Evaluation of Fluoride-18-Labeled Boronophenylalanine-Positron Emission Tomography Imaging for the Assessment of Boron Neutron Capture Therapy in Patients with Recurrent Head and Neck Squamous Cell Carcinoma
Received: 25-Oct-2016 / Accepted Date: 03-Nov-2016 / Published Date: 10-Nov-2016 DOI: 10.4172/2161-119X.1000277
Abstract
Introduction: A role for fluoride-18-labeled boronophenylalanine positron emission tomography (18FBPA-PET) in boron neutron capture therapy (BNCT) has not been fully elucidated. We investigated the role of 18FBPA-PET in BNCT for recurrent head and neck squamous cell carcinoma (HNSCC) patients. Materials and methods: 18FBPA-PET images were obtained from 10 histologically verified recurrent HNSCC patients who received BNCT. The intratumoral accumulation of 18FBPA was calculated as the ratio of maximum and minimum radioactivity counts to that of normal tissue (Tmax/N and Tmin/N ratios, respectively). The percentage volume at which the radioactivity count ratio was >2.5 compared to normal tissue was also calculated (Vo2.5). Moreover, mean and minimum irradiation dose to the tumor was calculated. We investigated which parameters could predict the treatment effect of BNCT. Results: Treatment effects of local lesions were as follows: complete remission (CR) in 5 cases and non-CR in 5 cases. Only Tmin/N ratio showed a significant difference between the CR and non-CR groups (P=0.008). Conclusion: The Tmin/N ratio of 18FBPA-PET can predict the treatment effect of BNCT for recurrent HNSCC.
Keywords: Recurrence head and neck SCC; BNCT; 18FBPA-PET; T/N ratio; 10B
259177Introduction
Boron neutron capture therapy (BNCT) is a type of high linear energy transfer radiation therapy. High irradiation doses can be selectively delivered to tumor cells without causing serious damage to surrounding normal tissue, due to high accumulation of 10B in the tumor compared to adjacent normal tissue. The results of clinical trials of BNCT that used L-10B-para-boronophenylalanine (L-BPA) as a boron delivery agent have been reported for the treatment of recurrent head and neck cancers [1-4].
The actual irradiation dose to the tumor during BNCT is determined by the intratumoral 10B concentration and the neutron flux to the tumor [5,6]. Since a fairly constant neutron flux is achieved in the irradiation field, the tumor irradiation dose can be largely determined from the intratumoral 10B concentration. Thus, estimation of 10B concentrations is required. Less invasive methods for measuring 10B concentration did not exist 2 decades ago. Therefore, using surgical specimens from malignant melanomas, Mishima and Fukuda et al. measured 10B concentrations directly to estimate the tumor/ normal tissue ratio (T/N ratio) [7,8].
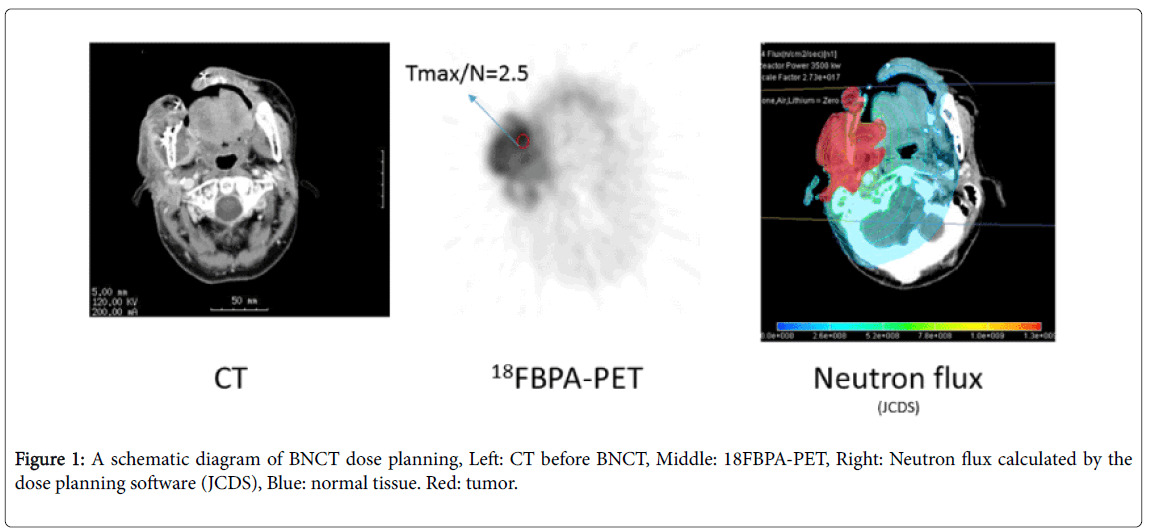
In 1998, Imahori et al. reported a less invasive method for estimating intratumoral 10B concentrations using positron emission tomography (PET) [9] and calculation of the T/N ratio of 10Bcontaining compounds using PET has since become the standard BNCT planning method. The tumor irradiation dose is calculated from the neutron flux and the T/N ratio of 18FBPA-PET (Figure 1). Clinically, a 18FBPA-PET T/N ratio of ≥ 2.5 is generally considered a requirement for use of BNCT in head and neck cancer [2,10]. This value was established to ensure that tumors with a depth ≥ 6 cm will receive >20 Gray equivalent (GyE), which is necessary for tumor control, while surrounding normal tissue (primarily skin) receives a tolerable irradiation dose [11].
