induction of immune responses was evaluated. Mice were orally immunized withunmodified liposomes containing ovalbumin (OVA) (group I), OVA-containing monophosphoryl lipid A (MPL)-modified liposomes (group II), OVA-having SA-modified liposomes (group III) or OVA alone (group IV). After immunization, significant OVA-specific antibodies were detected in the serum and intestine from mice of groups I to III, but not in group IV. Especially, intestinal IgG and IgA antibody responses against OVA were significantly higher in mice of group III than in groups I and II. When sera were analyzed for isotype distribution,OVA-specific IgG1 antibody responses were noted in mice of groups I and II, whereas the induction of OVA-specific IgG1 and IgG3 antibody responses was observed in mice of group III. Moreover, substantial production of IFN-γ(Th1-type) and IL-4 (Th2 type) was demonstrated in spleen cells from mice of group III in vitro. These results suggest that the SA-modified liposomes would serve effectively as mucosal vaccine adjuvant for inducing humoral and cell-mediated immune responses.
| Keywords |
| Liposomes; Vaccines; Protective immunity; Th1; Th2 |
| Introduction |
| Many pathogens cause initially disease by colonizing or penetrating through the mucosal surface of the enteric epithelium [1-4]. The mucosal immune system plays a central role in the primary defense against pathogens by preventing binding of the microbes or their toxins to the epithelium [5-7]. Induction of mucosal immune responses is achieved by the deposition of antigen via the mucosa (e.g., oral route) but not the systemic route [8]. Further, mucosal immunization has been shown to induce antigen-specific immune responses in both mucosal and systemic compartments [8-12]. Although systemic vaccination (e.g., intramuscular injection) can induce effective immune responses in the systemic compartment, it does not result in the generation of antigen-specific mucosal immune responses. Considering infection of pathogens, mucosal vaccination can offer two layers of immunity (mucosal and systemic immune responses) and provide an effective barrier against invasion of pathogens. Externally secreted IgA and local IgG antibodies produced in response to the mucosal invasion or administration of antigens perform important functions in this system [13,14]. It has been reported that these local antibodies are effective in inhibiting the binding of pathogen to the mucosal cells [13]. However, it has been shown that delivery of antigen alone is insufficient for the induction of maximum levels of antigen-specific immune response by mucosal vaccine [8,9]. Thus, it is necessary to coadminister with new adjuvants and carriers for the induction of mucosal immune responses. |
| Liposomes have been used as immunological adjuvants to enhance the immune response to several bacterial and viral antigens [15-18]. In particular, the potential usefulness of liposomes as adjuvants for developing vaccines has led to considerable interests during the last few years because the materials encapsulated within the liposomes are protected from degradation until they reach the target sites [19]. Several studies have demonstrated the potential of liposomes as adjuvants [20-29]. In these studies, it is revealed that, depending on the liposomal composition, charge and size, liposomes may have different pharmacokinetics and be formulated to obtain optimal retention and presentation of the vaccine antigens and are avidly taken up by the dendritic cells (DCs) owing to their particulate nature. It is well known that cationic liposomes are able to both enhance and modulate the immune responses. The adjuvant mechanisms of cationic liposomes have been reviewed elsewhere [30]. Different amphiphilic cationic compounds have been tested for inclusion into liposomes and hold promise for vaccine delivery [31-35]. However, relatively little data on their potential mucosal vaccine is inconclusive. Moreover, since many of them are very expensive, cationic liposome vaccines are in limited clinical application. Thus, for the clinical application of the cationic liposome vaccine, in expensive cationic compounds is required. |
