Research Article Open Access
Evaluation of Patients Receiving Jeeva® at an Integrative Pulmonary Care Center
Narinder Singh Parhar1*, Gloria St John1, Ajaipal Singh Gill1, Frank Son1 and Sachin A Shah1,2
1Parhar Health Systems, Roseville, California, USA
2Pharmacy Practice, Thomas J long School of Pharmacy and Health Sciences, University of the Pacific, Stockton, California, USA
- Corresponding Author:
- Narinder Singh Parhar
Sutter Independent Physician 584 N, Sunrise Avenue, #100, Roseville, California
Tel: (916) 773-2990
Fax: (916) 773-5154
E-mail: Parharmd@gmail.com
Received Date: October 17, 2016; Accepted Date: October 25, 2016; Published Date: October 31, 2016
Citation: Parhar NS, John GS, Gill AS, Son F, Shah SA (2016) Evaluation of Patients Receiving Jeeva® at an Integrative Pulmonary Care Center. J Tradi Med Clin Natur 5:195.
Copyright: © 2016 Parhar NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Introduction: Asthma and COPD contribute significantly to morbidity, mortality, and social-economic burden. Integrative Pulmonary Care center (IPCC) is a specialized program that has an integrative approach to respiratory care. Notably, eligible patients may receive a novel plant based therapeutic option (Jeeva®) in addition to standard of care. Jeeva® integrates several nutraceuticals known to have immune-modulatory, anti-inflammatory andantioxidantproperties. Methods: An evaluation of patient records was performed for all asthma/COPD patients enrolled in the IPCC program who had consented to consume Jeeva®. Demographic data, past medical history, and spirometry data (FEV1, FVC, FEV1/FVC, FEV1% predicted, FVC% predicted, FEV1/FVC% predicted) were collected along with a survey-based assessment of quality-of-life. The primary endpoint was the maximum change in FEV1 and FVC pre-bronchodilator after Jeeva® initiation. A paired students’ t-test was utilized to compare the maximum change post- Jeeva® from baseline. Intent-to-treat analysis was performed using the last-observation carried forward methodology. Results: A total of 26 patients were included for analyses. Median duration of Jeeva® consumption was approximately 6 months (range 1–12 months). There was a statistically significant change in FEV1 and FVC from baseline [1.64 ± 0.72 L to 1.80 ± 0.72 L; (p=0.019) and 2.26 ± 0.80L to 2.50 ± 0.74 L (p=0.004) respectively]. Quality-oflife improved statistically significantly and there was a notable decrease in medication burden. Conclusion: Patients receiving Jeeva® as part of the IPCC significantly improved pre-bronchodilator FEV1 and FVC from baseline. A small improvement in quality-of-life and medication burden was evident. Further studies looking at Jeeva® in a randomized, placebo-controlled, clinical-trial is warranted.
Keywords
COPD; Asthma; Nutraceuticals
Introduction
Asthma and Chronic Obstructive Pulmonary Disease (COPD) are major causes of morbidity and mortality globally with COPD being the third leading cause of death [1]. More recently, asthma and COPD are being recognized as overlapping conditions aptly termed “asthma– COPD overlap syndrome” (ACOS) [2]. Pharmacologic options for their management often include short and long acting β2-agonists, anticholinergics, inhaled glucocorticoids and Leukotriene modifiers. However, none of the existing medications or regimens has been conclusively shown to modify the long-term decline in lung function [3].
Every year, approximately $30 Billion is spent out-of-pocket by Americans on complementary health approaches. The Integrative Pulmonary Care Center (IPCC) is a private physician’s office program that specializes in the care of patients with respiratory conditions using traditional and integrative approaches to care. In addition to standardof- care, patients can be initiated on a supplement called Jeeva® [4]. Patients are also introduced to yoga based breathing exercises, and given the opportunity to work with an exercise physiologist to help correct any defects in posture that limit breathing.
Jeeva® is a novel plant based therapeutic option that integrates several nutraceuticals known to have immune-modulatory, antiinflammatory, and antioxidant properties [5-21]. It includes Arabinogalactan, Acai berry, concentrated Aloe polysaccharides, Bilberry, Gum Acacia, Star Anise, and Turmeric Root in varying doses and is available in a capsule form [16]. To assess the degree of clinical benefit and impact on quality-of-life, we evaluated patients enrolled in the IPCC program being treated with Jeeva®.
