Research Article Open Access
Evaluation of Intact Cell Matrix-Assisted Laser Desorption/Ionization Timeof-Flight Mass Spectrometry for Capillary Electrophoresis Detection of Controlled Bacterial Clumping
Pomastowski P1,2, Szultka M1,2, Kupczyk W3,2, Jackowski M3,2 and Buszewski B1,2*1Department of Environmental Chemistry and Bioanalytics, Faculty of Chemistry, Nicolaus Copernicus University, Poland
2Interdisciplinary Centre of Modern Technology, Nicolaus Copernicus University, Poland
3Department of Surgery, Collegium Medicum, Nicolaus Copernicus University, Poland
- *Corresponding Author:
- Buszewski B
Department of Environmental Chemistry and Bioanalytics
Faculty of Chemistry, Nicolaus Copernicus University
Gagarina 7, 87-100 Torun, Poland
Tel: +48566114308
Fax: +48566114837
E-mail: bbusz@chem.umk.pl
Received date: October 29, 2015; Accepted date: November 18, 2015; Published date: November 25, 2015
Citation: Pomastowski P, Szultka M, Kupczyk W, Jackowski M, Buszewski B (2015) Evaluation of Intact Cell Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for Capillary Electrophoresis Detection of Controlled Bacterial Clumping. J Anal Bioanal Tech S13:008. doi:10.4172/2155-9872.S13-008
Copyright: © 2015 Pomastowski P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been proposed as a technique of choice for quick classification of bacterial cells and precise characterization of microorganisms. The evaluated MALDI MS procedure was confronted with automated biochemical method using the VITEK 2 Compact system employed as a reference method. Moreover, the investigation aimed at describing and optimization of the environmental conditions having an impact on reproducibility and quality of one-dimensional intact-cell MALDI TOF spectra. The work presents off-line combination of capillary electrophoresis of microbial clumping with spectrometric detection.
Keywords
Identification of bacteria; Matrix-assisted laser desorption/Ionization mass spectrometry; Intact cell analysis; Capillary electrophoresis; Clumping
Introduction
One of the fundamental stages of microbial analysis is identification and characterization of microorganisms. In modern medicine early detection of pathogens provides an opportunity to determine the risk of neoplastic process in the infected organs, and to implement appropriate preventive and screening actions, which would minimize the risk of developing the disease [1]. In the case of food (e.g., dairy) and pharmaceutical industry, identification of microorganisms is an essential step of a technological process, which determines the quality of the manufactured product [2]. Furthermore, in medicine - detection of a disease in an early stage of development would allow to achieve better results of a treatment, in industry - identification of pathogens in early stages of a technological process saves money, production time and influences improvements in the decision-making processes [3]. Classical methods for identification of microorganisms commonly used in routine microbiological laboratories are based on a set of tests and analyses of biochemical properties of microorganisms, as well as on methods utilizing antigen-antibody reactions [4]. Recent years have brought increasing interest in automation of identification methods based on a study of biochemical characteristics of microorganisms, along with improvement of serological methods, causing a significant increase in the diversity of rapid identification tests [5]. Nevertheless, waiting for the result of identification is still long enough, and depending on the method and the analytical system, it ranges from 4-8 hours for automated methods to about 24 hours for semi-automatic ones [4,5]. Therefore, emphasis has been put on the search for new, precise and rapid methods of identification of microorganisms.
One of them is the use of capillary electrophoresis approaches for analysis of bacterial and yeast cells [6]. Unfortunately, this technique bases on conventional and restricted UV- or diode array detection (DAD) and requires long time for optimization of separation process [6,7]. Solving the problem of detection, raising selectivity and reducing the time to identify microorganisms is possible with the use of intact cell matrix-assisted laser-desorption/ionization (IC MALDI) time-of-flight mass spectrometry (TOF MS) method [1-5,8]. Spectrometric analysis of a sample with this method provides comprehensive information within a couple of minutes. In addition, one of the advantages of IC MALDI method is the fact that only one (single) colony is needed for microbiological analysis [1,8]. Moreover, some released protocols enable direct IC MALDI analysis of bacteria cells from infected blood [8] or milk [9]. This procedure is based on analysis of the unique protein profile of a microorganism, known as molecular fingerprint (MF) [1,9]. In this method, a pure culture of a microorganism is applied as a thin layer on a MALDI target. Then, solution of a matrix is deposited, mainly HCCA (α-cyano-4-hydroxycinnamic acid), DHB (dihydroxybenzoic acid), SA (synapic acid) dissolved in an organic solvent.