| Stearylamine (SA) is one of low-priced cationic compounds and SA modification of the liposomes represents an important factor for enhancing their immunoadjuvancy in the induction of antigen-specific immune responses by conventional (injection) route [36]. Therefore, SA-modified cationic liposomes are expected as mucosal (oral) vaccine adjuvant. However, data on the SA-modified cationic liposomes as oral vaccine adjuvant is relatively little. To know the usefulness of SA-modified cationic liposomes as oral vaccine adjuvant, the present study, mice were orally immunized with OVA-containing SA-modified cationic liposomes, and immune responses were evaluated. Our data suggests that SA-modified cationic liposomes can induce strong antigen specific humoral (Th2) and cell-mediated (Th1) immunity as oral vaccine adjuvant. |
| Materials and Methods |
| Materials |
| Dipalmitoyl phosphatidyl choline (DPPC), cholesterol (Chol), monophosphoryl lipid A (MPL), SA, trypsin inhibitor (soybean type I-S), ovalbumin (OVA), and bovine serum albumin (BSA) (SIGMA) were commercial products. |
| Animals |
| Female BALB/c mice (6 weeks old) were purchased from Charles River Japan, Tokyo, Japan. They were maintained according to the Standards Relating to the Care and Management of Experimental Animals of Japan. The experiments were carried out in accordance with the guidelines for animal experimentation in Osaka Prefecture University. |
| Preparation of liposomes |
| SA-modified liposomes that entrap OVA were prepared according to the method of Watarai et al. [37]. DPPC (4 μmol), Chol (4 μmol), and SA (0.4 μmol), each dissolved in an organic solvent, were mixed in a conical flask. The lipids were dried on a rotary evaporator, followed by standing for 30 min under high vacuum in a desiccator. After addition of 1 ml of PBS containing OVA (5 mg/ml) and incubation at an appropriate temperature for 3 min, the lipid film was dispersed by vigorous vortexing. Any unencapsulated OVA were removed by repeated centrifuging at 14,000 x g for 20 min at 4°C in PBS, and the resulting liposome suspension was used for immunization. MPL-modified liposomes were also prepared according to the above procedure using lipid mixture of DPPC (4 μmol), Chol (4 μmol), and MPL (16 μg). Furthermore, unmodified liposomes were prepared according to the above procedure using lipid mixture solution without SA and MPL. The amount of OVA entrapped in liposomes was determined by the following method. Ninety μl of isopropyl alcohol was added to a 10 μl suspension of liposome-entrapped OVA (at 3-fold dilution in PBS), followed by vortex mixing. The protein concentration of the resulting solutions was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories), with bovine plasma gamma globulin used as a standard. |
| Immunization of mice |
| Mice were divided into 3 groups (5 mice per a group). Each group was orally immunized as follows: group I, unmodified liposomes that entrap OVA (200 μg protein/150 μl/mouse); group II, MPL-modified liposomes that entrap OVA (200 μg protein/150μl/mouse); group III, SA-modified liposomes that entrap OVA (200 μg protein/150 μl/mouse); group IV, OVA alone (200 μg protein/150 μl/mouse). Immunization was repeated two times at 2-week intervals. Two weeks after final immunization, the mice were killed and sera, small intestine (30 cm from pylorus), and spleen were harvested. Intestinal antibodies from small intestine regions were collected as described previously [37]. Sera and intestinal antibodies obtained were used for antibody assay. Spleen cells isolated as described previously [38] were used for cytokine measurements. |
