Research Article Open Access
Evaluation of Factors Impacting Agrobacterium-Mediated Indica Rice Transformation of IR58025B - a Public Maintainer Line
| Huaping Gui, Xia Li, Yubo Liu and Xianggan Li* | |
| Syngenta Biotechnology China Co, Ltd 25 Life Science Park Road, Changping District, Beijing 102206, P.R. China | |
| *Corresponding Author : | Xianggan Li Syngenta Biotechnology China Co Ltd 25 Life Science Park Road, Changping District Beijing 102206. P. R. China Tel: +86 1080735909 E-mail: Xianggan.Li@syngenta.com |
| Received: February 01, 2016 Accepted: February 15, 2016 Published: February 20, 2016 | |
| Citation: Gui H, Li X, Liu Y, Li X (2016) Evaluation of Factors Impacting Agrobacterium-mediated Indica Rice Transformation of IR58025B - a Public Maintainer Line. J Rice Res 4:163. doi:10.4172/2375-4338.1000163 | |
| Copyright: © 2016 Gui H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Rice Research: Open Access
Abstract
n this study, we report our efforts to develop an efficient Agrobacterium-mediated transformation protocol for the transformation of Indica rice IR58025B with mannose as a selection agent. Various factors, such as basal medium, culture temperature, strains for infection, air-drying duration of calli after infection, and salt concentration of coculture medium, were systematically optimized so that the highest transformation frequency (TF) reached to 74%. About 94% of mature embryos were induced to generate calli of high quality on modified NB medium. Both callus quantity and quality were improved when callus was induced from endosperm-free embryos in comparison to intact mature seeds. Higher temperature (32°C) showed improvements when compared to lower temperatures at (25°C and 28°C) for both callus induction and transformation. Agrobacterium strains of EHA105 produced the best TF in comparison with EHA101, LBA4404 and AGL1. However, LBA4404 produced the highest single copy frequency. Prior to co-cultivation, air-drying of inoculated calli was performed in a laminar flow hood for 4-7 hours and produced a higher TF. Lower salt concentration for co-cultivation medium enhanced both DNA delivery and TF.
| Keywords |
| Agrobacterium-mediated transformation; Air-drying; Culture temperature; Co-transformation |
| Abbreviations |
| PMI: Phospho Mannose Isomerase; TF: Transformation Frequency; COC: Co-Cultivation Medium; T-DNA: Transfer DNA |
| Introduction |
| Rice is one of the most important cereal food crops in the world and Indica-type rice provides the staple food for more than half of the world’s population (http://faostat.fao.org/site/339/default.aspx). In spite of its large scale production, a number of abiotic and biotic factors have limited its productivity. To meet the growing demands of an ever-increasing global population, genetic transformation of Indica rice, particularly for public breeding materials, has become a matter of increased urgency. Many factors are known to affect the efficiency of T-DNA delivery to plant cells, such as different plant varieties [1,2], different explant types [3-5], the quality of explants and Agrobacterium strains [6], the cell density of Agrobacterium for infection, inoculation period, co-culture medium and desiccation stress of the tissue before or after infection [6-9]. |
| Indica rice is still known as recalcitrant for transformation although Japonica rice was transformed using the Agrobacteriummediated method since 1994 [10] and Indica rice in 1996 [11]. Few reports on the Agrobacterium-mediated transformation of IR58025B are available [12]. This public cultivar is commonly applied to generate hybrids by breeders in the south Asia since it has higher combining ability and generates good hybrids. Therefore, there is a need to evaluate various factors that affect tissue culture and to develop a highly efficient Agrobacterium-mediated transformation protocol for this public cultivar. Hiei et al. [13] reported a protocol for Group I Indica rice transformation including IR58025B