The matrix serves as a medium for extraction and crystallization of proteins (and other components such as lipopolysaccharides), mainly bacterial ribosomal proteins, which are present in the largest amount in microorganism cells [9,10]. During evaporation of the solvent the extracted bacterial proteins undergo co-crystallization with the matrix and thus formed crystals are subsequently subjected to pulsed laser beam irradiation. During this process the matrix crystals absorb highenergy photons of a specific wavelength which results in desorption and ionization of the matrix and desorption of protein molecules. In high vacuum of the ionization chamber the matrix protons collide with neutral molecules of proteins giving them a positive charge, so that protein and peptide molecules get ionized [1,11]. Positive ions of the matrix and the proteins are then accelerated in electrostatic field and move towards electrodes whose special design allows passage through the electrode. The rate of passage of ions through individual electrodes is inversely proportional to their mass and charge. After passing through the electrode, the ions migrate towards the detector, freely moving through the region, in which electrostatic field no longer operates (field-free drift region). The differences in molecular mass and charge cause that each molecule requires different time to reach the detector. The detector records the time of flight of ions and automatically generates a spectrum mainly corresponding to protein and peptide ions of different mass to charge ratio. The resulting spectrum of the tested strain is then compared with reference spectra of microorganisms, and automatically assigned to give identification of the organism [12,13].
Four commercial systems are mainly used in routine identification of microorganism, namely: BioTyper, SARAMIS, VITEK MS and Andromas, provided by Bruker, Shimadzu, bioMérieux and Andromas SAS, respectively [14-17]. Unfortunately, the cost of the software and the required licenses are often a limiting factor for a common use of these systems. Therefore, a homemade database (repository) can be developed and applied to targeted identification of microorganisms and as a complementary method to classical molecular or biochemical techniques. Moreover, a local repository of reference bacterial strains can also be used for statistic evaluation of one-dimensional IC MALDI spectra [18] or in a detection approach for separation analysis of microorganisms.
The aim of this study was evaluation of conditions influencing reproducibly and quality of bacterial spectra. Furthermore, the acquired spectra were to serve as a basis for a homemade repository. The investigation was to verify whether implementation of one-dimensional MALDI TOF MS system could be successful in identification and characterization of selected bacterial cells, also in the case of coupling of IC MALDI technique with capillary electrophoresis.
Materials and Methods
Chemicals
Acetonitrile (ACN), ethanol, trifluoroacetic acid (TFA), hydrochloric acid, sodium hydroxide solution, TRIS (tris(hydroxymethyl)aminomethane), boric acid (B) and calcium nitrate were purchased from Sigma Aldrich (Steinheim, Germany). Ultra-pure water from a Milli-Q water system (Millipore, Bedford, MS, USA) was used throughout the work.
All chemicals for MALDI-MS analyses were supplied at the highest commercially available purity from Fluka Feinchemikalien GmbH (part of Sigma Aldrich). Ground steel targets (Bruker Daltonik, Bremen, Germany) were used for sample deposition. α-cyano-4- hydroxycinnamic acid (HCCA), 2,5-dihydroxybenzoic acid (DHB) and sinapinic acid (SA) were employed as matrices for MALDI analysis of intact bacterial cells (dried droplet method), Bruker Bacterial Test Standard (BTS) were used for external calibration.
Culture media
Agar plates for bacterial growth were obtained from Pol-Aura, Dywity, Poland (Soybean Casein Digest Agar (SCDA), R2A Agar (R2A), Mueller Hinton (MH2), chocolate agar (CA), Schaedler Broth (SB)) and from bioMérieux, Warsaw Poland (Columbia CNA Agar supplemented with Colistin-Nalidixic Acid (COS), MacConkey Agar (MCK), Schaedler agar with 5% sheep blood (SCHE), SCDA).
Bacteria culture: The bacterial strains were used during investigations: Arthrobacter psychrolactophilus ATCC 700733, Klebsiella pneumoniae ATCC700603, Bacillus cereus ATCC 10876 and ATCC 13061, Bacillus subtilis ATCC 19659 and ATCC 11774, Escherichia coli ATCC 25922, ATCC 10536, Lactococcus lactis subsp. lactis ATCC 11454 and ATCC 19435, Micrococcus luteus ATCC 10240, Pseudomonas putida ATCC 31483 and Pseudomonas aeruginosa ATCC 27853 were obtained from Pol-Aura. Another strain of Lactococcus lactis subsp. lactis was obtained from the collection of the Department of Microbiology (Nicolaus Copernicus University, Torun, Poland).
Initially, the analyzed bacterial strains were cultivated at 37°C for 17 h on different solid (SCDA, R2A, MH2, CA, MCK, COS, SCHE) and liquid media (SB, TSB) to examine how different culture media influences the obtained MS spectra. In the next step, bacterial strains were cultivated at 37°C and seven different times of incubation: 12 h, 17 h, 24 h, 36 h, 48 h, 72 h and 96 h to control the effect of time on the composition and quality ICM MS spectra. After each incubation period increment of CFU (colony forming unit) was determined and additionally OD600 (optical density measured spectrophotometrically at 600 nm) was examined in the case of liquid media. Bacterial colonies were identified using Vitek 2 Compact system, according to the manufacturer’s instructions, with identification cards GP (Gram positive bacterial identification), GN (Gram negative bacterial identification) and ANC (anaerobic bacteria and coryneform bacteria identification) taking into account cultural, morphological and biochemical criteria.