| Antibody assay |
| Antibody assay was performed according to the method described previously [39]. |
| Cytokine measurements |
| Measurement of cytokine levels was performed according to the method described previously [39] using spleen cells from non-treated control and group III mice. |
| Statistical analysis |
| Student's T-test was employed in the statistical evaluation of the results. |
| Results |
| Immune responses in mice immunized orally with OVA-containing SA-modified liposomes: |
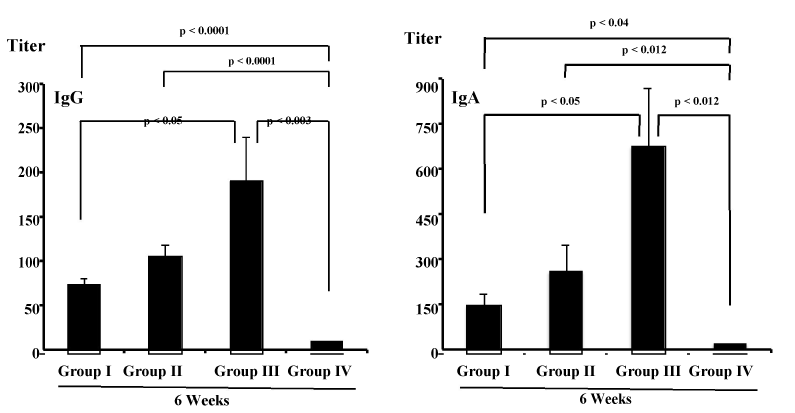
| Mice were administered orally with OVA antigen, such as unmodified liposomes containing OVA (group I), MPL-modified liposomes containing OVA (group II), SA-modified liposomes containing OVA (group III) or OVA alone (group IV), and antibodies against OVA were evaluated at 14 days after final immunization. As shown in Figure 1, in serum from mice receiving OVA alone (group IV), production of anti-OVA IgG and IgA antibody was not demonstrated. On the other hand, serum IgG and IgA activity against OVA could be seen in the groups I, II and III. IgG and IgA antibody responses against OVA in the groups I, II and III were significantly higher than those in group IV (IgG: p<0.05, p<0.0001, p<0.005 compared to groups I, II and III, respectively; IgA: p<0.0001 compared to groups I, II and III). Furthermore, mice immunized with OVA-containing MPL-modified liposomes (group II) showed significantly higher OVA-specific serum IgG antibodies than did mice immunized unmodified liposomes entrapping OVA (group I) (p<0.02) and mice immunized with OVA-having SA-modified liposomes (group III) (p<0.0095). |
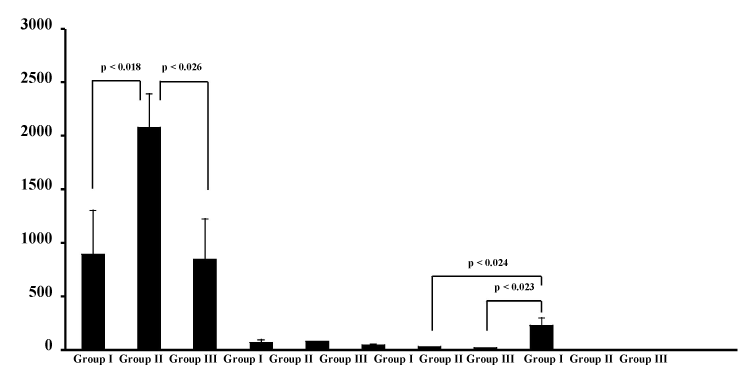
| Furthermore, serum antibody responses were characterized by analyzing the pattern of IgG subclasses present in sera from mice in groups I to III. As shown in Figure 2, OVA-specific serum IgG1 antibody responses were demonstrated in the serum from the three groups. In particular, production of IgG1 antibody against OVA was significantly enhanced by the oral administration of MPL-modified liposomes containing OVA (group II) than by unmodified liposomes entrapping OVA (group I) (p<0.018) or by SA-modified liposomes containing OVA (group III) (p<0.026). The induction of OVA-specific serum IgG3 antibody responses was demonstrated in sera from mice in group III (p<0.024 vs group I; p<0.023 vs group II), although the serum IgG2a antibody activities against OVA were not detected in any mice in groups I to III. |
| None of mice in groups I, II and III induced detect levels of OVA-specific IgE (Figure 2). Next, we investigated whether OVA-specific antibody responses were effectively induced in mucosal compartment by oral immunization. The intestinal anti-OVA antibody responses were evaluated after immunization. Figure 3 shows the OVA-specific intestinal IgG and IgA responses in mice of groups I, II, III and IV. Production of anti-OVA IgG and IgA antibody was demonstrated in intestinal fluid from mice in the groups I, II and III, but not in the group IV. The levels of OVA-specufic IgG and IgA in mice of groups I, II and III were much higher than those detected in the group IV (IgG: p<0.0001, p<0.0001, p<0.003 compared to groups I, II and III, respectively; IgA: p<0.04, p<0.012, p<0.012 compared to groups I, II and III, respectively). Among groups I to III, furthermore, IgG and IgA antibody responses against OVA were significantly higher in group III than in group I and group II (p<0.05). |
| Th1 and Th2 cytokine production by spleen cells from mice immunized intranasally with OVA-having SA-modified liposomes: |
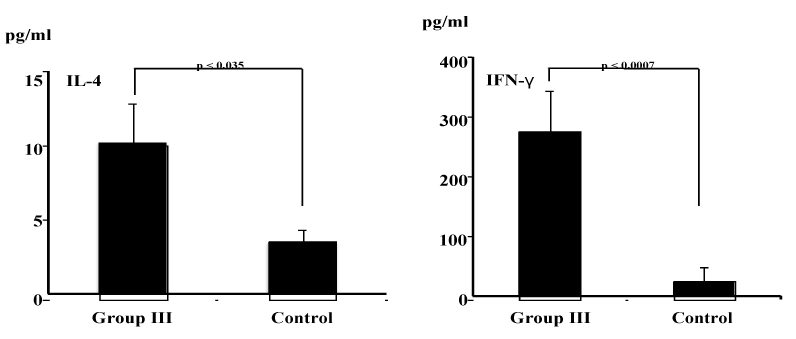
