Evaluation of Micropollutant (17Alpha Ethinylestradiol; EE2) Content in Waste Water in the Built Environment: Differences due to Varying Building Uses and Occupiers
Received: 25-Jun-2021 / Editor assigned: 01-Jan-1970 / Reviewed: 01-Jan-1970 / Revised: 01-Jan-1970 / Accepted Date: 09-Jul-2021 / Published Date: 16-Jul-2021
Abstract
The presence of pharmaceutical and personal care products (PPCPs) in the environment is an emerging field of study which has generated huge concerns, as these PPCPs are usually found in trace concentrations, below detectable limits and have been reported to result in negative physiological changes in fish. This project is aimed at developing and establishing a suitable sampling protocol for the ecotoxicological evaluation of municipal waste water from Glasgow Caledonian University (GCU), using samples from two teaching blocks in GCU – The Govan Mbeki and George Moore buildings. These locations were chosen based on the hypothesis that the Govan Mbeki building has a higher female student population than the George Moore building. The flow pattern of both streams of waste water was determined and time weighted composite sampling was carried out over a sampling period of five working days. The samples were characterized chemically, with no significant differences in the measured parameters. In comparison, samples from both locations were fairly consistent and representative of the student population during the sampling period. No EE2 was detected, as a result of low concentration of the calibration standard used, which lacks sensitivity to EE2 at lower concentrations. It is recommended that in future research, lower concentrations of standard solutions be used to calibrate measuring equipment and samples from more than two locations should be obtained, to provide a wider range of comparable results.
Keywords: Micropollutants; Oestrogen; Pharmaceuticals; Wastewater; Liquid Chromatography-Mass Spectrometry (LC-MS)
Introduction
Continuous release of pharmaceutical products into the environment is treacherous to the existence of aquatic organisms, particularly in receiving waters. These substances are designed to be biologically active, soluble in water and not easily biodegradable; thus, making the marine habitat an ultimate sink for natural and anthropogenic chemicals [1].
Oestrogens are female hormones which are vital to maintain health of the reproductive tissues, skin, and breasts and brain [2]. There is growing concern over the presence of these hormones in the environment as they have the ability to instigate a negative interference with the natural balance of the ecosystem. Oestrogens are more persistent than other steroid hormones in the environment, as a result of the four rings in their chemical structure, making the compound relatively stable [3]. EE2 is a synthetic oestrogen, commonly used as a chief component in the manufacture of oral contraceptives [4]. Its presence in the aquatic environment is as a result of waste water treatment plants to efficiently remove the said compound [5]. Ternes reports that it is one of the most significant xenoestrogens to be detected in domestic sewage, in concentrations ranging from 0.2-5.0 ng/L [6]. Studies indicate that:
• Feminization of male rainbow trout is observed from EE2 concentrations as low as 0.1 ng/L [7].
• Long term exposure of newly hatched common roaches to EE2 at a concentration of 4ng/L resulted in sex change of male fish to female [8].
The first review of pharmaceuticals and hormones in the environment was carried out by Richardson and Bowron (Aherne et al) with the use of a gas chromatograph-mass spectrometer unit (GCMS) [9]. Oral contraceptives were not detected, resulting in the conclusion that “those hormones should not warrant environmental concern”. Upon further research, 17 ng/L of Noresthiserone and 6 ng/L of Progesterone were detected in samples of river and potable water respectively. This renders the reports of Richardson and Bowron (cited in Aherne et al.) invalid because over the past decades, studies have been conducted with significant progress recorded in the detection and evaluation of the effects of a wide range of pharmaceutical substances at different concentrations, even below detection limits report that oestrogen containing compounds interfere with the non-reproductive physiological and behavioural processes in fish, such as inhibiting smelting of the Atlantic salmon [10]. In related research, oestrogen has been observed to affect the reproductive behavior of fish, such as aggressive courtship and feminization of male fish reduction in quality and quantity of the gametes of male and female zebra fish [11,12].
A joint study conducted by Scottish Water and Scottish Environment Protection Agency (SEPA)which was aimed at determining the concentrations of certain EDCs in samples of waste water from several sources including domestic, correctional, industrial, health care, livestock production and educational facilities recorded the various concentrations of oestrogen found in each sample. EE2 was detected in large concentrations at the educational and correctional facilities respectively. EE2 was not detected in the sample from the livestock production facility, which is to be expected because EE2 is an artificially synthesized compound.