Methods
Upon approval from the Institutional Review Board we performed a review of patients enrolled at an IPCC (http:// integrativepulmonarycarecenter.com/) at a private physician’s office in Roseville, California [16]. The primary practitioner specializes in internal medicine and holds board certification with the American board of Alternative and Integrative Medicine. All patients voluntarily signed an informed consent document before participating in the program.
Patients were selected for analysis if they had a diagnosis of COPD and/or asthma, and consumed Jeeva® (1 capsule twice daily) till at least the next follow-up visit (approximately 2-3 months after enrolment). Those under 18 years of age or pregnant were excluded. Patients were only included if they had a baseline and at least 1 follow up visit with spirometry data (FEV1 and FVC measurements). Forced expiratory volume in one second (FEV1), defines the volume of air that can be forced out in one second after taking a deep breath and forced vital capacity (FVC) measures the volume of air forcibly exhaled from the point of maximal inspiration both of which are accepted objective markers of pulmonary function.
A comprehensive chart review was conducted to collect age, race, gender, height, weight, smoking status, comorbidities, medication list, spirometry data and adverse effects. Pulmonary function test parameters (FEV1, FVC, FEV1/FVC, FEV1% predicted, FVC% predicted, and FEV1/FVC% predicted) were extracted at baseline and for each subsequent visit with available spirometry data.
Quality-of-life data was collected using the SF-8 survey. Each question was converted to a numerical score with the lowest possible score being 8 and the highest score being 42. It is important to note that a lower score denotes an improvement in quality-of-life.
The primary endpoint was the maximum change in FEV1 and FVC pre-bronchodilator from baseline. Secondary endpoints included the largest change from baseline in FEV1 post-bronchodilator, FEV1% predicted pre-bronchodilator, FEV1% predicted post-bronchodilator, FVC post-bronchodilator, FVC% predicted pre-bronchodilator, FVC% predicted post-bronchodilator, FEV1/FVC pre-bronchodilator, FEV1/ FVC post-bronchodilator, FEV1/FVC% predicted pre-bronchodilator, FEV1/FVC% predicted post-bronchodilator and SF-8 survey.
A paired students’t-test was utilized to compare all endpoints preand post- Jeeva® with a p<0.05 considered significant. For patients with missing data, an intent-to-treat (ITT) analysis was performed using the last-observation carried forward methodology.
Results
A total of 26 patients were included for analyses. Sixteen patients were male (62%) and 10 (38%) were Female. The average age and weight were 76 ± 10 years and 179 ± 40 pounds, respectively. Twenty had asthma and 18 had COPD with over 50% having a diagnosis of both. Fourteen (71%) were former smokers and two (4%) were current smokers. Seventeen patients (65%) had hypertension, nine (35%) had diabetes. Relevant comorbid past medical history included allergic rhinitis (38%), anxiety (31%), and chronic bronchitis (8%).
Thirteen patients were on short acting β-2 agonists (50%), four on long acting β-2 agonists (15%), fourteen on inhaled corticosteroids (54%), five on anticholinergics (19%), and one on a leukotriene modifier (4%). Seven were on a long acting β-2 agonist/corticosteroid combination (27%) and four (15%) on a β-2 agonist/anticholinergic combination.
Median duration of Jeeva® consumption was 175 days (range 28– 371 days).
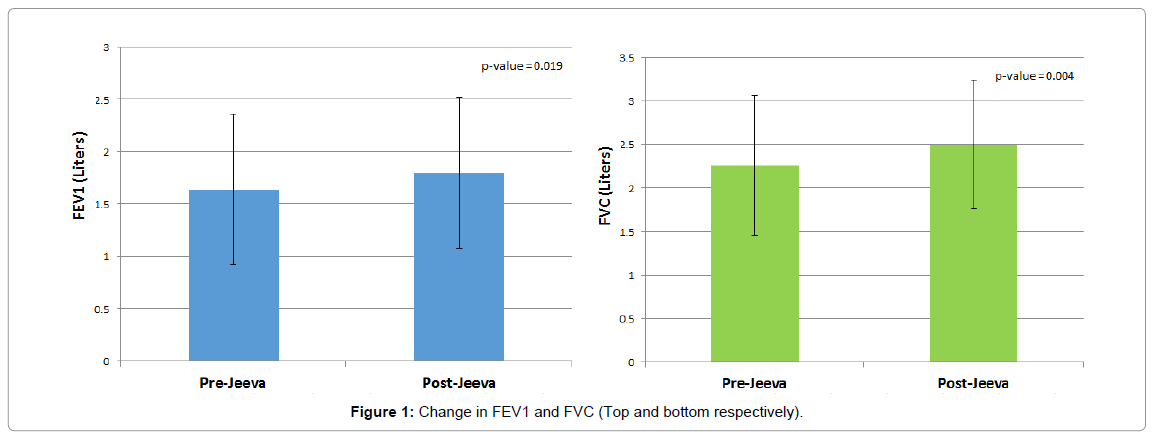
FEV1 pre-bronchodilator (Figure 1) improved significantly from baseline (1.64 ± 0.72 L to 1.80 ± 0.72 L; p<0.019). FEV1 postbronchodilator improved significantly from 1.77 ± 0.68 L to 1.88 ± 0.72 L (p=0.035). FEV1% predicted pre- and post-bronchodilator also improved significantly (Table 1).