Although the utility of the T/N ratio has been comprehensively reported in some clinical studies, actual intratumoral accumulation of 18FBPA can fluctuate depending on cell density and tumor cell activity [12] and this value is inhomogeneous in tumor tissue. Moreover, radiosensitivity differs for each pathologic tumor type. In this study, we investigated whether 18FBPA accumulation that has potentially inhomogeneous distribution in tumors can predict the treatment effect of BNCT for head and neck squamous cell carcinoma (HNSCC).
Patients and Methods
Study design
This study was a retrospective, single-institution review of HNSCC patients treated with BNCT.
Patients
We previously reported 20 recurrent head and neck cancer patients treated with BNCT [2]. We selected 10 of these 20 patients with squamous cell carcinoma for the present analysis; the remaining 10 patients with other pathologies were excluded, as their tumors had different degrees of radiosensitivity. Characteristics of the selected 10 cases are presented in Table 1. Patients included 8 men and 2 women, with a median age at time of treatment of 62.5 years (range, 39 to 77 years). Primary lesion sites were the oropharynx in 5 cases, tongue in 2 cases, and nasal ala, oral floor and larynx in 1 case each. All patients were undergoing third-line or later treatment, and had already received 40-66 Gy (median, 62.5 Gy) of radiotherapy prior to BNCT. The clinical stage was rN2a in 5 cases, rT4 in 3 cases and rT2 in 2 cases at the time of treatment based on the Union for International Cancer Control Disease Stage Classification (VIIth Edition).
| No. | Primary site | Age | Sex | Histology | TNM (Recurrence) | TNM (Initial) | Initial treatment | Salvage treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Oropharynx | 73 | M | SCC | rT2 | T1N2bM0 | CCRT | Chemo |
| 2 | Lingual | 39 | F | SCC | rT4 | T2N0M0 | CCRT | Op |
| 3 | Nasal ala | 71 | M | SCC | rN2a | T2N0M0 | OP | CCRT, Chemo |
| 4 | Oropharynx | 77 | M | SCC | rT2 | T1N2bM0 | CCRT | Op |
| 5 | Oropharynx | 73 | M | SCC | rT4 | T4aN2bM0 | CCRT | Op |
| 6 | Larynx | 64 | M | SCC | rN2a | T2N0M0 | CCRT | OP, Chemo |
| 7 | Oral floor | 57 | M | SCC | rT4 | T1N0M0 | OP+RT | Chemo |
| 8 | Oropharynx | 39 | F | SCC | rN2a | T2N2bM0 | CCRT | Chemo, 3DRT |
| 9 | Lingual | 45 | M | SCC | rN2a | T2N1M0 | Op+RT | Chemo |
| 10 | Oropharynx | 61 | M | SCC | rN2a | T2N2bM0 | CCRT | Chemo |
| Abbreviations: | SCC: Squamous Cell Carcinoma; CCRT: Concurrent Chemoradiotherapy; Op: Operation; Chemo: Chemotherapy; 3DRT: Three Dimensionsradiotherapy | |||||||
(Kawasaki Medical School IRB approval number 40).
