Evaluation of Pongamia pinnata Products against the Sclerotium rolfsii Extracted from Chickpea
Received: 28-Apr-2014 / Accepted Date: 23-Jun-2014 / Published Date: 03-Jul-2017 DOI: 10.4172/2329-8863.1000291
Abstract
This study was conducted to investigate antifungal properties of Pongamia pinnata products. Pongamia pinnata seed oils and leaf diffusates contain products that show fungi-static effect against Scelotium rolfssi on Chickpea. This study shows comparison between antifungal effects of Pongame seed oils and Leaves, as both of these potentially inhibit Sclerotial production and germination of S. rolfsii. It was noticed that ingredients of leaves were changed when autoclaved. It also showed that there were some ingredients that were altered in terms of composition when heated. These agents were thought to be proteins and carbohydrates. Similarly, ingredients of seed oil also undergo change in composition when autoclaved. Extract showed insignificant difference about 20% (v/v) in inhibiting concentrations when used before autoclaving, while there was a significant difference among various concentrations after autoclaving. This significantly increased inhibition was present in Sclerotial production, germination and growth of mycelia. Overall, leaf extract was more effective than seed oil extract.
Keywords: Pongamia pinnata, Fungi static, Sclerotium rolfsii, Chick pea
405297Introduction
Cicer arietinum L, commonly known as chickpea is very important grain and a legume crop which is mainly sown in rainy conditions in Pakistan. It is an important human food enriched with protein used as dietary protein source and used as animal feed predominantly for the population of Southeast Asia [1]. Chickpea is the source of energy as well as fuel. Chickpea is a main source of human and domestic animals food, predominantly in developing countries. Total cultivated area of chickpea is 12.0 million hectares globally with MT production rate of 10.9 million and an average yield of 913 kg ha-1 [2].
A number of biotic and abiotic factors are involved in decrease of chickpea production. Among the biotic factors, the collar rots disease caused by Sclerotium rolfsii Sacc, is a main contributing agent especially when weather conditions are favourable. These pathogens are very difficult to control with cultural practices and usually prohibited by chemical means.
Pongamia pinnata L. (Known as kerung) belongs to family Fabaceae which is medium size, an evergreen tree having short trunk and dispersing crown. The trees are planted for shade purposes and grown as an ornamental tree. It is also a nitrogen fixing tree which produces seeds containing 30-40% oil. It is a high-speed growing; deciduous, glabrous, trunk of diameter up to 60 cm, bark is smooth, grey in colour. Its leaves are imparipinnate, sometimes shiny, young, pinkish red, glossy and deep green when mature. Its many parts are used for timber, fuel production, medicinal and industrial purposes [3]. Pests are harmful to agriculture, forestry and public health which are causing losses in millions of dollars. Numerous approaches are used for the crop management against these pests. Physical, chemical, biological and resistance breeding are the most common. In chemical approach, pesticides are used with an extensive range and controlling numerous pests activities. Increasing awareness of public concern regarding a continued use of agro-chemicals that are damaging biotic and abiotic environment, driving the search of more environmentally safe methods that will contribute to the goal of sustainability in agriculture [4].
Traditionally, humans are exploiting biological sources in order to get economic benefits out of them. Chickpea was protected by using biological control agents against collar rot and its plant growth was promoted by these agents. Pongamia pinnata extracts are also known to possess insecticidal and nematicidal activities making it useful in management of agriculture and environment. Oil extract of Pongamia pinnata was found significantly to inhibit nematodes reproduction and exhibited favorable characters to promote growth of chickpea [5]. Similarly, Khurma and Mangotra [6] tested the usefulness of seeds taken out from 15 leguminosae towards the juveniles of root-knot nematode and originate that Pongamia pinnatacaused high mortality.
Methodology
This study was carried out at the partially at Department of Botany, PMAS-Arid Agriculture University Rawalpindi. Research was carried out to determine the antifungal activities of Pongamia pinnanta against pathogen Sclerotium rolfsii extracted from chickpea. Three products namely pongame seeds oil, pongame leaf decoction and diffusate were tested for their fungicidal properties against mycelial growth, sclerotial production and sclerotial germination of Sclerotium rolfsii .