| OVA-having SA-modified liposome-induced antigen-specific serum IgG1 and IgG3 responses suggest efficient major histocompatibility complex presentation of the antigen leading to both humoral (IgG1) (Th2) and cell-mediated (IgG3) (Th1) response (Figure 2). To confirm antigen-specific Th1 and Th2 responses, therefore, cytokine measurements were done by cytokine ELISA. As shown in Figure 4, higher levels of both Th1 (IFN-γ) and Th2 (IL-4) cytokines were detected in the culture supernatant harvest from in vitro OVA-stimulated spleen cells from mice in group III than did spleen cells from non-treated control mice (IFN-γ, p<0.035; IL-4, p<0.0007). |
| Discussion |
| The mucosal immune system plays a central role in the primary defense against pathogens by preventing binding of the microbes or their toxins to the epithelium [40-42]. Thus, the development of mucosal vaccines is of great importance in veterinary medicine and new adjuvants are essential to this aim. In addition, efficient vaccine delivery systems have also been required for achievement of protective immunity. Previously, we have reported that liposomes are an effective antigen-delivery vehicle for the induction of systemic and mucosal immune responses [18,24,37,43]. In addition, it has been shown that cationic liposomes are the most effective liposomal delivery systems for vaccine antigens compared with other liposome system (anionic and neutral liposomes) [30]. The use of cationic liposomes as antigen-delivery vehicles in vaccine is well-documented method to increase the immune recognition against otherwise inert or poorly immunogenic subunit proteins [44]. SA is one of cationic compounds. Incorporated in liposomal membrane, it leads to a positive (cationic) surface charge and can enhance liposomal immunoadjuvancy in the induction of antigen-specific immune responses [36]. However, data on the SA-modified cationic liposomes as mucosal vaccine adjuvant is relatively little. In this study, thus, we used the SA-modified cationic liposomes as adjuvant for mucosal vaccine, especially oral vaccine, and induction of systemic and local (mucosal) immune responses was evaluated. |
| It has been established that liposomes have an application possibility as an adjuvant for use in vaccines [15,16,43]. In this study, none of mice receiving OVA alone (group IV) showed the production of anti-OVA IgG and IgA antibody in serum and intestine (Figures 1 and 3). However, the oral administration of unmodified liposomes containing OVA (group I), MPL-modified liposomes containing OVA (group II), and SA-modified liposomes containing OVA (group III) induced not only good serum IgG and IgA responses against OVA (Figure 1), but also good intestinal IgG and IgA responses against OVA (Figure 3). In particular, MPL-modified liposomes containing OVA (group II) induced serum IgG responses in mice greater than those induced by OVA-containing unmodified liposomes (group I) and by OVA-containing SA-modified liposomes (group III) (Figure 1). Intestinal IgG and IgA antibody responses against OVA, on the other hand, were significantly higher in group III than in group I and group II (p<0.05) (Figure 3). These results indicate that liposomes function as effective mucosal adjuvant for increasing IgG and IgA responses in the serum and intestinal mucosa when immunized by oral route and that the adjuvanticity of liposomes can be further elevated by inclusion of MPL in liposomes for potentiating IgG antibody responses in the serum, whereas the adjuvant effect of liposomes resulted in further potentiating IgA antibody responses in the intestinal mucosa by inclusion of SA in liposomes. |