Spectrometric analysis of bacteria: Initially, bacterial colonies were smeared on a MALDI target as a thin layer and overlaid with 1.0 μl of matrix solution: DHB (50 mg/mL), HCCA (10 mg/mL) or SA (20 mg/mL) in a solvent mixture (EtOH/ACN/H2O, 1:1:1 (v/v/v) to examine the impact of the kind of a matrix on recorded spectra [16]. The TFA solution was added to solvent mixture to 0.1-2.5% v/v final concentrations. Since the obtained results were not satisfactory, the part of the protocol was modified. Bacterial samples were transferred from agar plate to the above-mentioned acidified solvent mixture so that OD600 of the final bacterial suspension ranged between 0.04-0.6 (109-1012 cells per mL). In order to evaluate how this change affects the amount of cells per spot, the bacterial suspension was diluted with the solvent mixture at 1:1, 1:10, 1:100, 1:1000 and 1:10 000.
Intact cell mass spectrometric analysis was conducted with the use of ultrafleXtreme MALDI-TOF/TOF mass spectrometer (Bruker Daltonik, Bremen, Germany) equipped with a modified Nd:YAG laser (smartbeam II™) operating at the wavelength of 355 nm and the frequency of 2 kHz. ICM MS spectra of were recorded in linear positive mode within m/z range of 300-30 000 and applying the acceleration voltage of 25 kV. All mass spectra were acquired and processed with the dedicated software: flexControl and flexAnalysis, respectively (both from Bruker). Cluster analysis with Ward’s method and Euclidean distances of the obtained ICM MS spectra, as well as verification of the indicated characteristic peaks was performed with the use of Statistica software (StatSoft, Krakow, Poland).
Electrophoresis of microbial clumping
Bacterial cells were rinsed with water twice, then suspended in 0.005M Ca(NO3)2 solution in order to modify the surface charges, and after 1 h the pellet with bacterial cells was washed again to remove free Ca2+aq ions [19]. The final bacterial sample was suspended in an outlet buffer. OD600 of the bacterial suspension was estimated between 0.04- 0.6.
Capillary electrophoresis (CE) experiments were performed with an HP3DCE system (Agilent Technologies, Waldbronn, Germany) equipped with DAD and fused silica capillaries (id=75 μm; Ltot=33.5 cm; Leff=25 cm; Composite Metal Services, Shipley, UK). New capillaries were rinsed before use with 1.0M NaOH, deionized water, and BGE (background electrophoretic buffer) for 10 min each. The electrophoretic analysis was performed in a nonlinear system, namely: outlet buffer: TB (CTRIS=4.5 mM, CB=50 mM, pH 8.3), inlet buffer: TB-HCl (CTRIS=4.5 mM, CB=50 mM, CHCl=4.4 pH 7.15), Imax=100 μA, U=15 kV, t=23°C, λ=214 nm, and injection in the pressure mode at 50 mbar for 25 s. Between runs, the capillaries were washed with 1.0M NaOH, deionized water - for 2 min each, and a running buffer for 4 min. A total volume of 0.5 mL stock bacterial suspension was used for electrophoretic measurements [19]. The focused fractions of bacterial clumping were collected in CE-MS mode, diluted and transferred to a MALDI target according to the above-mentioned procedure.
Results and Discussion
Spectrometric evaluation of environmental conditions
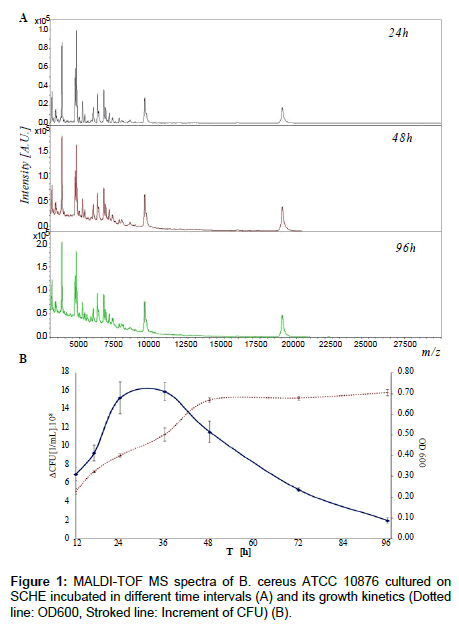
Initially, all 13 bacterial strains were cultured on various solid agar plates and two kinds of liquid media, at 37°C for 17 h in aerobic conditions to demonstrate the impact of different media (dedicated or not to culture examined microbial strain) on quality of IC MALDI spectra of cultured bacterial cell. It was observed that the best growth was reached in the case of Schaedler media, both agar and liquid, for all the examined microbial strains. Although Schaedler media are intended for cultivation of obligate anaerobe or strict anaerobe, the examined bacteria also exhibited stable, reproducible and fast growth. After optimization of the growth conditions the bacterial species were cultured for different time intervals: 12 h, 17 h, 24 h, 36 h, 48 h, 72 h and 96 h, in solid and liquid medium. After that, increment of CFU and OD600 was measured for all bacteria species which were cultured on SCDA and SCHE media (Figure 1B). The examined bacterial strains exhibited three stages of growth. The first phase, with the incubation time between 0-24 ± 12 h, was the growth stage - microbial cells were viable and growing. The second stage (24-36 ± 12 h) was the stationary (plateau) phase, and the last one was the death phase (48-96 h). For the last two stages the cells were non-growing and viable, and non-growing, non-viable, respectively [20]. IC MALDI spectra were registered for all bacterial colonies incubated in various incubation time intervals (Figure 1A).