Table 1: Patient and tumor characteristics.
(Kawasaki Medical School IRB approval number 40).
Quantitative measurement of PET data
18FBPA-PET scans were performed at Nishijin Hospital, Kyoto, Japan. BPA was synthesized as described previously [13,14]; the protocol for PET measurements using a HEADTOME III (Shimadzu Co., Kyoto, Japan) has also been described elsewhere [8,15,16]. We used PETViewer 2.0 (software.informer.com) and Amide software (SourceForge, Inc.) for data analysis.
Parameters
We placed a rectangular region of interest (ROI) (size: 4 pixels, 1 pixel=2 × 2 mm) on tumor and normal tissue. The left ventricle was designated as normal tissue. The contralateral cervical artery was also substituted for normal tissue in patients whose PET scans only evaluated the head and neck regions. The highest ROI count was defined as the Tmax, while the lowest was defined as the Tmin. The ratios of the Tmax or Tmin to the ROI count of normal tissue were calculated as the Tmax/N and Tmin/N ratios, respectively. The percentage intratumor volume with a T/N ratio ≥ 2.5 was defined as Vo2.5. All ROIs were drawn by the same physician, who has >13 years of experience in PET analysis.
Mean and minimum irradiation doses to the tumor were calculated by the BNCT planning system (mean tumor dose and minimum tumor dose, respectively).
Examinations
Patients were divided into two groups based on local treatment effect: the complete remission (CR) and the non-CR groups. The Tmax/N ratio, Tmin/N ratio, Vo2.5, mean tumor dose and minimum tumor dose were compared between the CR and non-CR groups.
Statistical analysis
Student’s t test was used for statistical analysis. A P-value <0.05 was considered statistically significant.
Results
All results are shown in Table 2. Of the 10 patients, recurrences consisted of local recurrence in 5 patients (2 of whom also had distant metastasis), regional recurrence in 2 patients and distant metastasis in 1 patient. Based on local treatment effect, 5 patients were assigned to the CR group and the remaining 5 were assigned to the non-CR group. Survival times ranged from 3 to 20 months; all patients died within 20 months. The 1 year survival rate was 20% and median survival was 10.5 months. Causes of death were local recurrence in 5 cases, distant metastasis in 3 cases and other (carotid blowout syndrome [CBS]) in 2 cases. (We have previously reported these 2 CBS deaths [17]).
| No. | Tmax/N | Tmin/N | Vo2.5 (%) | GTV (cm3) | Tumor mean dose (Gy-Eq) | Tumor minimum dose (Gy-Eq) | Tumor Response | Follow up (M) | Failure | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 1.9 | 83 | 12 | 18.9 | 12.4 | NC | 19 | Local | Local |
| 2 | 5 | 1.2 | 92 | 52 | 45.9 | 32.7 | PR | 11 | Local+Distant | Distant |
| 3 | 2.6 | 2.2 | 95 | 7 | 21.6 | 13.4 | NC | 12 | Local+Distant | Distant |
| 4 | 4 | 2.6 | 100 | 15 | 52 | 25.3 | CR | 20 | Distant | Distant |
| 5 | 5 | 1.9 | 97 | 24 | 54.6 | 25.6 | PR | 11 | Local | Local |
| 6 | 3.8 | 3.5 | 100 | 25 | 38.2 | 20.1 | CR | 7 | (-) | CBS |
| 7 | 2.5 | 2.1 | 73 | 43 | 55.2 | 36.5 | PR | 10 | Local | Local |
| 8 | 2.9 | 2.3 | 87 | 16 | 53.6 | 25 | CR | 7 | Local(out of field) | Local |
| 9 | 4.2 | 3.5 | 100 | 23 | 62.9 | 29.3 | CR | 6 | Local(out of field) | Local |
| 10 | 2.6 | 2.3 | 78 | 36 | 54.3 | 25.3 | CR | 3 | (-) | CBS |
| Abbreviations: | CR: Complete Remission; PR: Partial Remission; NC: No Change; CBS: Carotid Blowout Syndrome | |||||||||
Table 2: Dose evaluation after BNCT and Clinical results of all patients.