| Furthermore, we estimated whether OVA entrapped within liposomes induce IgE production, because IgE shows harmful effects, such as allergy. In the present study, an induction of IgE antibody against OVA was not observed in mice orally immunized with OVA in association with liposomes, such as unmodified liposomes containing OVA (group I), MPL-modified liposomes containing OVA (group II), SA-modified liposomes containing OVA (group III) (Figure 2). This suggests that liposomes might serve as a mucosal adjuvant without detrimental effects, such as allergic responses. |
| Th2-type cytokines are pivotal for regulation of IgG1 antibody responses, while Th1-type cytokines support IgG2a and IgG3 antibody responses [45]. In this study, it was shown that oral immunization with OVA-containing unmodified liposomes (group I), with MPL-modified liposomes having OVA (group II) and with SA-modified liposomes in association with OVA (group III) induced antigen-specific IgG1 antibody responses (Figure 2). Especially, a significant increase in the titer of IgG1 antibody was noted in mice immunized MPL-modified liposomes containing OVA (group II). This result suggests that oral immunization with antigen-containing MPL-modified liposomes induces antigen-specific Th2 responses predominantly. In this study, on the other hand, oral immunization with SA-modified liposomes in association with OVA (group III) induced not only antigen-specific IgG1 antibody production, but also IgG3 antibody production (Figure 2). This finding suggests that SA-modified liposomes were potent to induce both a humoral (Th2-type) (IgG1) and a cell-mediated (Th1-type) (IgG3) response. Actually, this was corroborated by the production of cytokines IFN-γ (Th1) and IL-4 (Th2) (Figure 4). After antigens are taken up by DCs, in general, most of the antigens are probably delivered into processing pathways for MHC class II presentation of peptides (Th2-type response), but some antigens might escape from phagosomes into the cytoplasmic compartments and be delivered into MHC class I presentation pathways (Th1-type response) [46-50]. Although little is known about this “leakage” from phagosome to the cytoplasm, it is conceivable that a large number of antigens should be incorporated into DCs in order for them to be presented MHC class I. Interactions between liposomes with immune cells such as DCs have been studied. Liposomal properties such as chaege, size, and lipid composition have been shown to affect liposomal uptake by macrophages [51]. Cellular uptake of liposomes is generally believed to be mediated by the adsorption of liposomes onto the cell surface and subsequent internalization and endocytosis. It would be accepted that positively charged SA-modified liposomes could be taken up in large quantities. Internalized SA-modified cationic liposomes may induce both a humoral (Th2-type) and a cell-mediated (Th1-type) response. |
| A new immunizing method using cationic compound (SA)-modified liposomes would clearly be worth. To our knowledge, this study is the first report about usefulness of cationic compound (SA)-modified liposomes as mucosal vaccine adjuvant. We have provided here evidence for induction of antigen-specific humoral and cell-mediated immune responses. The present results will provide useful information for the design of oral liosome vaccine. Furthermore, this cationic compound (SA)-modified liposome vaccine adjuvant would be effective in inducing protective immunity, thereby facilitating extirpation of the disease. |
| Conclusions |
| In conclusion, this study was carried out to evaluate the usefulness of cationic compound (SA)-modified liposomes as mucosal (oral) vaccine adjuvant. It was confirmed that SA-modified liposomes could serve effectively as mucosal (oral) vaccine adjuvant for inducing humoral and cell-mediated immune responses. |
| In summary, it is expected to use SA-modified liposomes as as mucosal (oral) vaccine adjuvant for the induction of protective humoral and cell-mediated immunity. |
References
- Beachey EH (1981) Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis 143: 325-345.