Collection and preservation of Pongamia pinnata products
Pongame (Pongamia pinnata ) seeds oil was obtained from Biodiesel Laboratory of Quaid e Azam University Islamabad and stored at 4 ± 1°C in refrigerator. Pongame leaves were collected from Botany Campus of Pir Mahr Ali Shah University of Arid Agriculture, Rawalpindi. Leaves were washed and dried for one day. Afterwards packed in envelope and preserved in oven at 6°C for 3 days to ensue removal of moisture.
Preparation of pongame leaves extract
Dried leaves weighted 100 g later on it was grinded separately in an electric grinder and powder was obtained which was soaked in 500 ml of distilled water. Mixture thus obtained was refluxed and then agitated for an hour at 200 rpm. The filtrate in distilled water was squeezed out by using muslin cloth.
Preparation of pongame leaves decoction
Dried leaves weighted 100 g were used for preparation of decoction. For preparation of decoction, shade dried leaves weighing about 100 g were boiled in double distilled water until it reduced to 4 ml [7]. Various v/v concentrations (5, 10, 20 and 30%) of decoction were used in each experiment. The decoction was prepared.
Preparation of pongame seeds oil
Seeds oil was obtained from Biodiesel Laboratory of Quaid-e-Azam University Islamabad. Oil was stored at 4 ± 1°C in refrigerator.
Sclerotium rolfsii isolation
Sclerotium rolfsii isolates used in study were obtained from mycological collection of Department of Botany of Arid Agriculture University Rawalpindi. Sclerotium rolfsii was refreshed with the help of sterilized forecep and plated on sterilized Potato Dextrose Agar (PDA) medium (potato starch: 20 g, dextrose: 20 g, agar: 20 g and distilled water to make the volume 1 litter. PDA medium was amended with antibiotic (streptomycin 100 μg/ml). Plates were incubated at 25°C and observed daily for emergence of colonies. Single spore inoculation method used for sub culturing in order to obtain pure culture. Pure cultures were kept at 4°C and sub-cultured once in a month.
Evaluation of pongame products
To carry out evaluation of Pongame products, variable concentrations of Pongamia (5, 10, 20 and 30 ml/L) amendments were applied on Potato Dextrose Agar PDA medium of Sclerotium rolfssi and then poured in petri dishes. Control was prepared by adding water and PDA media only. Each treatment solution was analyzed separately three times along with control. These plates were incubated at 25 ± 2°C followed by inoculation. In order to investigate the effect of heat on products of Pongamia pinnata , each product was separately evaluated and compared with other products with and without autoclaving (121°C at 15 psi).
Bioassay to evaluate the activity against mycelial growth and sclerotial production of Sclerotium rolfsii
Measurement of outspread growth of mycelium was done by drawing two lines, perpendicular to each other in a way that they coincide with mycelium boundaries. Mycelial growth in control and in treated group were compared to find out inhibition in mycelial growth. For this, formula given below was used:
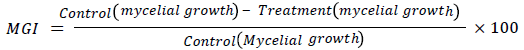
For the assessment of Sclerotial production in the Sclerotia harvested from replicates of each treatment after 15 days, sclerotial weight of each treatment was averaged to get mean weight of Sclerotia per plate. Inhibition of Sclerotial production (SPI) was calculated by following formula

Bioassay to evaluate activity against sclerotial germination
In case of Sclerotial germination, all Sclerotia were harvested from 15 days old cultures of Sclerotium rolfsii with the help of brush. Surface sterilized Sclerotia were placed on media mentioned with pongame products at the rate of 6 Sclerotia per plate. Three replicates were made for each treatment. Inhibition in Sclerotial Germination (SGI) was calculated in comparison with control by using following formula
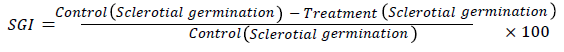
Mycelial growth Inhibition, sporangial production inhibition and sclerotial germination inhibition means were calculated along with standard errors.
Statistical analysis
All of the treatments were replicated thrice to express results with their calculated mean standard deviation. For this, ANOVA was performed by using software Prism 4 (GraphPad. Inc.) P<0.05 was taken as significant value.