| Endpoint | Baseline | Maximum change from baseline | p-value |
|---|---|---|---|
| FEV1 (Liters) | 1.64 ± 0.72 | 1.80 ± 0.72 | p=0.019 |
| FEV1 (Liters) [post- bronchodilator] |
1.77 ± 0.68 | 1.88 ± 0.72 | p=0.035 |
| FEV1% predicted | 60.92 ± 22.64 | 67.08 ± 22.23 | p=0.018 |
| FEV1% predicted [post-bronchodilator] |
64.83 ± 21.41 | 69.04 ± 23.03 | p=0.052 |
| FVC (Liters) | 2.26 ± 0.80 | 2.50 ± 0.74 | p=0.004 |
| FVC (Liters) [post- bronchodilator] |
2.45 ± 0.74 | 2.57 ± 0.76 | p=0.054 |
| FVC% predicted | 61.72 ± 18.77 | 69.40 ± 16.03 | p=0.002 |
| FVC% predicted [post- bronchodilator] |
66.13 ± 15.77 | 70.04 ± 15.37 | p=0.045 |
| FEV1/FVC | 71.73 ± 14.28 | 74.29 ± 14.19 | p=0.221 |
| FEV1/FVC [post-bronchodilator] |
71.85 ± 14.59 | 75.05 ± 14.92 | p=0.056 |
| FEV1/FVC% predicted | 101.32 ± 20.18 | 103.64 ± 20.23 | p=0.363 |
| FEV1/FVC% predicted [post-bronchodilator] |
101.25 ± 20.55 | 105.54 ± 21.33 | p=0.081 |
Table 1: Change in Pulmonary Function Endpoints
FVC pre-bronchodilator (Figure 1) improved significantly from baseline (2.26 ± 0.80 L to 2.50 ± 0.74 L; p=0.004). FVC postbronchodilator improved from 2.45 ± 0.74 L to 2.57 ± 0.76 L (p=0.054). FVC% predicted pre and post-bronchodilator also improved significantly (Table 1).
FEV1/FVC pre-bronchodilator (Table 1) improved nonsignificantly from baseline (71.7 ± 14.3 to 74.3 ± 14.2; p=0.221). A trend towards significant improvement was seen with the change in FEV1/ FVC post-bronchodilator from 71.9 ± 14.6 to 75.1 ± 14.9 (p<0.056).
FEV1/FVC% predicted pre-bronchodilator improved non-significantly from baseline (p=0.363). FEV1/FVC% predicted post-bronchodilator showed a trend towards significant improvement (p=0.081).
Quality of life score improved significantly from 27.4 ± 6.49 to 26.4 ± 6.19 (p<0.017). Nine (35%) of the patients had a reduction or elimination of their pulmonary medication (rescue or maintenance). One (4%) had a reduction in their oxygen use. None of the patients had a respiratory related emergency room visit or hospitalization while in the IPCC program.
Discussion
Asthma and COPD result in a significant social and economic burden [22]. According to the CDC, asthma accounts for one-quarter of all emergency room visits in the U.S. each year and over 3,000 deaths each year [23]. The estimated total cost for the two conditions was approximately $68 billion in 2008 [24]. Recently, the Centers for Medicare and Medicaid Services (CMS) expanded the Hospital Readmission Reduction Program (HRRP) to include COPD. The burden of illness of these patients is such that the CMS will now penalize hospitals for what they deem as unplanned readmission for COPD [25]. This is driven by the fact that 23% of COPD hospitalizations are subsequently readmitted within 30 days post discharge [26].
