Research Article Open Access
Evaluation of Effect of Ethanol Extract of Gongronema latifolium and Piper guineense on Lipid Profile in Ethanol Intoxicated Wistar Rats
| Ali Fredrick U1*, UA Ibiam2, Ominyi MC1, N Wankwo OVU1, Ogbanshi ME2 and Eze US3 | |
| 1Department of Biotechnology, Ebonyi State University, Abakaliki, Nigeria | |
| 2Department of Biochemistry, Ebonyi State University, Abakaliki, Nigeria | |
| 3Biology Department Ebonyi State College of Education, Ikwo, Nigeria | |
| Corresponding Author : | Ali Fredrick Department of Biotechnology Ebonyi State University, Abakaliki, Nigeria Tel: +2348063893022 E-mail: alifredrick13@gmail.com |
| Received: April 13, 2015; Accepted: July 15, 2015; Published: July 22, 2015 | |
| Citation: Ali Fredrick U, Ibiam UA, Ominyi MC, N Wankwo OVU, Ogbanshi ME, et al. (2015) Evaluation of Effect of Ethanol Extract of Gongronema latifolium and Piper guineense on Lipid Profile in Ethanol Intoxicated Wistar Rats. Biochem Physiol 4:171.doi:10.4172/2168-9652.1000171 | |
| Copyright: © 2015 Ali Fredrick U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Aim: The study was carried out to investigate effects of administration ethanol leaf extract of Gongronema latifolium and Piper guineense on lipid profile parameters against ethanol – induced hepatic injury in rats.
Method: Forty male albino rats (weighing 150-220 g) were used. The animals were divided into four groups A, B, C and D with C and D subdivided into four subgroups; C1, C2, C3 and C4; D1, D2, D3 and D4 for G. latifolium and Piper guineense