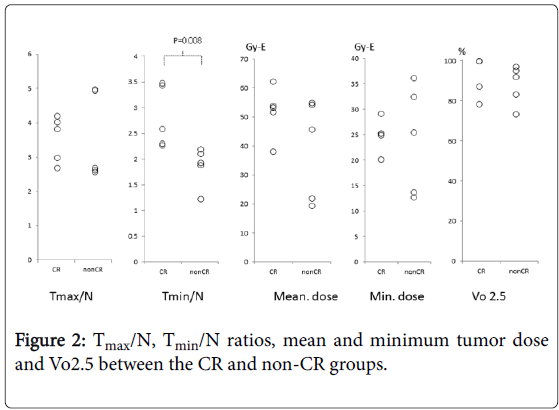
In the CR group, the Tmax/N ratio ranged from 2.6 to 4.2 (median, 3.8) and the Tmin/N ratio ranged from 2.3 to 3.5 (median, 2.6). Vo2.5 ranged from 78% to 100% (median, 100%). Mean tumor dose ranged from 38.2 to 62.9 (median, 53.6) GyE and minimum tumor dose ranged from 20.1 to 29.3 (median, 25.3) GyE.
In the non-CR group, the Tmax/N ratio ranged from 2.5 to 5 (median, 2.6) and the Tmin/N ratio ranged from 1.2 to 2.2 (median, 1.9). Vo2.5 ranged from 73% to 97% (median, 92%). Mean tumor dose ranged from 18.9 to 55.2 (median, 45.9) GyE and minimum tumor dose ranged from 12.4 to 36.5 (median, 25.6) GyE.
Of these five parameters, a statistically significant difference between the CR and non-CR groups was only noted for the Tmin/N ratio (Figure 2, P=0.008).
Discussion
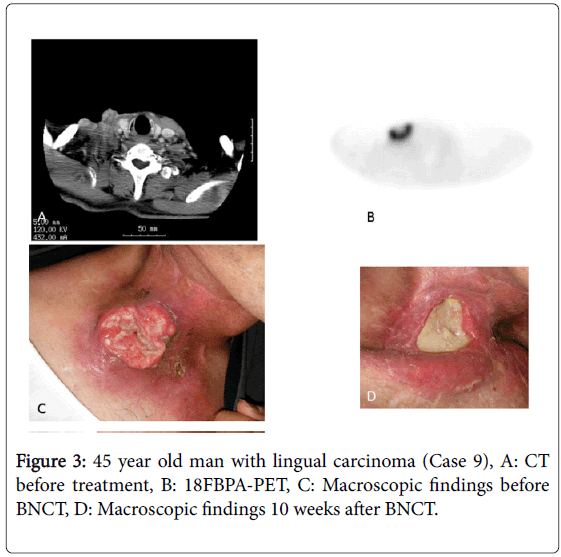
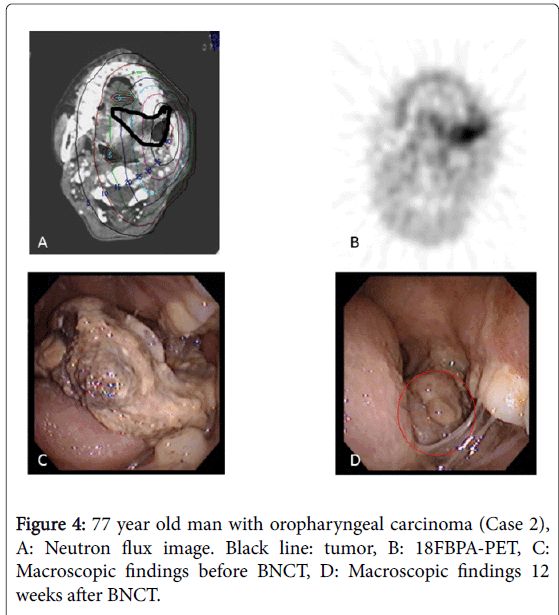
In our institute, the BNCT irradiation dose for head and neck cancers is determined using the T/N ratio of 18FBPA-PET in the following manner: (1) skin dose ≤ 18 GyE and (2) tumor dose ≥ 20 GyE. Biologically, the effect of the boron neutron capture reaction does not differ much in normal tissue between individuals [18], but largely differs in tumor tissues. As shown in this manuscript, the only parameter that predicted treatment effect was the Tmin/N ratio (P=0.008); none of the other parameters appeared to be associated with treatment effect. We consider that in patients with high Tmin/N ratios, a sufficient dose could be administered to the large tumor volume, so satisfactory local treatment effect was achieved. In contrast, in patients with low Tmin/N ratios, the volume to which a sufficient irradiation dose was not delivered was large, residual tumor cells survived and the patients failed to achieve a CR. For example, the tumor disappeared 10 weeks after BNCT in a patient with a high Tmin/N ratio (3.5) and a high Tmax/N ratio (5; Case 9, Figure 3). In contrast, the tumor remained 12 weeks after BNCT in a patient with a low Tmin/N ratio (1.2). The tumor irradiation dose was calculated as 32.7 GyE based on the Tmax/N value of 5 at the actual BNCT planning and 18FBPA accumulation varied largely in the tumor in this patient (Case 2, Figure 4).