- Edwards RA, Puente JL (1998) Fimbrial expression in enteric bacteria: a critical step in intestinal pathogenesis. Trends Microbiol 6: 282-287.
- Khan AS, Mühldorfer I, Demuth V, Wallner U, Korhonen TK, et al. (2000) Functional analysis of the minor subunits of S fimbrial adhesion (SfaI) in pathogenic Escherichia coli. Mol Gen Genet 263: 96-105.
- Klemm P, Schembri MA (2000) Bacterial adhesins: function and structure. Int J Med Microbiol 290: 27-35.
- Williams RC, Gibbons RJ (1972) Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science 177: 697-699.
- Winner LSIII, Mack J, Weltzin R, Mekalanos JJ, KraehenbuhlJP,et al. (1991) New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridomatumors protects against Vibrio cholera infection. Infect Immun 59: 977-982.
- Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, et al. (1995) Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in murine model of Chlamydia trachomatis genital tract infection. Infect Immun 63: 4704-4714.
- Mestecky J, McGhee JR (1989) Oral immunization: past and present. Curr Top MicrobiolImmunol 146: 3-11.
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, et al. (1992) The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10: 75-88.
- Kunisawa J, Kurashima Y, Kiyono H (2012) Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev 64: 523-530.
- Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, et al. (2012) Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines 11: 367-379.
- Sato S, Kiyono H (2012) The mucosal immune system of the respiratory tract. CurrOpinVirol 2: 225-232.
- Bouvet JP, Fischetti VA (1999) Diversity of antibody-mediated immunity at the mucosal barrier. Infect Immun 67: 2687-2691.
- Bouvet JP, Decroix N, Pamonsinlapatham P (2002) Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol 23: 209-213.
- Gregoriadis G (1990) Immunological adjuvants: a role for liposomes. Immunol Today 11: 89-97.
- Brito LA, Malyala P, O'Hagan DT (2013) Vaccine adjuvant formulations: a pharmaceutical perspective. SeminImmunol 25: 130-145.
- Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3: 13.
- Fukutome K, Watarai S, Mukamoto M, Kodama, H (2001) Intestinal mucosal immune response in chickens following intraocular immunization with liposome-associated Salmonella entericaserovarEnteritidis antigen. Dev Comp Immunol 25: 475-484.
- Romero EL, Morilla MJ (2011) Topical and mucosal liposomes for vaccine delivery. Wiley Interdiscip Rev NanomedNanobiotechnol 3: 356-375.
- Gregory RL, Michalek SM, Richardson G, Harmon C, Hilton T, et al. (1986) Characterization of immune response to oral administration of Streptococcus sobrinus ribosomal preparations in liposomes. Infect Immun 54: 780-786.
- Kahl LP1, Lelchuk R, Scott CA, Beesley J (1990) Characterization of Leishmania major antigen-liposomes that protect BALB/c mice against cutaneous leishmaniasis. Infect Immun 58: 3233-3241.
- Bülow R, Boothroyd JC (1991) Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol 147: 3496-3500.
- Li W, Watarai S, Iwasaki T, Kodama H (2004) Suppression of Salmonella entericaserovarEnteritidis excretion by intraocular vaccination with fimbriae proteins incorporated in liposomes. Dev Comp Immunol 28: 29-38.
- Irie T, Watarai S, Iwasaki T, Kodama H (2005) Protection against experimental Aeromonassalmonicida infection in carp by oral immunisation with bacterial antigen entrapped liposomes. Fish Shellfish Immunol 18: 235–242.
- Rhalem A, Bourdieu C, Luffau G, Pery P (1988) Vaccination of mice with liposome-entrapped adult antigens of Nippostrongylusbrasiliensis. Ann Inst Pasteur Immunol 139: 157-166.
- O'Hagan DT, Rappuoli R (2004) Novel approaches to vaccine delivery. Pharm Res 21: 1519-1530.
- Kersten GF, Crommelin DJ (2003) Liposomes and ISCOMs. Vaccine 21: 915-920.
- Gregoriadis G, Bacon A, Caparros-Wanderley W, McCormack B (2002) A role for liposomes in genetic vaccination. Vaccine 20 Suppl 5: B1-9.