using immature embryos as explants. Our study mainly focused on callus explants derived from mature seeds and systematically optimized several factors that could influence transformation frequency. These factors include the basal medium for generating high quality calli, the optimized tissue culture temperature, different strains, desiccation after infection, different salt concentration in co-cultivation (COC) media, and the size of transformation constructs. By optimizing these factors, a highly efficient and reproducible Agrobacterium-mediated transformation protocol was developed for Indica rice-IR58025B, a public maintainer line used in hybrid rice production. |
| Materials and Methods |
| Callus induction |
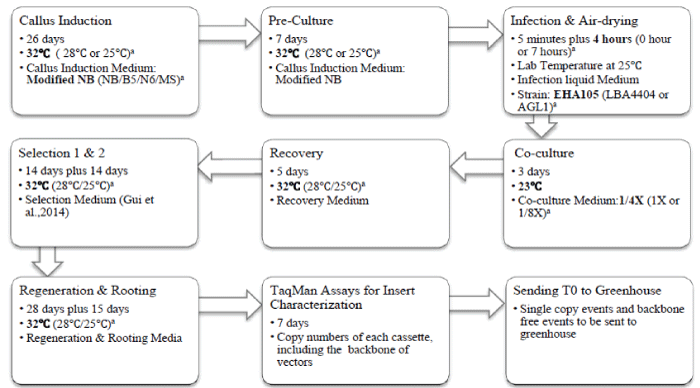
| Tissue culture was performed as previously reported by our lab [14,15]. De-husked mature seeds of IR58025B were surface sterilized, soaked in autoclaved water for 4-6 hours at room temperature (28°C) before endosperms were removed with a scalpel blade. Embryos were cultured on callus induction medium with a scutellum side contacting the callus induction medium. Rice embryos were cultured at 32°C unless specified otherwise. |
| Transformation method |
| Transformation procedures were performed as previously reported [14]. PMI-09 (maize gene codon optimized pmi) driven by the maize Ubiquitin promoter was used in all of the transformation experiments with the exception of the strain comparison experiments in which PMI- 01 (native pmi gene from E. coli) driven by the rice Actin promoter was used [14]. Embyogenic calli were inoculated with the Agrobacterium tumefaciens strain EHA105, LBA4404 and AGL1 respectively in those experiments to compare the three strains. After infection with Agrobacterium, the calli were placed onto an empty plate with 3 pieces of dry filter paper on the bottom and a piece of dry filter paper on the top of infected calli for 0, 4, 7 hours respectively. The petri dishes were covered with the plate lid during air-drying in a hood. After air-drying for 0-7 hours, the calli were transferred to the co-culture medium. Three types of co-culture media were tested; normal basal medium, 1/4 salts level and 1/8 salts level medium. All the calli on the co-culture media were cultured at 23°C for Agrobacterium infection. The infected calli were divided into three groups and maintained at 25°C, 28°C, and 32°C throughout all of the following transformation steps: recovery, selection, regeneration, and rooting (Figure 1). |
| Callus induction and COC media with treatments are introduced in corresponding experiments. The recovery medium is similar to the COC medium with following differences: 300 mg/l instead of 800 mg/ lcasamino acids; 30 g/l instead of 20 g/l maltose; 3 g/l Phytagel to replace 8 g/l agarose; removal of 10 g/l glucose and 20 mg/l acetosyringone, with an additional 500 mg/l proline. The mannose selection pressure is the same as described in our previous report [14]. The shoot regeneration medium is similar to NBKA reported by Duan et al. [15] with the following modifications: 1 mg/l kinetin to replace 0.5 mg/l Kinetin, 200 mg/l Timentin to replace 250 mg/l Carbenicillin, and 3 g/l Phytagel to replace 2 g/l Phytagel, 20 g/l maltose and 5 g/l mannose to replaced 30 g/l sucrose and 30 g/l sorbitol. The rooting medium consisted of ½ MS basal salts and vitamins, MS iron, 30 g/l sucrose, and 4 g/l Phytagel. Figure 1 outlines our optimized process, with current standard operation conditions in bold and treatments in parenthesis. |
| Co- transformation |
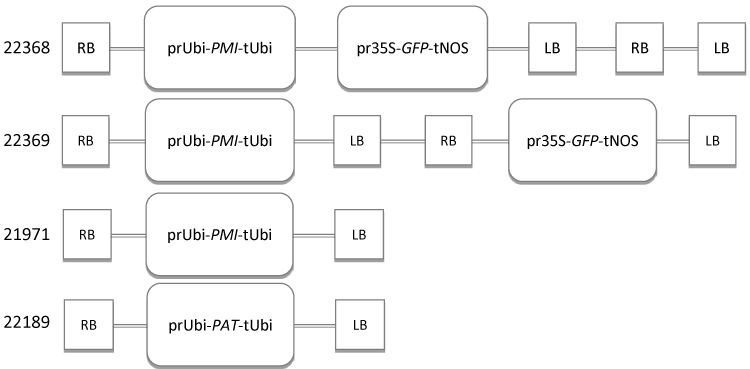
| The map of vectors used in co-transformation is shown in Figure 2. The vector of 22189 is transformed with 4 mg/l bialaphos in the selection medium. OD660 of Agrobacterium suspension for 22189 and 21971 was adjusted to approximately 0.1, respectively, and then mixed together for co-transformation (Figure 2). |
| Analysis method |