Results
Inhibition results of Sclerotium rolfssi after treatment with Pongamia pinnata products obtained from in vitro experimentation are shown in Figures 1-9. Maximum inhibition of mycelia growth, sclerotial production and germination was recorded at higher concentrations of Pongamia products extracts and the effect of all the products was fungi static rather fungi toxic. Various v/v concentrations (5, 10, 20 and 30%) of leaves extracts and seed oils were used to inhibit growth of mycelium production and germination of Sclerotium. Results thus obtained were statistically analyzed at P=0.05. There was a noteworthy difference between effectiveness of seeds oils and leaves of Pongamia pinnata that were used after autoclaving and without autoclaving throughout fungal (S. rolfssi ) life period. However, treatment with control showed no inhibition at all. Furthermore, the effectiveness of both seed oils and leaves was minimized after exposure to heat.
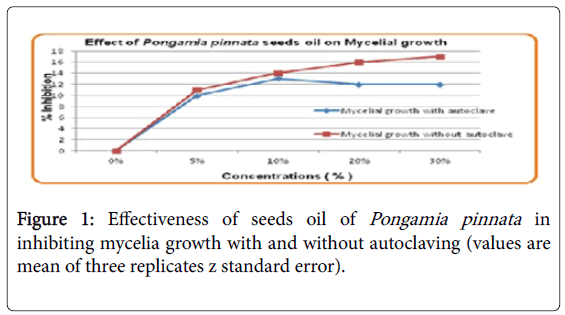
Effect of Pongamia pinnata seeds oil on mycelial growth
When seeds oil was used without autoclaving, it showed greater inhibition of mycelial growth as compared to when used after autoclaving as shown in Figure 1. Inhibition observed by seeds oil without autoclaving was 12% whereas this inhibition was increased to 16% when used without autoclaving.
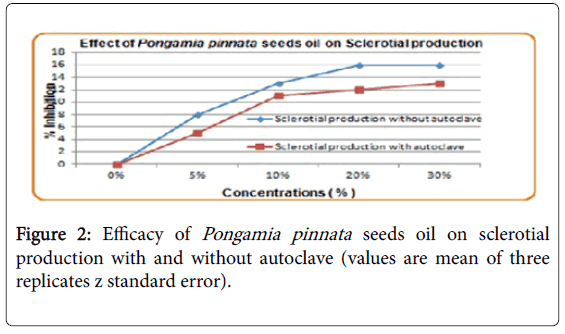
Effect of Pongamia pinnata seeds oil on sclerotial production
Effectiveness of leaf diffusate for inhibition of Sclerotial production was enhanced double when concentration was doubled from 5 to 10%. However, there was slight increase in efficacy with further increase in concentrations (20-30%). Autoclaving also reduced efficacy of leaf diffusate as shown in Figure 2.
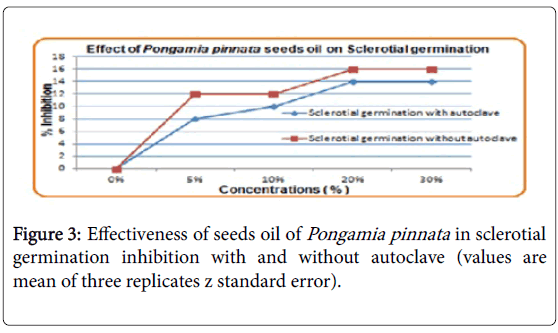
Effect of Pongamia pinnata seeds oil on sclerotial germination
At 20% concentration Pongame seed oil with autoclaving there was 14% inhibition of sclerotial germination and was observed same at 30% concentration with autoclaving. Highest inhibition was observed at 20 and 30% concentrations with and without autoclaving shown in Figure 3.