respectively, each contained four rats. Group A and B served as positive and negative controls, group B, subgroup C and D were exposed with 70% ethanol for 7 days to induce liver damage and later treated group C and D with ethanol extracts for 21 days while 0.9% normal saline were administered to the controls. The sub-groups were administered 200, 400, 600 and 800 mg kg-1 b.wt. of ethanol extract of G. latifoliujm and Piper guineense leaf, respectively. After 21 days blood sample were collected and the serum were used to evaluate lipid profile spectrophotometrically.
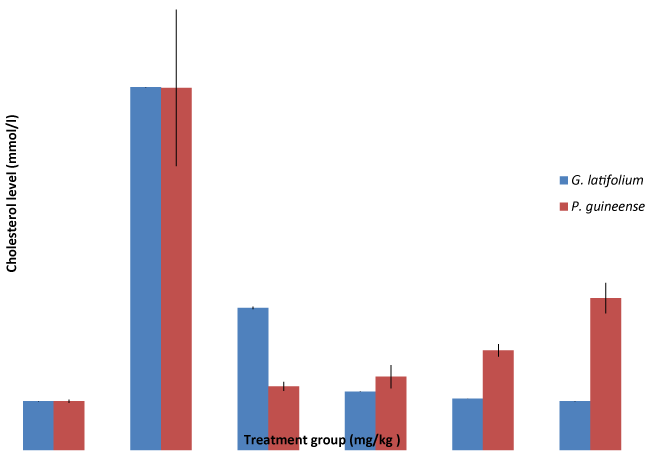
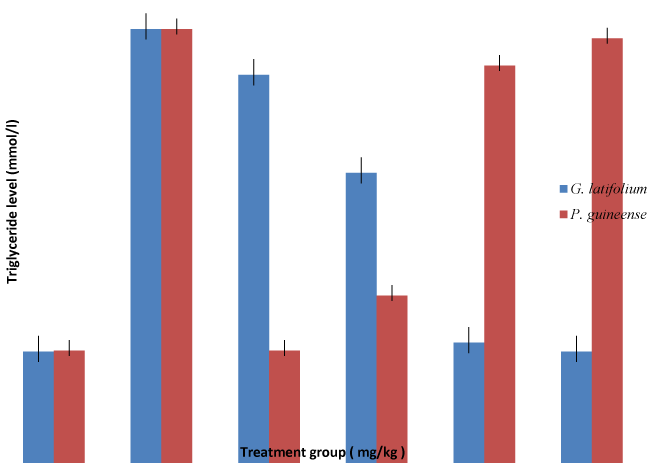
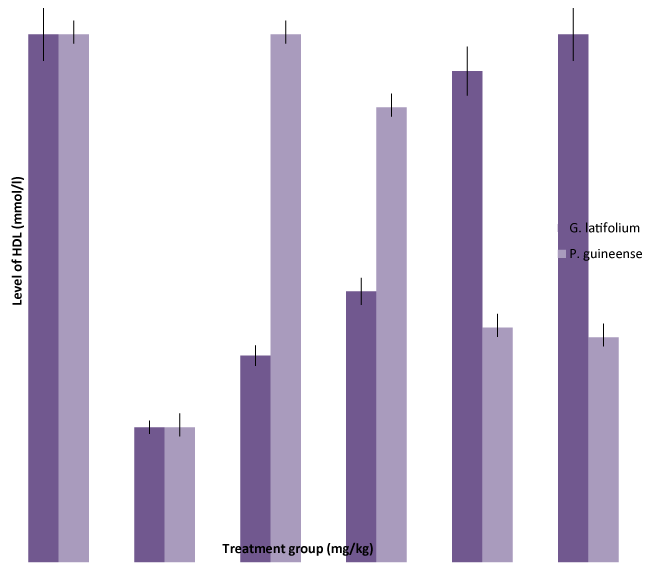
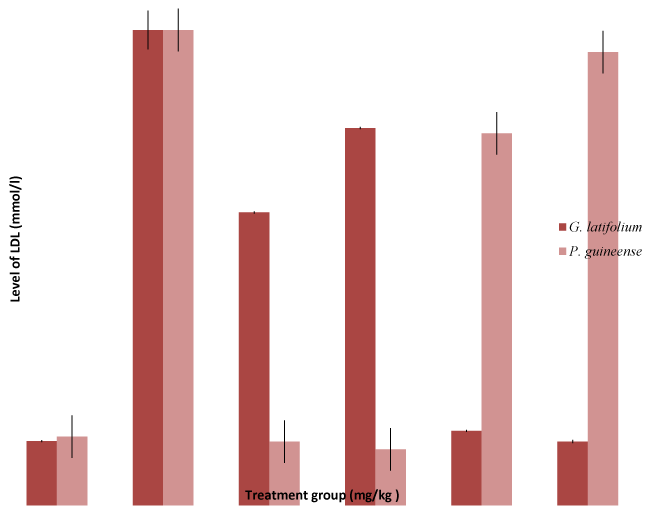
Results: Significant (P < 0.05) increase of serum levels of total cholesterol (CHL), triglycerides (TG), low density lipoprotein (LDL), and decrease in high density lipoprotein (HDL) in ethanol intoxicated rats were observed. There were significant (P < 0.05) decrease of serum levels of low density lipoprotein (LDL) and increase in HDL, total cholesterol in ethanol intoxicated rats were restored to normal levels when treated with Gongronema latifolium and Piper guineense in a dose dependent fashion. The study demonstrated that Gongronema latifolium and Piper guineense possesses significant hepatoprotective effects and may be the source of lead compound in the management of liver diseases. Hence, the hypolipidaemic effects could represent a protective mechanism against the development hyperlipidaemia characteristic of ethanol.