It was shown for all bacterial species that various incubation time intervals influenced quality of the registered spectra. No major qualitative changes in peak patterns were detected, however increase of signal intensity over time (100% per 24 h) and drift of baseline were observed. This results directly from the current stage of microbial growth [1,2,21]. In the plateau and the death phase degradation of some enzyme complexes is initiated, and in the case of e.g., Bacillus species in the plateau phase, a spore is formed [1,20]. Qualitative hanges in the composition of cells translates directly into the quality of the registered spectrum. The obtained results are in accordance with previous studies [1,2,21]. In the case of the culture of E. coli or S. aureus it is possible to register reproducible and good quality spectra between the time intervals of 5-48 h [21]. On the other hand, in routine IC MALDI identification of microorganisms it is necessary to keep the time of bacterial cell incubation the same as in the case of reference bacteria. Otherwise, it may result in misidentification of bacterial species [1-7,14-17].
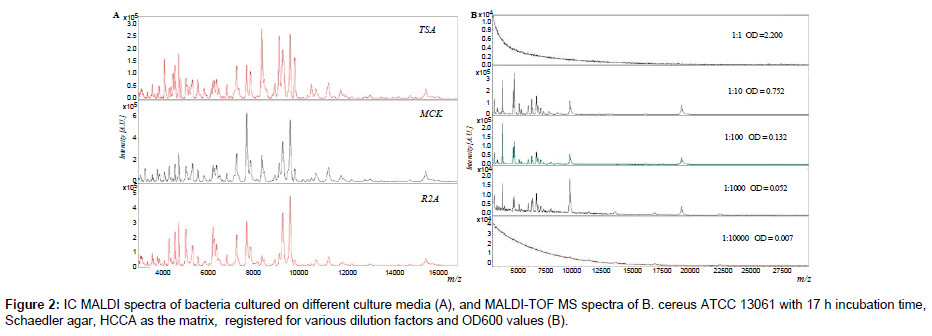
Initially, spectra were recorded for bacterial colonies coming from agar plates and liquid media deposited directly on a MALDI target. It was observed that IC MALDI spectra of the bacteria cultured in liquid media exhibited better quality, reproducibility and intensity. The spectra registered for smeared colonies from diverse agar plates were relatively irreproducible and many peaks originating from media themselves were observed. In order to improve the reproducibility and to eliminate the medium effect, each bacterial colony was placed in a solvent mixture and then onto a MALDI target. The obtained spectra were more intensive, reproducible and the signals coming from culture media were eliminated. Subsequently, influence of culture media on the quality of the obtained IC MALDI spectra was tested (Figure 2A).
It was shown that MALDI spectra of bacteria cultured on different agar plates were mutually consistent and only few peaks altered in intensity or were missing. Subsequently, the number of cells per spot was optimized (Figure 2B). This criterion is crucial in evaluation of IC MALDI spectra [1,2]. The optimal OD600 value of all analyzed bacterial suspensions was in the range of 0.80-0.04. These values represent 105- 109 cells per spot. Too small amount of microbial cells (less than 104) is insufficient to generate a spectrum, while in the case of excessive amount of cells (greater than 1010), it is impossible to reach desorption and ionization of bacterial components, and hence to register a spectrum [2,22]. It has been shown that one loopful of bacterial cells per spot, or 1 mL of suspended solution (e.g., E. coli, E. faecalis), is sufficient for registering good quality spectra [1,8]. In the case of IC MALDI analysis of bacteria cell taken directly from a biological sample (blood, milk) the minimal value is 103-105 cells per μL [8,9].