Furthermore, the continued presence of these substances in the environment could adversely affect plant life [13-15]. A study conducted by Shore et al. revealed that high levels of phytoestrogens were observed on a batch of alfalfa-a perennial forage crop, watered with effluent which contained oestrogens. Thus, it is necessary to determine if significantly elevated levels of endocrine disruptors present in the environment could be harmful to all living organisms [16].
This study involved exposure to and direct contact with raw human waste. To prevent associated illnesses, vaccination shots were taken as a health and safety precaution against Hepatitis A and B, typhoid, polio and tetanus. Furthermore, leptospirosis awareness was undertaken as there are currently no vaccines available for its prevention.
Experimental
Flow pattern, usage and student occupancy determination
Using a PRECISION 190P Ultrasonic flow meter, a week’s worth of flow data was obtained from both sampling locations to establish the flow patterns of waste water in both locations. The flow meter was installed via attachment to a water pipe in the waste water discharge chamber of the George Moore building. The Govan Mbeki building had a pre-installed flowmeter; thus, the flow data was extracted from the online data log. The sampling pattern and times were established, based on the flow data generated from both locations.
Swipe data was collected, which quantifies the presence of students in both teaching blocks during the sampling period via attendance monitoring. It presents the number of male and female students present during the sampling period in each location.
Sampling location, preparation and collection
The sampling points were located at the waste water discharge points of the George Moore and Govan Mbeki teaching blocks. George Moore building is home to the School of Engineering and Built Environment, while the Govan Mbeki building houses the School of Nursing and Life Sciences. Aquacell P2-COOLBOX automated samplers were used to obtain the samples. A sampler was installed at each sampling point and 4-Litre glass jars were used as the collecting vessels. A rubber-hose pipe was connected to the sampler, to provide an opening for the waste water to flow through. Baffles were connected at the end of the pipe which served to hold the end of the pipe in place and also create a small pool of water after toilet flush(es) to ensure sufficient mixing.
In order to establish a sampling protocol that is considered “fit for purpose”; some factors were considered, as suggested by Jones-Lepp et al. [17] namely:
a) Best fit sampling method which provides working samples that truly represent the area(s) of interest.
b) Suitability of the sample for achieving the set research objectives.
c) Ability of the sample size to satisfy the minimum detection limits requirements of the chosen analytical methods.
Following the aforementioned considerations, composite sampling was used, based on equal time intervals, following a flow pattern.
Samples of raw waste water were collected daily between the hours of 8.30 am to 2.00 pm from both sampling locations over a period of 5 working days (Monday to Friday). The composite samples comprised 34 grab samples, collected from each location at 12-minute interval. Additional spot samples were taken every hour to ensure that “on the hour” activities were captured. The samples were conveyed to the laboratory in cooler boxes, to maintain the temperature and integrity of the samples.
1.5 L of the sample was filtered within 24 hours by suction, using 100 μm, 0.7 μm and 0.45 μm Whatman filter papers respectively and kept refrigerated 4oC. Further filtration was carried out on 2 ml of the filtered sample, using 0.2 μm Whatman filter paper and the resultant filtrate was kept refrigerated at -20oC.
Characterization and wet chemistry analysis
The samples were analyzed in triplicates, with dissolved Oxygen, pH and temperature measured immediately after collection. The other parameters for which analyses were carried out include: Conductivity, colour, turbidity, total dissolved solids (TDS), total solids (TS), total fixed solids (TFS), total volatile solids (TVS), chemical oxygen demand (COD), biochemical oxygen demand (BOD), UV-Vis spectrometric determination, total organic carbon (TOC), Ammonia (NH3), Phosphate (PO4) and Nitrate (NO3).