| Keywords |
| Piper guineense; Gongronema latifolium; Ethanol; Lipids; Triglycerides; Cholesterol; Lipoprotein; Serum; liver |
| Introduction |
| Good health is a major resource and an important dimension of the quality of life. In present times, changes in lifestyle and dietary pattern stemming from rapid modernization have favored an increase in the occurrence of non-communicable yet chronic and degenerative diet related diseases among which cardiovascular diseases occupy a primary place [1]. Throughout the twenty first century the health science focused primarily on curing diseases with powerful drugs, more sophisticated diagnostic tests and more effective therapies [2]. Today the emphasis is on health expectancy rather than the life expectancy. Synthetic drugs may show serious side effects on health. Therefore attention has been directed to use the herbal medicine as an alternative. Cholesterol is a fat (lipid) which is produced by the liver and is crucial for normal body functioning [3]. The word “cholesterol” comes from the Greek word chole, meaning “bile”, and the Greek word stereos, meaning “solid, stiff ” [4]. Cholesterol exists in the outer layer of every cell in our body and has many functions. It is a waxy steroid and is transported in the blood plasma of all animals [5]. It is the main sterols synthesized by animalssmall amounts are also synthesized in plants and fungi [6]. |
| Cholesterol carried in the blood by molecules is called lipoproteins [7]. A lipoprotein is any complex or compound containing both lipid (fat) and protein [8]. The two main types are: HDL (high density lipoprotein): people often refer to it as good cholesterol. Experts say HDL prevents arterial disease. HDL does the opposite of LDL. HDL takes the cholesterol away from the cells and back to the liver. In the liver it is either broken down or expelled from the body as waste [9]. |
| G. latifolium is a rainforest plant which could occur as a herb, shrub or rarely treelike, with milky or less often, clear latex. Leaves simple, opposite or occasionally whorled, very rarely alternate, usually without obvious stipules, margin nearly always entire [10]. Inflorescences terminal, axillary, or extra-axillary, cymose, often condensed and umbel-like, occasionally a raceme like bostrychium. Flowers bisexual, ovaries 2, free, superior; ovules numerous. Seeds numerous, strongly compressed [11]. |
| G. latifolium is rich in phytochemicals and nutrients, all of which has been linked to its therapeutic and nutritional relivance in Nigeria. Report has shown that G. latifolium contains flavonoids, terpenes, tannins, saponins and alkaloids [12]. Other authors reported that G. latifolium contains essential oils, saponins and pregnanes and moderate amount of polyphenols, glycosides and alkaloids, and trace amounts of tannin, flavonoids and reducing compounds [13]. They also reported that G. latifolium extract can enhance the production of blood cells, especially at the doses of 200 and 300 mg/Kg body weight [14]. |
| The traditional and scientific relivance of P. guineense is numerous. It is endowed with therapeutic phytochemicals and nutrients which confer therapeutic effects on it and nutritional relivance as well [15]. Research has shown that P. guineense contains aromatic substances; insecticides, arachnicides, alkaloids, salt and substitutes and is used for genal purposes as ornamental, cultivated or partially tended, chewsticks, for food as special diets, sauces, condiments, spices, flavouring agents [16]; and as medicine in cases of arthritis, rheumatism, laxatives, sedatives, skin, mucosae, stomach troubles; tumours, cancers [17]; vermifuges, naso-pharyngeal affections, pregnancy, anti-aborifacients, small-pox, chicken-pox, measles, menstrual cycle; venereal diseases, pulmonary troubles, kidneys, diuretics, intestines, genital stimulants or depressants [18]; oral treatments; pain-killers [19]. Our earlier report has shown that the leaf of P. guineense is rich in flavonoids, flavonols and phenolic compounds [20] and that the seeds could therefore be employed as a natural antioxidant booster hence its relevance in food industry and justification for its ethno-medicinal uses [21]. Piper species exhibit a marked polyphenolic concentration and dose dependent free radical scavenging activity, hence can modulate free radicals induced disorders [22]. The objective of the study was to compare effects of ethanol extract of Gongronema latifolium and Piper guineense on lipid profile of ethanol exposed Wistar rats. |
| Materials and Methods |
| Plant materials |
| The leaves of Gongronema latifolium and Piper guineense plants were purchased from Abakpa Market, Abakaliki and brought to Department of Biochemistry Laboratory Ebonyi State University and dried under room temperature (25°C to 27°C) for two weeks. After which the leaves were pulverized into coarse form with a milling machine [23]. The coarse form was sieved using standard sieve of 1 mm size, and then 200 g was macerated in absolute ethanol (95%), which was contained in a 4-litre percolator. The system was left to stand for 48 hours and then filtered out with the help of a white filter cloth. The resulting ethanol extract was allowed to evaporate to dryness at room temperature. A known weight of the dry extract was determined. This was made into aqueous (saline) solution and ready for biochemical assays. |