| The TaqMan analysis was performed as previously reported by Gui et al. [14], using a relative quantification analysis to get the copy number of transgenic plants against a standard curve. Fam was used as the reporter of target genes and TET as the reporter of internal control and TAMRA was used as the quencher for either the target genes or the internal control. |
| PMI: Primer-forward: CCGGGTGAATCAGCGTTT |
| Primer-reverse: GCCGTGGCCTTTGACAGT |
| Probe: TGCCGCCAACGAATCACCGG |
| PAT: Primer-forward: TGGAAGCTCGAAATCTCTTTGC; |
| Primer-reverse: AACTCTTAGAGCTGCTGGATATAAGCA; |
| Probe: AGAATCCAACATCATGCCATCCTCCATG |
| GFP: Primer-forward: TTGCGCTGCCAGTTCGA |
| Primer-reverse: AAGTGCCAGTCGGGCATCT |
| Probe: CCGTGTACAAGGCCAAGTCCGTGC |
| JMP statistical discovery software was used for statistical analysis. |
| Results |
| Callus quality affected by different media and explant types |
| Five types of basal media were tested: N6 [16], B5 [17], MS [18], NB (combination of N6 macronutrient components and B5 micronutrient and B5 organic components), and modified NB (with 10 times B5 micronutrient) (Figure 3). Based on the visible assessment of callus appearance, such as colour, texture and size, modified NB medium was observed as the best medium for callus induction of this cultivar. Calli grown on modified NB medium appeared to be of better quality as dry, yellowish white in colour, compact in structure and globular in shape (as shown with the red arrows in Figure 3A). Also, more calli were shown to be generated in modified NB medium than that induced on other basal salts. The other four kinds of media produced fewer embyogenic calli (Figure 3B-E) with poor quality as being watery, frequently turning brown and necrotic (as shown with the blue triangles in Figure 3B). Explant type is also a very important factor in the transformation of this line. The intact mature seed without removal of the endosperm generated a reduced number of embryogenic calli (Figure 3F) and introduced more contamination. Average callus induction frequency for mature embryos without endosperm indicated the highest callus induction frequency from modified NB medium and the least from B5 medium (Table 1). |
| Higher temperature for callus growth and transformation |
| Mature embryos without endosperm were cultured on modified NB medium at the three temperatures, 25°C, 28°C, and 32°C. For Treatment 32°C, embryogenic calli grew to about 2 mm in diameter after 26 days and were ready for sub-culture. To yield a similar size of callus to that at 32°C, it required 29 days at Treatment 28°C and 33 days at Treatment 25°C. More calli with higher quality were produced at higher temperature, averaging five embyogenic calli generated from each mature embryo at 32°C. Less embyogenic calli were generated at lower temperatures, with an average of three embyogenic calli grown from each embryo at 28°C and on average, less than one embyogenic callus produced at 25°C. |
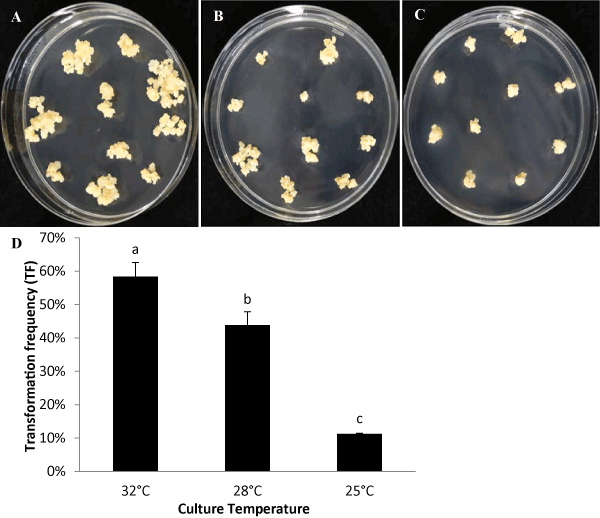
| In addition, we compared the impact of culture temperatures on the following-up transformation steps after co-cultivation with Agrobacterium. The same callus lines induced at 32°C were infected with Agrobacterium and co-cultivated at 23°C for 3 days. Then, these infected calli were divided and grown at three different culture temperatures, 32°C, 28°C, and 25°C, throughout all remaining four transformation steps (recovery, selection, regeneration and rooting). The results show Treatment at 32°C generates the most resistant transgenic calli after the selection step, where the calli grow quicker and the whole transformation process requires about 86 days from infection to T0 plants ready for greenhouse. Treatment at 28°C requires 2 weeks longer than that at 32°C. The calli at Treatment at 25°C grew very slow and required 5 weeks longer than that at 32°C treatment. Treatments at 32°C, 28°C, and 25°C produces the TFs at 58%, 44%, and 11% respectively (Figure 4). |
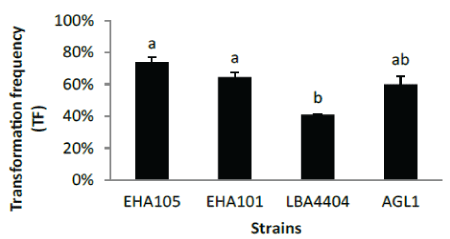
| Strains tested for transformation of IR58025B |