EE2 Determination using Liquid Chromatograph-Mass Spectrometry (LCMS)
Samples for EE2 measurement were kept refrigerated at -20oC for 7 days for the samples to be totally frozen, after which they were concentrated via 24 hour freeze drying process using a Christ Alpha LD Plus freeze drying unit. This resulted in the total evaporation of all liquid content, leaving behind a fine powder residue. The residue was dissolved in 0.05% Ammonium Hydroxide and methanol. LC-MS analysis was conducted using a Thermo Scientific: Dionex Ultimate 3000 LC-MS unit; Thermo Accucore C18 (100 × 2.1 mm) column was used against 12 point concentrations in ng/L at 0.1, 0.25, 0.5, 1,2.5,5,10,25,50,100,250 and 500.
Results
Student occupancy and water flow patterns determination
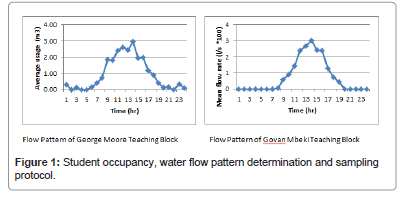
The student data and water flow obtained from both teaching blocks are presented respectively in the table below: (Table 1 and Figure 1).
| George Moore | Govan Mbeki | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 8.30 am-2.15pm | 3645 | 3559 | 865 | 3021 |
| Full day (Total) | 4275 | 3883 | 911 | 3291 |
Table 1: Student occupancy and water flow patterns determination.
EE2 determination
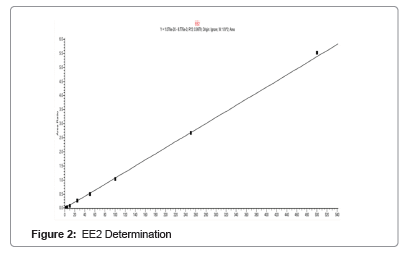
A calibration line plot of area ratio against concentration was obtained, showing the different concentrations against which the samples were tested. The line of best fit was extrapolated and also shows the concentrations at which the calibration line is valid as seen below. EE2 was not detected samples at any of the set concentrations (Figure 2).
Discussion
Student occupancy, water flow pattern determination and sampling protocol
This research was carried out based on the hypothesis that the Govan Mbeki building, which is home to the School of Nursing and Life Sciences will have a higher female population than the George Moore building which has the School of Engineering and Built Environment. The student swipe data revealed that the reverse was the case, as the George Moore building was seen to contain more female students than the Govan Mbeki building, slightly faulting the postulation with a difference of 592 and an approximate ratio of 1:1. This is contrary to the hypothesis made about the student population, but provides results within a workable comparable range as the female population of both buildings is not equal.
As seen in Figure 1 above, there are more students in both locations between the hours of 8-10 am, than other hours; with emphasis on the ninth (9 am) and thirteenth (1 pm) hours. At least, 85% of student presence was recorded between of 8.30 am to 2.15 pm, confirming that the sampling protocol devised for evaluation of 17α-ethinyl estradiol (EE2) will have matched the period where the majority of the students were in both teaching blocks. This is further supported by a rise in the mean flow rates in both the George Moore and Govan Mbeki buildings between the 8.30 am till 2.15 pm, as illustrated in Figure 2. The peaks in the flow pattern are particularly distinct on the hour, indicating heavy toilet usage as these are the hours in which the students are about to start or have just finished a class and this justifies the spot samples being taken on the hour. However, at 1 pm, there were 1150 students present in the George Moore building, which can be said to be a large number and result in a peak flow rate of 2.50 m3/hr seen in Figure 1 above, relative to the number of students in the Govan Mbeki building, which was 690 (approximately half the size of those in the George Moore building) with a flow rate of about 2.4 m3/hr. Thus, a sampling scheme such as that used in this research thesis will capture samples that are truly representing the location(s) of interest.
The results obtained from measurement of the seventeen parameters (as listed in xxx above) did not reveal any significant deviations, thus making the working samples comparable.
EE2 determination
The calibration plot for the EE2 measurement was a 12 point concentration line as in above. Only 9 out of the 12 standard concentrations were used because the other three were invalid. The concentrations of 0.1, 0.25 and 0.5 ng/l were rejected because the resulting internal standard areas were not within the selected criteria, as they were either too high or too low, as seen in the calibration line in above. Thus, the lowest standard concentration used in this measurement was 1 ng/l. No EE2 was detected in any of the samples run against the standard concentrations. This is as a result of the calibration concentrations used, limit of quantification and the environmental quality standards of EE2.