| Volume to be administered = [weight of animal (g) x Dose of drug (mg/kg)]/ [1000 x Concentration of drug (mg/ml)] |
| Experimental animals |
| Forty male rats age eight weeks weighing 115-180 g were sourced from the animal house of Pharmacy Department University of Nigeria Nsukka and brought to animal house of Biotechnology Department Ebonyi State University, Abakakliki, Nigeria. The animals were taking care of according to the university ethics on animal care. |
| Experimental design |
| The animals were divided into four groups and four subgroups made up of four rats each. Group A represented the normal control, which was fed with the normal rat diet and water ad libitum. Group B represented the negative control, which was exposed to 70% ethanol (kg body weight) only for seven days. |
| Group C and D were subdivided into C1, C2, C3 , C4, D1, D2, D3 and D4 exposed to 5 ml/kg of 70% ethanol for seven days and later treated (o.i) daily, separately with 200, 400, 600 and 800 mg/kg body weight of Gongronema latifolium and Piper guineense extracts for twenty one (21) days. The route of administration (exposure) was oral intubation (o.i). |
| Assay for lipid profile |
| Total cholesterol: The Assay of total cholesterol using Randox Commercial kit is based on the assay principle that cholesterol is determined after enzymatic hydrolysis and oxidation of cholesterolester and cholesterol, respectively [24]. The indicator quinineimine is formed from hydrogen peroxide and 4-aminoantipyrine in the presence of phenol and peroxidase. |
| Distilled water (10 μl) was pipette into test tubes labelled B (reagent blank) only. Standard solution of 10 μl was pipette into test tubes labelled ST (standard). Serum sample (10 μl) from the various groups/ rats was correspondingly pipette into the last set of test tubes labelled SA (sample). Finally, 1 ml of the reagent was added to all the three sets of test tubes (reagent blank, standard and sample), mixed thoroughly and incubated for 10 minutes at 25°C. Absorbance of the Sample (Asample) against the reagent blank was read within 60 minutes at. The concentration of cholesterol in the serum sample was determined as follows: |
| Concentration of cholesterol in Sample = [ΔAsample / ΔAstandard] * Conc. of Standard |
| High Density Lipoproteins (HDL)-cholesterol: Reagent B (2 X 50 ml. Phosphate 35 mmol/l, cholesterol esterase > 0.2 U/ml, cholesterol oxidase > 0.1 U/ml, peroxidase > 1 U/ml, 4-aminoantipyrine 0.5 mmol/L, sodium cholate 0.5 mmol/L, dichlorophenol-sulfonate 4 mmol/L, pH 7.0. HDL Cholesterol standard: 1 x 5 ml, cholesterol 15 mg/dl) was brought to room temperature. Distilled water (50 μl) was pipette into the Blank test tubes (B). HDL cholesterol standard (50 μl) and sample supernatant was pipette into the standard (ST) and the sample (SA) test tubes, respectively. Reagent B (1.0 μl) was added to all test tubes and thoroughly mixed and then incubated for 30 minutes at room temperature. The absorbance (A) of the standard and sample at 500 nm wavelength was measured against the Blank. The colour was stable for at least 30 minutes according to Reithman and Frankel [25]. |
| Low Density Lipoprotein (LDL)-cholesterol: The serum (100 μl) was pipette into the centrifuge tube which was immediately accompanied with the addition of 1 ml of the precipitation reagent to the centrifuge tube. The contents were well mixed and left to stand for 10 minutes at 25°C and then centrifuged for 15 minutes at 3500 rpm. The cholesterol concentration of the supernatant was determined within 1 hour after centrifugation. |
| Distilled water (50 μl) was pipetted into a reagent blank test tube (B) of a new set of test tubes. The standard solution (50 μl each) and the supernatant were pipetted into the standard test tube (ST) and the sample test tube (SA), respectively. This was accompanied with the addition of 1 ml each of the reagent solution to all the three sets of test tubes. The test tubes, contents were well mixed and incubated for 10 minutes at 25°C.The absorbance of the Sample (Asample) against the reagent blank was recorded or measured at 550 nm. |
| Statistical analysis |
| The results were expressed as mean ± SD and tests of statistical significance were carried out-using one-way ANOVA. The statistical package used was Statistical Package for Social Sciences (SPSS for windows, version 20). Values of p < 0.05 were considered significant. |
| Results and Discussion |