- Alving CR, Peachman KK, Rao M, Reed SG (2012) Adjuvants for human vaccines. CurrOpinImmunol 24: 310-315.
- Christensen D, Korsholm KS, Andersen P, Agger EM (2011) Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines 10: 513-521.
- Chen W, Yan W, Huang L (2008) A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer ImmunolImmunother 57: 517-530.
- Jiao X, Wang RY, Feng Z, Alter HJ, Shih JW (2003) Modulation of cellular immune response against hepatitis C virus nonstructural protein 3 by cationic liposome encapsulated DNA immunization. Hepatology 37: 452–460.
- Christensen D, Foged C, Rosenkrands I, Nielsen HM, Andersen P, et al. (2007) Trehalose preserves DDA/TDB liposomes and their adjuvant effect during freeze-drying. BiochimBiophysActa 1768: 2120–2129.
- Guy B, Pascal N, Françon A, Bonnin A, Gimenez S, et al. (2001) Design, characterization and preclinical efficacy of a cationic lipid adjuvant for influenza split vaccine. Vaccine 19: 1794-1805.
- BarnierQuer C, Elsharkawy A, Romeijn S, Kros A, Jiskoot W (2012) Cationic liposomes as adjuvants for influenza hemagglutinin: more than charge alone. Eur J Pharm Biopharm 81: 294-302.
- Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, et al. (1997) Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. BiochemBiophys Res Commun 240: 793-797.
- Watarai S, Han M, Tana, Kodama H (1998) Antibody response in the intestinal tract of mice orally immunized with antigen associated with liposomes. J Vet Med Sci 60: 1047-1050.
- Watarai S, Kushi Y, Shigeto R, Misawa N, Eishi Y, et al. (1995) Production of monoclonal antibodies directed to Hanganutziu-Deicher active gangliosides, N-glycolylneuraminic acid-containing gangliosides. J Biochem 117: 1062-1069.
- Watarai S, Iwase T, Tajima T, Yuba E, Kono K (2013) Efficiency of pH-sensitive fusogenic polymer-modified liposomes as a vaccine carrier. ScientificWorldJournal 2013: 903234.
- Lingwood CA (1996) Role of verotoxin receptors in pathogenesis. Trends Microbiol 4: 147-153.
- Watarai S, Tana, Inoue K, Oguma K, Naka K, et al. (2000) Inhibitory effect of intestinal anti-Gb3 IgA antibody on verotoxin-induced cytotoxicity. LettApplMicrobiol 31: 449-453.
- Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, et al, (2010) Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. ProcNatlAcadSci USA 107: 8794-8799.
- Han M, Watarai S, Kobayashi K, Yasuda T (1997) Application of liposomes for development of oral vaccines: study of in vitro stability of liposomes and antibody response to antigen associated with liposomes after oral immunization. J Vet Med Sci 59: 1109-1114.
- Christensen D, Korsholm KS, Rosenkrands I, Lindenstrøm T, Andersen P, et al. (2007) Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines 6: 785-796.
- Snapper CM, Mond JJ (1993) Towards a comprehensive view of immunoglobulin class switching. Immunol Today 14: 15-17.
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C (1995) Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3: 783-791.
- Nelson D, Bundell C, Robinson B (2000) In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol 165: 6123-6132.
- Chen W, Masterman KA, Basta S, Haeryfar SM, Dimopoulos N, et al. (2004) Cross-priming of CD8+ T cells by viral and tumor antigens is a robust phenomenon. Eur J Immunol 34: 194-199.
- Bevan MJ (1976) Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 143: 1283-1288.
- Bevan MJ (2006) Cross-priming. Nat Immunol 7: 363-365.
- Aramaki Y, Akiyama K, Hara T, Tsuchiya S (1995) Recognition of charged liposomes by rat peritoneal and splenic macrophages: Effects of fibronectin on the uptake of charged liposomes. Eur J Pharm Sci 3: 63-70.
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14152
- [From(publication date):
June-2014 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 9540
- PDF downloads : 4612