The Scientific Committee on Health and Environmental Risks (2011) adopted an EQS of 0.035 ng/l for EE2. In a study of European surface waters, the mean LOQ for EE2 was reported as 0.8 ng/l (Kuck and Ballschmitter, 2001). Furthermore, a study conducted by Scottish Water on waste water effluents across the domestic, commercial and industrial sectors revealed an LOQ of 0.1 ng/l for EE2 (Zyndul and Gillman, 2013). It can be seen that there is a range of the detection limits for EE2, from as low as 0.1 ng/l (ZYNDUL AND GILLMAN, 2013) to as high as 4.3 ng/l [18-21].
this thesis, EE2 was not detected because the calibration of the LC-MS spectrometer began at a standard concentration of 1.0 ng/l. With the possibility of detection of EE2 at such a minute concentration of 0.035 ng/l, it can be said that calibration at that concentration may greatly enhance the detection of EE2.
Furthermore, non-detection of EE2 can be as a result of matrix effect. This occurs when other compounds in the sample interfere with the compound of interest and suppress, or very rarely, enhance the characteristics (for example, mass spectroscopic signal) of that particular compound to be used in its detection [22,23]. Matrix effect can also be a direct result of the presence of the compound of interest in such small concentrations as to support the interference [24]. Paracetamol and caffeine were detected in very high concentrations in the samples, which can both be sources of interference with the EE2. Thus, increasing the sample volume to be concentrated for use in EE2 measurement could enhance its detection.
Conclusion
The flow data/pattern of both sampling locations showed peaks, which were mostly on the hour and this, was used to determine the sampling times for this project; to ensure that representative samples were taken. Hence, the chosen sampling protocol: 8.30 am to 2 pm grab sampling scheme from both locations can be adopted.
The student data from both locations revealed that comparatively, the numbers of female students in the George Moore and Govan Mbeki buildings are not as marginally different as hypothesised, with an approximate 1:1 ratio. This did not provide data/results for effective comparison.
Comparatively, the samples from both locations were fairly consistent as there were no significant differences in the chemical parameters measured.
The non-detection of EE2 in any of the samples could have been as a result of matrix effect or the use of high standard concentrations, which were well above the LOQ of EE2, in obtaining the calibration line.
In future studies, a larger volume of the samples should be concentrated for the LC-MS measurement of EE2 and known standard concentrations as low as 0.035 ng/l (which is the EQS set by the SCHER for EE2) should be used in obtaining the calibration line. Furthermore, more than 2 sampling locations should be used, to enable comparison of a wider range of waste water streams and a suitable student distribution for optimum results.
Limitations
There was significant loss of data for the flow rate of the George Moore building. Flow data for two days was lost due to a malfunction of the flow meter, which resulted in a wipe off of the data for two days during the sampling period. It was a set back because there was no room for complete comparison with the Govan Mbeki building on all the sampling days.
The LC-MS could only be calibrated to as low as 1.0 ng/l, which was above the 0.035 ng/L stipulated LOQ for EE2. Also, the sample volume concentrated for EE2 measurement was not sufficient enough to overcome possible interferences from other compounds.
The study was restricted to waste water samples of only two locations which did not have a significant difference in the number of female students as earlier hypothesised and did not provide enough room to evaluate samples from a range of different waste stream sources for extensive comparison.
References
- Sumpter JP. (1995) Xenoendocrine Disrupters: Environmental Impacts. Toxicol Lett 102-103: 337-342.
- Ying GG, Kookana RS, Ru YJ. (2002) Occurrence and Fate of Hormone Steroids in the Environment. Environ Int 28:545-551.
- Vaicunas RC. (2012) Hormone and Antibiotic Concentrations in Surface Water of Delaware. MSc thesis, University of Delaware.
- Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, et al. (2010) Daily Bisphenol Excretion and Associations with Sex Hormone Concentrations: Results from the Inchianti adult population study. Environ Health Perspect 118: 1603-1608.
- Clouzot L, Marrot B, Doumenq P, Roche N (2008) 17a-ethinylestradiol: An endocrine disrupter of great concern. Analytical methods and removal processes applied to water purification: A review Environ Prog 27: 383-396.