Our findings indicated a significant benefit in pulmonary function markers in patients enrolled in the IPCC program. Benefits in spirometry endpoints were in line with data from previous analyses where Jeeva® improved pre-bronchodilator FEV1 and FVC by 264 mL and 314 mL from baseline, respectively [4]. This current analysis builds on previous work as it also shows a small improvement in quality-of-life. There was also a reduction in medication use in 38% of the patients. This is important as frequency of rescue inhaler use is a surrogate marker of exacerbation of disease [27].
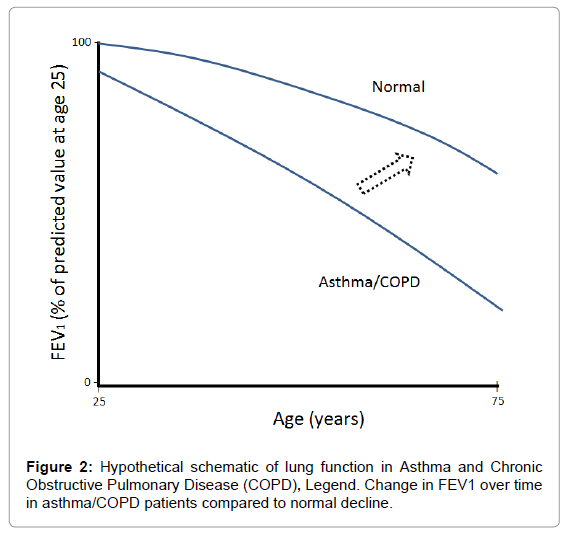
The respiratory system undergoes various physiological, immunological, and anatomical changes with age [28]. The estimated rate of decline in FEV1 is 25-30 mL/yr starting at age of 35-40 years and can double to 60 mL/yr after the age of 70 years. FVC generally declines at approximately 22 mL/yr in healthy subjects (20 to 60 years) [29]. Figure 2 depicts the hypothetical decline in FEV1 over time [2]. It is possible that the incorporation of Jeeva® to standard-of-care in patients with asthma or COPD can direct them away from the typical decline in FEV1 (depicted by the dotted line arrow) but this needs further evaluation.
The findings from this report and our previous work together support conducting randomized, double-blinded, placebo-controlled trials with Jeeva®. Other novel, albeit expensive, approaches to optimizing pharmacotherapy include nanoparticle-based drug delivery and innovative inhalers [30,31]. In the future, it would be important to perform cost-effectiveness analyses to assess the ideal integrative approach to minimize the disease burden and optimize quality of life in patients with asthma and COPD.
There are several limitations of note. Primarily, there are innate limitations of a retrospective analysis such as this and it does not infer causality. A lack of placebo arm rules in the possibility of patients simply benefiting from the improved attention received when enrolled in a specialized center such as IPCC. There is compelling data suggesting that a specialized care management model can have a positive outcome on patient health [32]. The compliance rate of yoga breathing was low which could diminish the magnitude of efficacy seen from the IPCC program. However, it strengthens our case for supporting the benefits being primarily driven by Jeeva®. Hospital admissions were not assessed from a review of medical records but from patient recall which is not always accurate. A lack of a control arm and a small sample size pose inherent limitations in data extrapolation and wide applicability.
Conclusion
Patients receiving Jeeva® as part of the IPCC significantly improved pre-bronchodilator FEV1 and FVC from baseline. A small improvement in quality-of-life and medication burden was evident. Further studies looking at Jeeva® in a randomized, placebo-controlled, clinical-trial are warranted.
Acknowledgement
The authors would like to thank Athena Xides, Pharm.D. and Kunal Shah, Pharm.D. Candidate for their assistance.
References
- http://www.who.int/mediacentre/factsheets/fs310/en/
- Postma DS, Rabe KF (2015) The Asthma-COPD Overlap Syndrome. N Engl J Med 373: 1241-9.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272: 1497-505.
- Shah SA, Lee JJ, Son F, St John G, Parhar NS (2016) Impact of a Novel Plant-based Treatment Option in Improving Pulmonary Function Markers in Patients with Chronic Obstructive Pulmonary Disease and Asthma. AlternIntegr Med 2: 215.
- http://integrativepulmonarycarecenter.com/
- Kelley GS (1999) Larch Arabinogalactan: clinical relevance of a Novel Immune-Enhancing Polysaccharide. Altern Med Rev 4: 96-103.