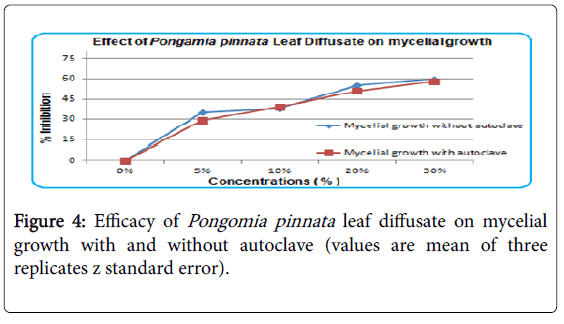
Effect of Pongamia pinnata leaf diffusate on mycelialm growth
Inhibition of Mycelial growth and sclerotial germination was effected more by Pongame leaves as compared to seeds oil. Four different concentrations 5, 10, 20 and 30% (v/v) of Pongame leaves decoction and diffusate were used. The results have shown that that heating effects were insignificant and normal control was without inhibition (Figure 4). The inhibition rate of mycelia growth was same for autoclaving and without autoclaving of Pongame leaf diffusate. Leaf diffusate with 30% concentration have shown highest inhibition rate.
Effect of Pongamia pinnata leaf diffusate on sclerotial production
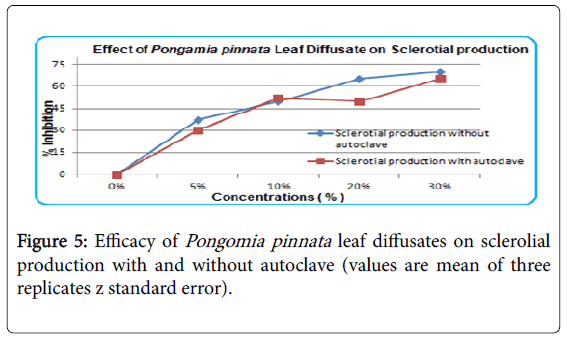
Pongamia pinnata leaves diffusate shown highest inhibition during Sclerotial production. Autoclaving and without autoclaving effects were same (Figure 5). 30% concentration was observed as highest.
Effect of Pongamia pinnata leaf diffusate on sclerotial germination
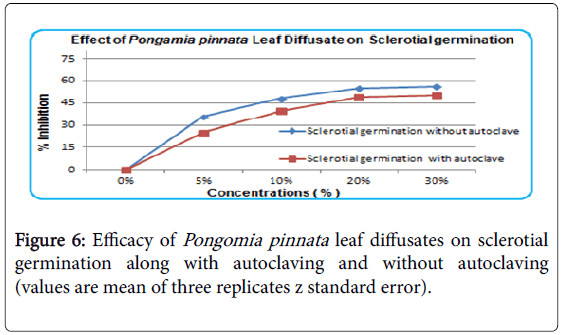
The 5% concentration of leaf diffusate inhibited at rate of 20% and it’s doubled at concentration of 10% without autoclaving. Almost same and highest inhibition observed at 20 and 30% concentration of leaf diffusate against sclerotial germination (Figure 6).
Effect of Pongamia pinnata leaf decoction on mycelial growth
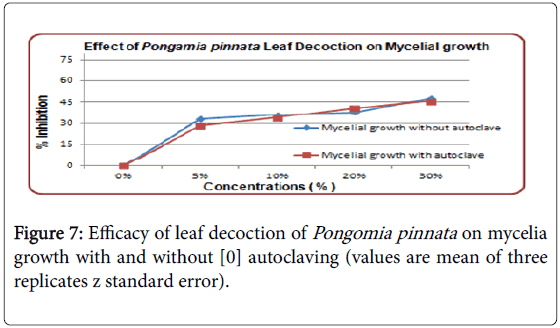
With 30% concentration of leaf decoction highest inhibition rate observed on mycelial growth. With and without autoclaving has no effect on efficacy of leaf decoction. There was 28% inhibition at 5% concentration of leaf decoction with autoclaving on mycelia growth and almost 32% inhibition observed without autoclaving shown in Figure 7.
Effect of Pongamia pinnata leaf decoction on sclerotial production
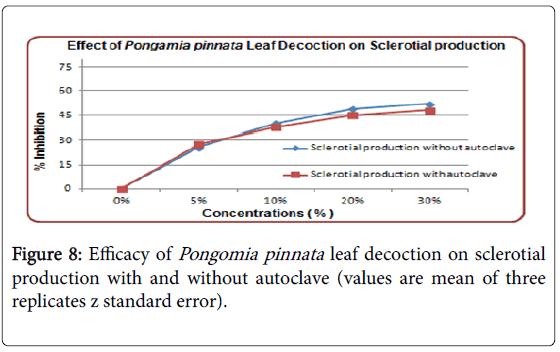
Effect of leaves decoction have shown same inhibition on sclerotial production and mycelia growth. With or without autoclaving has no significant variation in efficacy of leaf decoction. From 5 to 60% inhibition rate observed in sclerotial production by leaf diffusate (Figure 8).