| The transformation frequency of four Agrobacterium strains, EHA105, EHA101, LBA4404 and AGL1 were compared. EHA105 and EHA101 perform better than LBA4404 on transformation frequency. Considering the quality of transgenic events (single copy frequency)(Figure 5), LBA4404 is the best among four strains, as high as 59.5% single copy frequency, significantly higher than other three strains. Backbone free frequency is similar, around 60% for all four strains. The single copy frequency is defined as the percentage of single copy events among total positive events produced (Table 2). |
| Impact of air-drying treatment on TF |
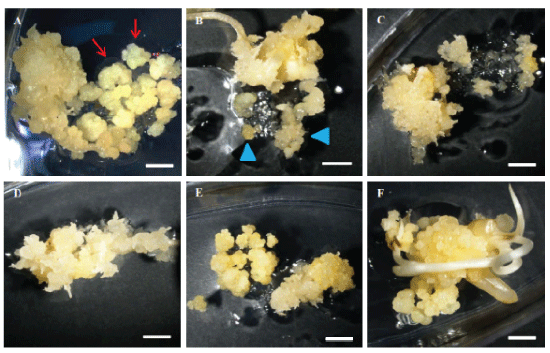
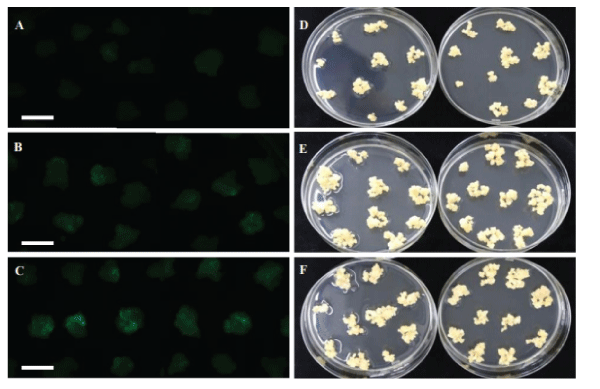
| The rice calli were collected in a tube and mixed with Agrobacterium suspension culture for infection for 5 minutes. After infection, the liquid Agrobacterium solution was drained and then the infected calli were placed on COC medium for co-culturing. Different airdrying durations were tested immediately after infection and before co-cultivation. Air-drying for 4 or 7 hours in a laminar air flow hood improves TF to 62%, in contrast to poor TF of 46% without air-drying. Figure 6 displays the treatments of 4 and 7 hours air-drying, leading to more transiently expressing green fluorescence protein (GFP) cells (Figure 6B and 6C) in contrast to the zero hour (Figure 6A) and more resistant calli on selection medium (Figure 6E and 6F) in comparison with the zero hour (Figure 6D). We concluded that a longer air-drying time of 4-7 hours could benefit Agrobacterium infection compared to no air-drying treatment (Figure 6). |
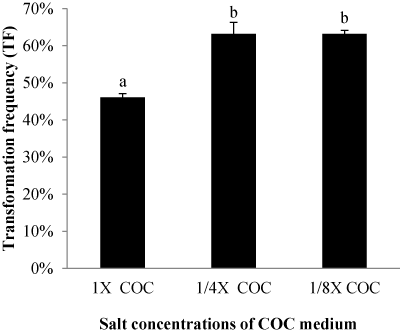
| Effect of different salt concentrations of COC medium |
| We evaluated different salt concentrations in the COC medium. The normal salt concentration of basal medium (N6 macronutrients, B5 micronutrients, MS iron salts, and B5 organic components) in the COC medium was kept as 1X COC or reduced to 1/4 (1/4X COC) or 1/8 (1/8X COC). Other components as 500 mg/l glutamine; 800 mg/l casamino Acids; 20 g/l maltose; 10 g/l glucose; 2 mg/l 2, 4-D; 8 g/l agarose; 20 mg/l acetosyringone were kept the same. GFP expression indicated that lower salt concentrations of COC medium enhanced T-DNA delivery. Calli exposed to either 1/4 COC or 1/8 COC produce a higher TF of 63% than that in the normal 1X salt concentration (Figure 7). |
| Co-transformation efficiency |
| Marker free transformation is preferred since the selectable marker is not a trait but a tool in the transformation process. By co-transformation, marker-free transgenic plants are obtained by segregation in next generation due to two T-DNAs insertions which may integrate into different positions at different chromosomes. Co-transformation of 2 T-DNAs in one Agrobacterium strain or 2 T-DNAs in two different Agrobacterium strains was designed for generating marker free events. The co-TF was compared for 2 T-DNAs co-transformation (22369 in Table 3) vs. 2 Agrobacterium strains co-transformation (using PMI selection), and the 2 T-DNAs cotransformation was more efficient than the 2 strains co-transformation (Table 3). |
| Discussion |
| To establish an efficient Agrobacterium-mediated transformation system, high quality callus is a prerequisite and so choosing the best callus induction medium is one of the most important factors. Hiei et al. [19] demonstrated that the induction of embryogenic calli with the potential for higher rates of cell division is an important factor in determining transformation frequency. The commonly used basal medium for tissue culture is MS, N6, B5 and NB medium. Different plant species and genotypes require different kinds and amounts of nutrients, even requiring specific nutrient elements during specific stages [20]. The number of common medium is limited for our process; therefore, we decided to make some modifications based on the commonly used media for the specific variety. |