The actual tumor irradiation dose is less than the calculated dose, based on the value of the Tmax/N ratio. In particular, in cases in which the difference between the Tmax/N and Tmin/N ratios is large, large discrepancies in BNCT treatment effects may exist. In fact, the calculated irradiation dose in the area with the lowest 18FBPA accumulation was 25-85% of the highest in the 5 non-CR cases. Of 5 non-CR patients, the total amount of neutron flux was determined by the normal skin irradiation dose in 1 patient and the tumor irradiation dose in 4 patients. In those 4 patients, the tumor irradiation dose was calculated to be 12.4-32.7 GyE using the Tmax/N ratio for actual BNCT and the skin irradiation dose was 4-9 GyE. The minimum tumor irradiation dose was only 7.8-11.4 GyE based on our simulation using the Tmin/N ratio. Considering the skin irradiation dose was much lower than the possible tolerated dose, a higher treatment effect could be obtained if the Tmin/N ratio had been used for dose calculation.
Some issues regarding the use of 18FBPA results for BNCT planning remain to be resolved. First, whether 18FBPA accumulation correctly reflects BPA distribution during BNCT remains controversial. Mitsuyoshi et al. demonstrated that 18FBPA was taken up into cells via the same amino acid transporter L-system as BPA in human glioblastoma cells [19]. 18FBPA and BPA showed almost identical pharmacokinetics in the body in our clinical study (unpublished data). Thus, we have used 18FBPA-PET in BNCT planning as an indicator of 10B concentration. Second, it is difficult to reflect the inhomogeneous 10B intratumoral concentration with the current BNCT dose planning system (SERA [5] and JCDS [6]). We anticipate the development of a BNCT planning system that can support inhomogeneous 18FBPA accumulation in the tumor. Alternatively, we propose use of the Tmin/N ratio rather than the Tmax/N ratio to increase the treatment effect of BNCT.
The source of neutrons in Japan has been the long-employed nuclear reactors; however, BNCT accelerators that can provide an alternative to nuclear reactors are currently under development [20]. Detailed analysis of previous clinical cases is required to properly administer next-generation BNCT using accelerators. We hope such endeavors will lead to the development of highly effective head and neck cancer treatment. This is the first study in which the results of 18FBPA-PET imaging have been shown to predict a treatment effect of BNCT against recurrent HNSCC.
Conclusion
The Tmin/N ratio of 18FBPA-PET is a useful parameter for predicting the BNCT treatment effect in patients with recurrent HNSCC.
Acknowledgement
This work was supported by JSPS Grant-in-Aid for Scientific Research (C) Number 15K10797.
Conflicts of Interest
None to declare.
References
- Kato I, Ono K, Sakurai Y, Ohmae M, Maruhashi A, et al. (2004) Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot 61: 1069-1073.
- Aihara T, Morita N, Kamitani N, Kumada H, Ono K, et al. (2014) Boron neutron capture therapy for advanced salivary gland carcinoma in head and neck. Int J Clin Oncol 19: 437-444.
- Kankaanranta L, Koivunoro H, Seppälä T, Saarilahti K, Atula T, et al. (2007) Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int J Radiat Oncol Biol Phys 69: 475-482.
- Haapaniemi A, Kankaanranta L, Saat R, Koivunoro H, Saarilahti K, et al. (2016) Boron Neutron Capture Therapy in the Treatment of Recurrent Laryngeal Cancer. Int J Radiat Oncol Biol Phys 95: 404-410.