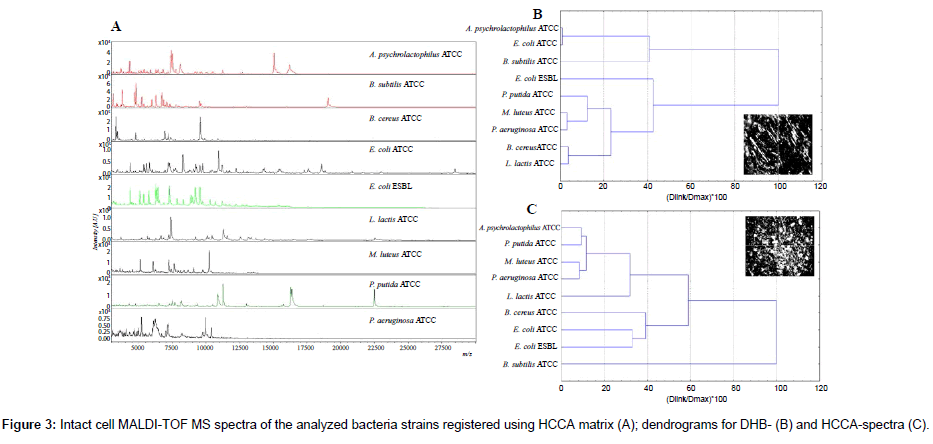
In this investigation IC MALDI spectra were registered using various matrices, namely: SA, HCCA and DHB. The worst results were obtained for sinapinic acid. Application of HCCA and DHB gave reproducible spectra with high resolution and stable ionization (Figure 3A).
In screening analysis of microbial cells the use of these two matrices is common [23-32]. However, the spectra recorded with different matrices had different distribution of peaks (Figure 3B, 3C). Spectra recorded using DHB as a matrix (Figure 3B) were richer in signals in the case of analysis of Bacillus sp. and L. latcis. Worse results were reported for HCCA spectra (Figure 3A) of Gram-positive bacteria. Peptidoglycan is the main component of cell walls of these bacteria. Probably disruption of cell walls during the laser ablation and ionization process was hindered by the multi-layer nature of peptidoglycan [14]. Therefore, it was impossible to ionize a sufficient number of proteins. DHB matrix has been reported to facilitate cell wall disruption and protein extraction [1,14,27-32].
The presented dendrograms exhibit different relations between various IC MALDI spectra registered using various matrix (Figure 3B, 3C). In the case DHB-based spectra the highest similarity was reported for A. psychrolactophilus and E. coli, M. luteus and P. aeruginosa, B. cereus and L. lacis. However, in the case of cluster analysis of HCCA-based spectra, the highest similarity was observed for A. psychrolactophilus and P. putida, M. luteus and P. aeruginosa. Nevertheless, HCCA spectra exhibited more significant differences between similar clusters. This may result from different nature of the two matrices [1]. DHB is characterized with large needles (Figure 3B), whereas HCCA forms fine crystals (Figure 3C). The different nature of crystallization process results in different extraction mechanism of bacterial components. The signals obtained for ICM measurement were mostly of ribosomal-protein origin [25-28]. Circa 50% of the peaks of all bacteria unambiguously represented basic ribosomal proteins [1]. The rest are cold-shock proteins, DNA-binding proteins and outermembrane lipoproteins [14]. Therefore, DHB will be the most favorable matrix for the analysis of lipoproteins, whereas in the case of intact cell measurements of DNA-binding proteins the use of HCCA will result in a higher number of signals [14,20,25-29]. The similarities of spectra are not correlated in genetic genetic origin of phylogenetic trees.
Identification of selected bacterial cells
In this study 13 different bacterial species, 510 various independent biological samples (8 replicates for each sample) were examined. All IC MALDI results of the tested bacterial stains were confirmed directly by classical standard microbiological testing using biochemical identification system (Vitek, bioMérieux) (Table 1).
| Bacterial strain | VITEK 2 | IC-MALDI TOF MS | CE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Match (P,%) | Mismatch | n | Match (P,%) |
Mismatch | Characteristic signals ± SD (m/z) | n | T ± SD (min) |
|
| Arthrobacter psychrolactophilus ATCC 700733 | 5 | 4(80) | 1 | 40 | 39(98) | 1 | 7393 ± 2, 15056 ± 3, 16202 ± 3 | 10 | 1.88 ± 0.09 |
| Bacillus cereus ATCC 10876 Bacillus cereus ATCC 13061 |
4 4 |
4(100) 4(100) |
0 0 |
50 40 |
50(100) 40 (100) |
0 0 |
4774 ± 2, 6939 ± 3, 9563 ± 3, 13630 ± 2 | 10 | 2.20 ± 0.03 |
| Bacillus subtilis ATCC 19659 Bacillus subtilis ATCC 11774 |
4 4 |
4(100) 4(100) |
0 0 |
50 50 |
50(100) 50(100) |
0 0 |
3799 ± 3, 9532 ± 3, 19070 ± 5, 19734 ± 1 | 10 | 2.02 ± 0.04 |