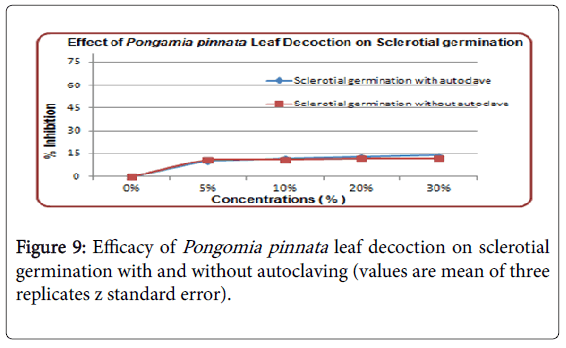
Effect of Pongamia pinnata leaf decoction on sclerotial germination
Very low effect of leaf diffusate on Sclerotial production was observed. All the concentrations from 5, 10, and 20 to 30% have same rate of inhibition on Sclerotial germination (Figure 9). Overall very low rate of inhibition have shown by leaf decoction.
Discussion
Thirty percent concentration of leaf diffusate of Pongamia pinnata without autoclaving have shown highest inhibition (Figure 6) while Pongame leaf decoction have shown lowest inhibition (Figure 9). Heat significantly reduces the effect of Flavonoid extracts. This increase was significant when higher concentrations of seeds oil was used during amendment of media. It shows that heat significantly affects the active ingredients involved in inhibition of sclerotial production. The effect of seed oil extracted from Pongamia pinnata on sclerotial production was considerably effective and that effect was increased with increase in its concentration. Effect of seeds oil of Pongamia pinnata was different on sclerotial germination with and without autoclaving (Figure 3). Inhibition was less after autoclaving of amended media. Effectiveness of leaf based products was more that seed product extracts.
With increase in concentration, effect of seed oil increased on inhibition of sclerotial germination but is was considerably less effective than as on mycelial growth. Efficacy of leaf diffusate with and without heating remained unchanged It is observed that efficacy of leaf diffusate is not effected by heat and there is no change in its composition. Efficacy of leaf diffusate increases with increase in its concentration. The effect of leaf diffusate is same on sclerotial growth at highest concentration i.e., 30%. It indicates that leaf diffusate have almost no effect of heat. The effect of Pongamia pinnata leaf diffusate on sclerotial germination was observed positive. It was slightly high with high concentration and effect of heat and without heat was considerably low (Figure 6). As compared to leaf decoction Pongame leaves diffusate was more effective against all three stages of pathogen Sclerotium rolfsii.
The effect of heating was not significant and unamended control was without inhibition. Mycelial growth inhibited by leaf decoction but as compared to leaf diffusate its rate of inhibition was low (Figure 7). Even increase in concentration from 5 to 30% had not played vital role in inhibition of mycelia growth. Effect of heating was also not observed. Leaf decoction has shown considerably less effect as compared to leaf diffusate. Sclerotial production inhibition was least observed by seeds oil. Effect of concentration increase was also observed, it shows that increased concentration contains more active ingredients in Pongamia pinnata products which ultimately effect the mycelia growth, sclerotial production and germination (Figure 8).
Cause behind this effect is active ingredients present in large concentration in leaf diffusate. Thus effectiveness of leaf based Pongame products were more than its seeds based products. Efficacy and antifungal activities of leaves are high because of presence of chemicals i.e., flavonoids, thermostatic nature [8]. Flavonoids were found to have effective antimicrobial and antifungal activities. Flavonoids are important to inhibit cytoplasmic membrane function, nucleic acid synthesis and also slow down energy metabolism in microorganisms [9]. Present study reveals a new idea and method of managing diseases of chickpea through natural and biological control. Pongame plant showed antifungal activities which can help in future for the isolating, purifying and concentrations active compounds in biochemical perspective. Appropriate selection of concentrations, formulation and application method could also help in further exploration of Pongamia pinnata plant related research.