- Nigg DW, Wessol DE, Wemple CA, Wheeler FJ (1999) SERA an advanced treatment planning system for neutron therapy and BNCT. Trans ANS 80: 66-68.
- Kumada H, Yamamoto K, Yamamoto T, Nakai K, Nakagawa Y, et al. (2004) Improvement of dose calculation accuracy for BNCT dosimetry by the multi-voxel method in JCDS. Appl Radiat Isot 61: 1045-1050.
- Mishima Y (1973) Neutron capture treatment of malignant melanoma using 10B-chlorpromazine. Pigment Cell Res 1: 215-221.
- Fukuda H, Honda C, Wadabayashi N, Kobayashi T, Yoshino K, et al. (1999) Pharmacokinetics of 10B-p-boronophenylalanine in tumours, skin and blood of melanoma patients: A study of boron neutron capture therapy for malignant melanoma. Melanoma Res 9: 75-83.
- Imahori Y, Ueda S, Ohmori Y, Kusuki T, Ono K, et al. (1998) Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med 39: 325-333.
- Aihara T, Morita N, Kamitani N, Kumada H, Ono K, et al. (2014) Boron neutron capture therapy for advanced salivary gland carcinoma in head and neck. Int J Clin Oncol 19: 437-444.
- Aihara T, Hiratsuka J, Morita N, Uno M, Sakurai Y, et al. (2006) First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 28: 850-855.
- Tani H, Kurihara H, Hiroi K, Honda N, Yoshimoto M, et al. (2014) Correlation of (18)F-BPA and (18)F-FDG uptake in head and neck cancers. Radiother Oncol 113: 193-197.
- Ishiwata K, Ido T, Mejia AA, Ichihashi M, Mishima Y (1991) Synthesis and radiation dosimetry of 4-borono-2-[18F]fluoro-DL-phenylalanine: a target compound for PET and boron neutron capture therapy. Int J Rad Appl Instrum 42: 325-328.
- Mishima Y, Imahori Y, Honda C, Hiratsuka J, Ueda S, et al. (1997) In vivo diagnosis of human malignant melanoma with positron emission tomography using specific melanoma-seeking 18F-DOPA analogue. J Neurooncol 33: 163-169.
- Takahashi Y, Imahori Y, Mineura K (2003) Prognostic and therapeutic indicator of fluoroboronophenylalanine positron emission tomography in patients with gliomas. Clin Cancer Res 9: 5888-5895.
- Miyashita M, Miyatake S, Imahori Y, Yokoyama K, Kawabata S, et al. (2008) Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol 89: 239-246.
- Aihara T, Hiratsuka J, Ishikawa H, Kumada H, Ohnishi K, et al. (2015) Fatal carotid blowout syndrome after BNCT for head and neck cancers. Appl Radiat Isot 106: 202-206.
- Ono K (2016) An analysis of the structure of the compound biological effectiveness factor. J Radiat Res 57 Suppl 1: i83-83i89.
- Yoshimoito M, Kirihata H, Honda N, Kawai K, Ohe K, et al. (2013) Predominant contribution of L-type amino acid transporter to 4-borono-2-18F-fluoro-phenylalanine uptake in human glioblastoma cells. Nucl Med Biol 40: 625-629.
- Kumada H, Kurihara T, Yoshioka M, Kobayashi H, Matsumoto H, et al. (2015) Development of beryllium-based neutron target system with three-layer structure for accelerator-based neutron source for boron neutron capture therapy. Appl Radiat Isot 106: 78-83.
Citation: Aihara T, Hiratsuka J, Fukumitsu N, Ishikawa H, Morita N, et al. (2016) Evaluation of Fluoride-18-Labeled Boronophenylalanine-Positron Emission Tomography Imaging for the Assessment of Boron Neutron Capture Therapy in Patients with Recurrent Head and Neck Squamous Cell Carcinoma. Otolaryngol (Sunnyvale) 6:277. DOI: 10.4172/2161-119X.1000277
Copyright: ©2016 Aihara T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5132
- [From(publication date): 0-2016 - Apr 08, 2025]
- Breakdown by view type
- HTML page views: 4163
- PDF downloads: 969