| Escherichia coli ATCC 25922 Escherichia coli ATCC 10536 |
5 5 |
5(100) 4(80) |
0 0 |
30 30 |
30(100) 30(100) |
0 0 |
3323 ± 2, 7695 ± 7, 10451 ± 7, 18576 ± 6, 20867 ± 2, | 10 | 3.21 ± 0.05 |
| Klebsiella pneumoniae ATCC700603 | 5 | 5(100) | 0 | 40 | 40(100) | 0 | 4363 ± 2, 6258 ± 4, 9545 ± 3 | 10 | 3.56 ± 0.06 |
| Lactococcus lactis subsp. lactis ATCC 11454 Lactococcus lactis subsp. lactis ATCC 19435 |
3 3 |
2(67) 3(100) |
0 0 |
30 30 |
30(100) 30(100) |
0 0 |
6632 ± 4, 7352 ± 6, 11256 ± 5, 22550 ± 2 | 10 | 2.36 ± 0.03 |
| Micrococcus luteus ATCC 10240 | 3 | 3(100) | 0 | 30 | 30(100) | 0 | 5104 ± 2, 7237 ± 4, 10225 ± 4 | 10 | 2.45 ± 0.04 |
| Pseudomonas putida ATCC 31483 | 3 | 2(67) | 0 | 30 | 30(100) | 0 | 16284 ± 6, 22511 ± 3, 10485 ± 2 | 10 | 3.74 ± 0.03 |
| Pseudomonas aeruginosa - ATCC 27853 | 3 | 3(100) | 0 | 30 | 30(100) | 0 | 5203 ± 3, 7173 ± 5, 9967 ± 6 | 10 | 3.69 ± 0.07 |
n-number of identification; P-percentage of success identification (mach/n*100%), t-electromigration time
Table 1: Identification of selected bacterial cells using biochemical and ICM MS methods.
Reference spectra were registered for all bacterial species. Then, the species were randomly selected for identification based on comparison of characteristic peak patterns and characteristic signal. The characteristic peaks were indicated using U-Mann-Whitney test (UMW) - one of the nonparametric test for independent samples. It is often used by researchers for statistical evaluation of spectrometric data [22]. The experimental data did not meet the assumptions of the Student’s t test and therefore this test could not be applied. Characteristic peaks were identified via independent comparison within a group. Table 1 summarizes the values of m/z which met the criteria of UMW test.
The obtained results of spectrometric identification of microbial species were in agreement with the ones of the biochemical method. Nevertheless, the spectrometric procedure is faster, more precise and efficient. In the case of biochemical analysis, the time and the use of expensive identification cards were the limiting factors. Moreover, spectrometric analysis was performed in a larger number of repetitions, due to lower cost of a single analysis, shorter time and easiness of preparation.
Capillary electrophoresis of microbial clumping with IC MALDI detection
According to the electrokinetic theory, the charge on a surface, determined by the characteristic properties of the membrane components (e.g., proteins, lipopolysaccharides), influences the behavior of microorganisms in the electric field [7]. This phenomenon determines electrophoretic mobility, which enables separation of bacteria cells (biocolloids) [6]. However, analysis of such complex systems is related to a number of problems, such as uncontrolled clumping (aggregation) and/or adhesion to the inner surface of a capillary. Another problem is detection of microbes. Common UV and DAD system do not allow for obtaining specific spectra that would enable a satisfactory identification of separated bacterial cells. The obtained spectra do not provide sufficient information about the aggregation of microorganisms [7]. Moreover, conventional linear buffer system is not effective in this case.
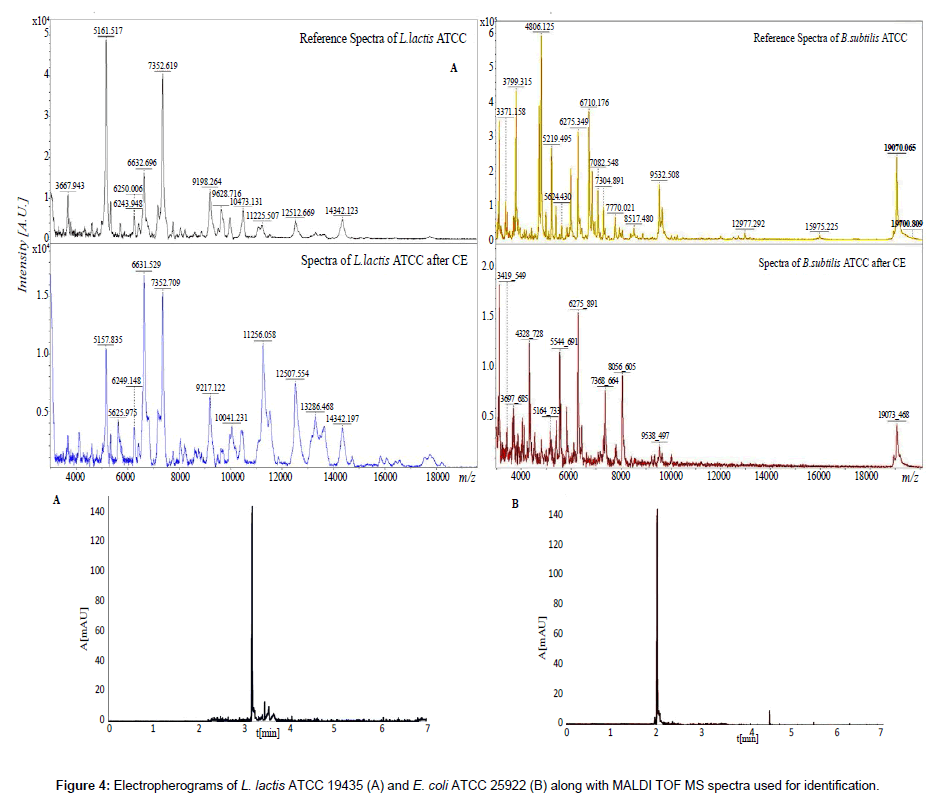
In the presented study a specially designed homemade databases of intact cell spectra were used for development of a method of capillary electrophoresis separation of microbial clumping coupled with IC MALDI identification. Figure 4 presents the example electropherograms and spectra of L. lactis and E. coli.