- Ternes TA. ( 1998) Occurrence of Drugs in German Sewage Treatment Plants and Rivers. Water Res 32(11): 3245-3250.
- Purdom CE, Hardiman PA, Bye VV, Eno NC, Tyler CR, et al. (1994)  Estrogenic effects of effluents from sewage treatment works. Chem Ecol 8: 275 – 285.
- Lange A, Paul GC, Coe TS, Katsu Y, Urushitani H, Iguchi T et al. (2009) Sexual Reprogramming and Estrogenic Sensitization in Wild Fish Exposed to Ethinylestradiol Environ Sci Technol 43: 1219-1225.
- Aherne G, English J, Marks V (1985) The Role of Immuno-assay in the Analysis of Micro-contaminants in Water Samples. Ecotox Environ Safe 9: 79-83
- Madsen SS, Mathiesen AB, Korsgaard B (1997) Effects of 17β-Estradiol and 4-nonylphenol on Smoltification and Vitellogenesis in Atlantic salmon (Salmo salar). Fish Physiol Biochem 1: 303-312.
- Saaristo M, Craft JA, Lehtonen KK, Lindstrom K. (2010) Exposure to 17α-Ethinyl estradiol Impairs Courtship and Aggressive Behaviour of Male Sand Gobies (Pomatoschistus minutus). Chemosphere 79: 541-546.
- Santos EM, Paull GC, Van-Look KJW, Workman VL, Holt WV, et al.( 2007) Gonadal Transcriptome Responses and Physiological Consequences of exposure to Eestrogen in Breeding Zebra fish (Danio rerio). Aquat Toxicol 83: 134-142.
- Lim R, Gale S, Doyle C. Endocrine disrupting compounds in sewage treatment plant (STP) effluent reused in agriculture: is there a concern?
- Shore LS, Correll DL, Chakraborty PK ( 1995) Animal Waste and the land-water Interface. Lewis Lub: Boca Raton, Florida P:155-162.
- Ying GG, Kookana RS, Ru YJ (2002) Occurrence and Fate of Hormone Steroids in the Environment. Environ Int 28: 545-551.
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP(1998) Widespread Sexual Disruption in Wild Fish Environ Sci Technol 32: 2498-2506.
- Jones-Lepp TL, Alvarez DA, Englert B, Batt AL ( 2006) Pharmaceuticals and Hormones in the Environment. In Encyclopedia of Analytical Chemistry; John Wiley and Sons; Meyers RA: 1-59.
- (2011) SCHER (Scientific Committee on Health and Environmental Risks), Opinion on draft environmental quality standards under the Water Framework Directive – Ethinylestradiol, 30 March 2011
- Zyndul A, Gillman S (2013) The Chemical Investigation Programme (CIP) Scottish Water Contribution Report 2013; Revision 2.
- Kuck H, Ballschmitter K ( 2001) Determination of Endocrine Disrupting Phenolic Compounds and Estrogens in Surface and Drinking Water Env Sci and Tech 35: 3201–3206.
- Belfoid A, Van der Horst A, Vethaak A, Schafer A, Rijs G, et al.( 1999) Analysis and Occurrence of Estrogenic Hormones and their Glucuronides in Surface Water and waste water in The Netherlands. Sci Total Environ 225:101-108.
- Patel D. (2011) Matrix Effect in a View of LC-MS/MS: An overview. Int J Pharma Bio Sci 2 (1): 559-564.
- Durve SJ, Naik S (2014) Matrix Effect - A Review. International Journal of Universal Pharmacy and Bio Sciences 2014, (3).
- Burham D, Price P, Rudewicz P (1996) Matrix Effects and LC-MS-MS. J Am Soc Mass Spectrom 7: 1099-1105.
Citation: Onwuchekwa C (2021) Evaluation of Micropollutant (17α- Ethinylestradiol; EE2) Content in Waste Water in the Built Environment: Differences due to Varying Building Uses and Occupiers. J Ecosys Ecograph 11:302.
Copyright: © 2021 Onwuchekwa C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1495
- [From(publication date): 0-2021 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 890
- PDF downloads: 605