| In this study, the minor salts based on the NB medium were modified according to the report by Lin and Zhang [20] and resulted in enhanced callus proliferation and generated more embryogenic calli for this cultivar. As for the isolated mature embryos performing better than the intact mature seeds for callus induction, Lin et al. [21] considered that a regulation system or buffer action may exist in the mature rice seeds, which may protect the embryo from de-differentiating into calli if the endosperm was not removed. Removal of endosperms can break the buffer system so that embryos are more exposed to the culture medium, especially the growth hormone. Furthermore, improved quality and quantity of calli can be produced. |
| Temperature is one of the key factors which affect the growth rate of calli. A temperature of 32°C was deployed to be optimal for callus growth of this rice line. Higher temperature not only increases the callus induction and proliferation but also improves the quality of calli in comparison with lower temperature. However, temperatures higher than 32°C are not suitable for callus growth of this maintainer line (data not shown). Calli of higher quality can be infected by Agrobacterium more efficiently. It can also withstand the infection, recover better and produce a higher TF [22]. Air-drying treatment of calli after the Agrobacterium infection can improve the transformation frequency. We found that the air-drying of the calli after infection can significantly suppress the over growth of Agrobacterium, and reduce contamination of Agrobacterium. This process may protect the calli from the injury during Agrobacterium infection. Another possible explanation for improved TF is that air-drying may cause cell plasmolysis so that the T-DNA delivery is enhanced as well [23]. |
| It has been reported that the use of low salt media during the infection and co-cultivation can enhance T-DNA delivery in many species such as maize [24,25], canola [7], and wheat [26]. In 2012, Tie et al. [27] reported that Agrobacterium infection appeared to have both up-regulatory and down-regulatory effects on thousands of genes. Fewer nutrients with starvation may lead to calli stress and trigger biochemical changes which can lead the plant cells to be more susceptible to Agrobacterium infection. |
| In a summary, an efficient Agrobacterium-mediated transformation protocol for the transformation of Indica rice IR58025B was developed. The modified NB medium was the best basal medium for induction with about 94% induction rate from mature embryos. Both callus quantity and quality were improved when callus was induced from endosperm-free embryos in comparison to intact mature seeds. Higher temperature (32°C) showed improvements when compared to lower temperatures at (25°C and 28°C) for both callus induction and transformation. Agrobacterium strains of EHA105 produced the best TF in comparison with other strains. Prior a higher TF Lower salt concentration for co-cultivation medium enhanced both DNA delivery and TF. |
| Acknowledgement |
| We are indebted to Erik Dunder, Thad Cherry and Qiudeng Que for providing the project closure report on “Indica Rice Transformation Technology Development” and “Rice Transformation Protocol”. Thanks to Wenqian Mei for earlier efforts on the Indica rice transformation system development. Thanks for all the support from Trait Research Groups, Plant Analysis Group, and Greenhouse Group at SBC. We particularly thank the Rice Insect Control Project Team for keeping timely communications and patient support during this endeavour. Additionally, we thank all support, suggestions and encouragement from Media and Glassware lab, Soybean, Corn, and Special transformation teams. The help of Erik Dunder, Sivamani Elumalai, and other colleagues for their internal reviews and comments is also greatly appreciated. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 11834
- [From(publication date):
February-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10757
- PDF downloads : 1077