The spectra of reference species were in agreement with the ones obtained after electrophoretic separation. The characteristic spectrometrics peak of the focused bacterial cells was consistent with the reference values. The observed small increase and decrease of the signal intensity and modification of some signals, result from sorption of calcium ions onto the proteins [14,33]. Modification of bacteria with calcium ions determines the change in their electrophoretic mobility and reduces repulsive forces. This results in creation of controlled clumping and signal amplification [19]. Application of buffers with different ions mobilities (an isotachophoretic mode) and without bacterial surface modification with Ca2+ allowed focusing the zones [19]. Therefore, it was possible to collect focused microbial cells and to detect their IC MALDI spectra [34]. The electrophoretic analysis was reproducible, as evidenced by the low standard deviation of electromigration time (Table 1). Moreover, it was shown the higher electromigration time of analyzed Gram-negative in comparison with Gram-positive bacterial cells (Table 1). It probably results from higher zeta potential value of Gram-positive bacteria in used buffer system and as a consequences weaker interaction with deprotonated silanol groups [7]. The performed investigation proves that application of CE-MALDI TOF MS approach may be useful in quantitative analysis of controlled clumping in routine bioanalysis of bacterial cells.
Conclusion
One-dimensional IC MALDI is has recently found application as a fast and alternative method of microbial identification to classical biochemical methods. An important advantage of ICM MS is very short time of analysis and the possibility of full identification of the microorganisms of interest. However, this strategy requires thorough evaluation of environmental conditions having strong impact on reproducibly of fingerprint spectra. Repeatability of ICM MS spectra largely depends on the type of the matrix, the incubation temperature and the amount of cells per spot. The kind of a culture medium does not influence significantly the quality of spectra. Apart from signal matching, searching for specific biomarkers of a given species is an effective method of microbial identification. MALDI TOF MS has proven to be a promising detection system for capillary electrophoresis of bacterial clumping.
Acknowledgements
This work was supported by NCU 2352-Ch and Symfonia I grant No. 2013/08/W/N28/0070, Maestro-6, Preludium grants No.: 2013/11/N/ST4/01835 and 2012/07/N/ST4/01856 from the National Science Centre, Poland, as well as by grants from the European Social Found, the Polish National Budget and the budget of the Kujawsko-Pomorskie Region as a part of the “Krok w przyszlosc V” (Step into the Future V) Sectoral Operational Program - Human Resources, 2013-2015.
References
- Mellmann A, Cloud J, Maier T, Keckevoet U, Ramminger I, et al. (2008) Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J Clin Microbiol 46: 1946-1954.
- Cherkaoui A, Hibbs J, Emonet S, Tangomo M, Girard M, et al. (2010) Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J Clin Microbiol 48: 1169-1175.
- Marklein G, Josten M, Klanke U, Müller E, Horré R, et al. (2009) Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J Clin Microbiol 47: 2912-2917.
- Smieja M, Mahony JB, Goldsmith CH, Chong S, Petrich A, et al. (2001) Replicate PCR testing and probit analysis for detection and quantitation of Chlamydia pneumoniae in clinical specimens. J Clin Microbiol 39: 1796-1801.
- Clarridge JE 3rd (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 17: 840-862.
- Armstrong DW, Schneiderheinze JM, Kullman JP, He L (2001) Rapid CE microbial assays for consumer products that contain active bacteria. FEMS Microbiol Lett 194: 33-37.
- Buszewski B, Szumski M, Klodzinska E, Dahm H (2003) Separation of bacteria by capillary electrophoresis. J Sep Sci 26: 1045-1049.
- Stevenson LG, Drake SK, Murray PR (2010) Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 48: 444-447.
- Barreiro JR, Braga PA, Ferreira CR, Kostrzewa M, Maier T, et al. (2012) Nonculture-based identification of bacteria in milk by protein fingerprinting. Proteomics 12: 2739-2745.