| The oxidation of ethanol via the alcohol dehydrogenase (ADH) pathway results in the production of acetaldehyde and the reduced form of nicotinamide adenine dinucleotide (NADH) [26]. A major contribution to the adverse effects of ethanol arises from the changes in the redox status, due to the increase in NADH and decrease in nicotinamide adenine dinucleotide (NAD+) concentration [26]. The generation of a large amount of reducing equivalents in liver, via ADH pathway, has been correlated with a number of metabolic disorders, including hyperlipidemia [27]. Previous studies have shown that a significant consequence of chronic ethanol administration was the increase of ADH activity in the rat liver, and the subsequent decrease of NAD+/NADH [28]. Thus, the capacity of the liver to promote hyperlipidemia and fat accumulation after ethanol administration was of great interest. In order to clarify the changes of the lipid profile, the level of triglycerides, cholesterol, low density lipoprotein and high density lipoprotein were investigated. Alcohol feeding is known to increase the biosynthesis and to decrease the catabolism of fatty acid and cholesterol, resulting in hypertriglyceridemia and hypercholesterolemia [29]. In the present study, we reported that triglyceride; low density lipoprotein and cholesterol level was increased in serum of ethanol-fed rats, mainly after the administration of 70% ethanol for 7days. Fatty liver results mainly from the accumulation of TG [30]. Increased TG levels after ethanol ingestion may be due to the increased availability of FFA, glycerophosphates, decreased TG lipase activity, and decreased fatty oxidation. These increased TG levels may lead to increased availability of FFA for esterification [31]. Free fatty acids are the principal components present in most lipids of biological importance. The observed increase in the levels of FFA may be directly due to lipid breakdown and indirectly due to the oxidation of ethanol to acetate, which in turn forms FFA [32]. Reports show that an increase in FFA level can increase the synthesis of other major lipids and activate NADPH- or NADH-dependent microsomal peroxidation [33]. Probably alcohol ingestion may produce the formation of esterification products of fatty acids and alcohol, which may be the mediators of end- organ damage. Increased levels of FFA may be due to lipid breakdown, which can increase the synthesis of other major lipids and activate NADPH- or NADH-dependent microsomal peroxidation [34]. The decrease in the high density lipoprotein concentration may be due to an accelerated degradation, and can result in the modification of composition, structure and stability of bio membranes thus leading to cellular dysfunction [35]. Multiple functional abnormality of liver may be associated with ethanol-induced changes in membrane composition and lipid peroxidation. The peroxidation of liver lipids is a factor in the pathogenesis of ethanol-induced liver injury [36]. We assume that the liver is susceptible to the peroxidative damage than other organs because of its specific metabolism and physiology, and also because the peroxidative process is facilitated by the accumulation of iron in the liver after chronic ethanol ingestion [37]. |
| Administration of the extract decreased significantly (P<0.05) reduced LDL-cholesterol and increased HDL- cholesterol level in all the experimental groups compared with the negative control values (p < 0.05) (Figures 3 and 4). Decreased plasma LDL- cholesterol concentration has been associated with reduced risk for condiovascular diseases [38]. Previous studies have demonstrated that aqueous extract of G.latifolium and Piper guineense has blood pressure lowering effects in rats [39,40] and in humans [41]. However, mechanisms responsible is not clear, although reduced vascular resistance due to enhanced endothelial function and inhibition of Ca2+ influx have been proposed in rats [42]. However, diminished endothelium-dependent vasodilation has been observed in both clinical and experimental hypercholesterolemia [43]. Hence [44] reported that agents that have the ability to lower cholesterol would reduce vascular resistance by improving endothelial function. The finding in this study that administration of ethanol extract of both G. latifolium and P. guineense lowers plasma cholesterol in a dose dependent fashion is in consonance with previous studies [45]. The significant decrease in plasma LDLcholesterol level after administration of extract may be due to alterations in the small intestine, which might increase conversion of VLDL to LDL [46]. Because there is no corresponding decrease in HDL-cholesterol levels, the plasma total cholesterol concentration observed in this study is attributed to decreased plasma level of LDL-cholesterol. |
| Conclusion |
| The hypolipidaemic effects observed in these plants represent protective mechanism against disorders of lipid metabolism in rats exposed to ethanol. |
| Acknowledgements |
| The authors are grateful to Mr. Ezenwali Moses of Caritas University Enugu State Nigeria for using his private laboratory for this work. |
References
|
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15185
- [From(publication date):
September-2015 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 10513
- PDF downloads : 4672