Wagh et al. [10] evaluated antibacterial and antifungal activity of Pongamia pinnata oil with different concentrations against Aspergillus fumigatus , A. niger , Pseudomonas aeruginosa and Staphylococcus aureus by employing dry-weight method and Minimum Inhibitory Concentration (MIC) determination. Presence of fatty acid has shown by performing chemical analysis of P. pinnata oil. They suggested to use fatty oil of Pongamia pinnata for developing plant derived antimicrobial drugs. Heating effects also showed that leaves have their ingredients not always constant after autoclaving. Ingredients affected by heat indicated that there are some agents present which are sensitive to heat and alters their composition with effect of heat. These agents may be composed of proteins and carbohydrates. Pongame seeds oil was less effective as compared to Pongame leaves. It means that seeds oil cannot retain their ingredients constant after autoclaving. When the extract was used without autoclaving the difference in inhibition among the concentrations was non-significant except 20% (v/v) where significant increase in inhibition was detected in case of mycelial growth, inhibition in sclerotial production and germination.
Conclusion
Present results from this study indicated that there are antifungal activities present in Pongamia pinnata products at certain concentrations that are fungi static rather than fungi toxic in inhibiting the mycelia growth, Sclerotial production and germination of S. rolfsii. Heating effects also indicated that leaves ingredients are not always constant after autoclaving.
Pongame seeds oil was less effective as compared to leaves. This study gives a new idea and method of managing disease of chickpea through biological control. The Pongame plant shows antifungal activity which will help in future for the isolating, purifying and concentrations active compounds in biochemical perspective. Appropriate selection of concentrations, formulation and method of application could help in further investigation and exploration of Pongamia pinnata related research.
References
- Hules JH (1991) Nature, composition and utilization of grain legumes. Uses of Tropical Grain Legumes: Proceedings of a Consultants Meeting, ICRISAT Center, India, pp: 11-27.
- Sheehy T, Sharma S (2012) The nutrition transition in the Republic of Ireland: trends in energy and nutrient supply using Food and Agriculture Organization food balance sheets. Brit J Nutr 106: 1078-1089.
- Das Z, Alam S (2001) Synthesis and studies of antibacterial activity of ponga glabol. J Chem Sci 116: 29-32.
- Herman GE, Howell CR, Viterbo A, Lorito M (2004) Trichoderma species opportunistic, avirulent plant symbionts. Nature Rev Micro 2: 43-56.
- Yadav YS, Siddiqui AU, Parihar A (2005) Management of root-knot nematodes. Asian Productivity Org, pp: 19-93.
- Khurma UR, Mangotra A (2004) Screening of some Leguminosae seeds for nematicidal location of Fusarium moniliforme in the seeds. Seeds Sci Techno 3: 683-690.
- Thakkur CG (1976) The Art and Science of Pharmacy. In: Introduction to Ayurveda: Basic Indian Medicine, 2nd (edn.). Gulakunverba Ayurvedic Society, Jamnagar, India. pp: 107-111.
- Sadri NL, Vibhavari Y, Deshtande KN, Mendulkar D, Nandal H (1983) Male antifertility activity of Azadirachta indica in different species. In: Natural Pesticides from Neem tree (Azadirachta indica A. Juss) and other tropical plants. Deutsche Gesellschaft for Technische Zusammenarbeit (GTZ), Eschborn, Germany, pp: 473-482.
- Tim TP, Lamb AJ (2005) Antimicrobial activity of flavonoids. Internat J of Antimicrobial Agents 26: 343-356.
- Wagh P, Rai M, Deshmukh SK, Durate MCT (2007). Bio-activity of oils of Trigonella foenum-graecum and Pongamia pinnata. African J Biotech 6: 1592-1596.
Citation: Shaheen I, Parveen S, Parveen Z (2017) Evaluation of Pongamia pinnata Products against the Sclerotium rolfsii Extracted from Chickpea. Adv Crop Sci Tech 5: 291. DOI: 10.4172/2329-8863.1000291
Copyright: © 2017 Shaheen I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4580
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3675
- PDF downloads: 905