- Ilina EN, Borovskaya AD, Malakhova MM, Vereshchagin VA, Kubanova AA, et al. (2009) Direct bacterial profiling by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for identification of pathogenic Neisseria. J Mol Diagn 11: 75-86.
- Nagy E, Maier T, Urban E, Terhes G, Kostrzewa M (2009) ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Species identification of clinical isolates of Bacteroides by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. Clin Microbiol Infect 15: 796-802.
- Couturier MR, Mehinovic E, Croft AC, Fisher MA (2011) Identification of HACEK clinical isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49: 1104-1106.
- Saffert RT, Cunningham SA, Ihde SM, Jobe KE, Mandrekar J, et al. (2011) Comparison of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometer to BD Phoenix automated microbiology system for identification of gram-negative bacilli. J Clin Microbiol 49: 887-892.
- Krásny L, Hyneka R, Hochela I (2013) Responses to glycemic control therapy according to age, gender, level of adiposity, and duration of diabetes in type 2 diabetic patients. IJMS 67: 61-69.
- Sogawa K, Watanabe M, Sato K, Segawa S, Ishii C, et al. (2011) Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganisms. Anal Bioanal Chem 400: 1905-1911.
- Lohmann C, Sabou M, Moussaoui W, Prévost G, Delarbre JM, et al. (2013) Comparison between the Biflex III-Biotyper and the Axima-SARAMIS systems for yeast identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51: 1231-1236.
- Marko DC, Saffert RT, Cunningham SA, Hyman J, Walsh J, et al. (2012) Evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of nonfermenting gram-negative bacilli isolated from cultures from cystic fibrosis patients. J Clin Microbiol 50: 2034-2039.
- Sauer S, Freiwald A, Maier T, Kube M, Reinhardt R, et al. (2008) Classification and identification of bacteria by mass spectrometry and computational analysis. PLoS One 3: e2843.
- Dziubakiewicz E, Buszewski B (2014) Capillary electrophoresis of microbial aggregates. Electrophoresis 35: 1160-1164.
- Kearns DB, Losick R (2005) Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19: 3083-3094.
- Reich M, Bosshard PP, Stark M, Beyser K, Borgmann S (2013) Species Identification of Bacteria and Fungi from Solid and Liquid Culture Media by MALDI-TOF Mass Spectrometry. J Bacterio Parasitol S5: 002.
- Vlek AL, Bonten MJ, Boel CH (2012) Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7: e32589.
- Inglis TJ, Healy PE, Fremlin LJ, Golledge CL (2012) Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis for rapid confirmation of Burkholderia pseudomallei in septicemic melioidosis. Am J Trop Med Hyg 86: 1039-1042.
- Hu Y, He LH, Xiao D, Liu GD, Gu YX, et al. (2012) Bacterial flora concurrent with Helicobacter pylori in the stomach of patients with upper gastrointestinal diseases. World J Gastroenterol 18: 1257-1261.
- Dridi B, Raoult D, Drancourt M (2012) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of Archaea: towards the universal identification of living organisms. APMIS 120: 85-91.
- Bessède E, Solecki O, Sifré E, Labadi L, Mégraud F (2011) Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin Microbiol Infect 17: 1735-1739.
- El Khéchine A, Couderc C, Flaudrops C, Raoult D, Drancourt M (2011) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS One 6: e24720.
- Hrabák J, Walková R, Studentová V, Chudácková E, Bergerová T (2011) Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49: 3222-3227.
- He Y, Chang TC, Li H, Shi G, Tang YW (2011) Matrix-assisted laser desorption ionization time-of-flight mass spectrometry and database for identification of Legionella species. Can J Microbiol 57: 533-538.
- Grosse-Herrenthey A, Maier T, Gessler F, Schaumann R, Böhnel H, et al. (2008) Challenging the problem of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Anaerobe 14: 242-249.
- Salplachta J, Kubesová A, Moravcová D, Vykydalová M, Süle S, et al. (2013) Use of electrophoretic techniques and MALDI-TOF MS for rapid and reliable characterization of bacteria: analysis of intact cells, cell lysates, and "washed pellets". Anal Bioanal Chem 405: 3165-3175.
- Pennanec X, Dufour A, Haras D, Réhel K (2010) A quick and easy method to identify bacteria by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 24: 384-392.
- Pomastowski P, Sprynskyy M, Buszewski B (2014) The study of zinc ions binding to casein. Colloids Surf B Biointerfaces 120: 21-27.
- Dieckmann R, Graeber I, Kaesler I, Szewzyk U, von Döhren H (2005) Rapid screening and dereplication of bacterial isolates from marine sponges of the sula ridge by intact-cell-MALDI-TOF mass spectrometry (ICM-MS). Appl Microbiol Biotechnol 67: 539-548.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 11436
- [From(publication date):
specialissue-2015 - Nov 22, 2024] - Breakdown by view type
- HTML page views : 10683
- PDF downloads : 753