- Chatterjee UR, Ray S, Micard V, Ghosh D, Ghosh K, et al. (2014) Interaction with bovine serum albumin of an anti-oxidative pecticarabinogalactan from Andrographispaniculata. CarbohydrPolym 101: 342-348.
- Domej W, Oettl K, Renner W(2014) Oxidative stress and free radicals in COPD – implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis 9: 1207-1224
- Skyberg JA, Rollins MF, Holderness JS (2012) Nasal Acai Polysaccharides Potentiate Innate Immunity to Protect against Pulmonary Francisellatularensis and Burkholderiapseudomallei Infections. PLoS Pathogens 8: e1002587.
- Manvitha K, Bidya B (2014) Aloe vera: a wonder plant its history, cultivation and medicinal uses. J Pharmacogn Phytochem 2: 85-88.
- Zhong JS, Huang YY, Zhang TH (2015) Natural phosphodiesterase-4 inhibitors from the leaf skin of aloe barbadensis Miller. Fitoterapia 100: 68-74.
- Habeeb F, Shakir E, Bradbury F (2007) Screening methods used to determine the anti-microbial properties of Aloe vera inner gel. Methods 42: 315-20.
- Hohtola A (2010) Bioactive compounds from northern plants. AdvExp Med Biol 698: 99-109.
- Burdulis D, Sarkinas A, Jasutiene I (2009) Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vacciniummyrtillus L.) and blueberry (Vacciniumcorymbosum L.) fruits. Acta Pol Pharm 66: 399-408.
- Braga PC, Antonacci, R, Wang YY, Lattuada N, Dal Sasso M, et al. (2013) Comparative antioxidant activity of cultivated and wild Vaccinium species investigated by EPR, human neutrophil burst and COMET assay. Eur Rev Med PharmacolSci 17: 1987-1999.
- Ali BH, Ziada A, Blunden G (2009) Biological effects of gum Arabic: A review of some recent research. Food ChemToxicol48 :1-8.
- Benmalek Y, Yahia OA, Belkebir A, et al. (2013) Anti-microbial and anti-oxidant activities ofIlliciumverum, Crataegusoxyacantha sspmonogyna and Allium cepa red and white varieties. Bioengineered 4: 244-48.
- De M, De AK, Sen P (2002) Antimicrobial properties of star anise (Illiciumverum Hook f). Phytother Res 16: 94-5.
- Kang P, Kim KY, Lee HS, Min SS, Seol GH, et al. (2013) Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci 93: 955-961.
- Shishodia S, Sethi G, Aggarwal BB (2015) Curcumin: getting back to the roots. Ann N Y AcadSci 1056: 206-17.
- Bengmark S, Mesa MD, Gil A (2009) Plant-derived health: the effects of turmeric and curcuminoids. NutrHosp 24: 273-81.
- http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
- Bousquet J, Bousquet P, Godard P, Daures JP (2005) The public health implications of asthma. Public Health Reviews. Bull World Health Organ 83: 548-554.
- http://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook.pdf
- Braman SS (2015) Hospital readmissions for COPD: We can meet the challenge.J COPD F2: 4-7
- Jencks SF, Williams MV, Coleman EA (2009) Rehospitalizations among Patients in the Medicare Fee-for-Service Program. NEJM 360: 1418-28.
- Jenkins CR, Postma DS, Anzueto AR, Make BJ, Peterson S, et al. (2015) Reliever salbutamol use as a measure of exacerbation risk in chronic obstructive pulmonary disease. BMC Pulm Med 15: 97.
- Sharma G, Goodwin J (2006) Effect of aging on respiratory system physiology and immunology. ClinInterv Aging 1: 253-260.
- Swanney MP, Stanton JD, O’Reilly-Nugent A (2014) Natural decline in FEV1 and FVC: Self versus reference equations. ERJ 42:1787.
- Yhee JY, Im J, Nho RS (2016) Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J Clin Med 20: 5.
- Virchow JC, Akdis CA, Darba J, Dekhuijzen R, Hartl S, et al. (2015) A review of the value of innovation in inhalers for COPD and asthma J Mark Access Health Policy 3: 28760.
- Ciccone MM, Aquilino A, Cortese F, Scicchitano P, Sassara M, et al. (2010) Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc Health Risk Manag 6: 297-305.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 11058
- [From(publication date):
November-2016 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 10233
- PDF downloads : 825