Research Article Open Access
Evaluation of Bioethanol Production from Ulva lactuca By Saccharomyces cerevisiae
Waleed M M El-Sayed1*, Hassan A H Ibrahim2, Usama M Abdul-Raouf3 and Manal Mahmoud El-Nagar21Microbiology Department, National Institute of Oceanography and Fisheries (NIOF), Red Sea, Egypt
2Microbiology Department, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
3Microbiology Department, Faculty of Science, Al-Azhar University, Assuit Branch, Egypt
- *Corresponding Author:
- Waleed M M El-Sayed
Microbiology Department, Marine Environmental Division
National Institute of Oceanography and Fisheries (NIOF), Red Sea, Egypt
Tel: +201224526982
E-mail: walled_mohamed78@yahoo.com
Received date: April 19, 2016; Accepted date: May 13, 2016; Published date: May 20, 2016
Citation: El-Sayed WMM, Ibrahim HAH, Abdul-Raouf UM, El-Nagar MM (2016) Evaluation of Bioethanol Production from Ulva lactuca By Saccharomyces cerevisiae. J Biotechnol Biomater 6:226. doi:10.4172/2155-952X.1000226
Copyright: © 2016 El-Sayed WMM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Ulva lactuca acts a vital potential marine energy crop. Reducing sugars from U. lactuca were obtained and evaluated for the bioethanol production by Saccharomyces cerevisiae. The optimization process was investigated by Plackett-Burman experimental design followed by immobilization technique on supported solid materials. Results show that the sugar concentration, pH level and the inoculums size have a significant effect on the bioethanol production by S. cerevisiae to give concentration (12 ± 0.5 g/g of sugar/l) with conversion efficiency (47.1%).
The immobilization of yeast cells upon luffa pulp shows the highest bioethanol productivity (13.3 g/g of sugar/l) with conversion efficiency (52%). Therefore, the immobilized yeast upon luffa pulp was recommended in the current work. Moreover, the supportive luffa pulp was efficiently used and recycled for several times in the bioethanol production.
Keywords
Ulva lactuca; Bioethanol; Plackett-Burman design; Immobilization
Highlights
• Green algae "Ulva lactuca" acts a vital potential marine energy crop
• Plackett-Burman design is essential tools to evaluated bioethanol production from green biomass "Ulva lactuca"
• Immobilization technique increase conversion efficiency of fermentationprocess
Introduction
Incrementing the industrialization and motorization of the world has led to a steep elevate for the authoritative ordinance of petroleum-based fuels. Moreover, increasing crude oilprices necessitate development the development of clean alternative energy sources and environmentally friendly is significant interest [1-3]. The marine biomasses, including algae, are recently gaining consideration as a countermeasure to global warming and as an alternative to traditional fuel as biofuel resources, because they can exhibit high productivity [4,5]. Marine algal biomass conversions into bioethanol may be financially feasible, since some algae hydrolysate can contain abundance in total carbohydrates more than some terrestrial lignocellulosic biomass feedstock [6,7]. In general, the utilization of non-edible macroalgae for the bioethanol creation has other advantages such as reduced competition with agricultural food and feed crops, significant returns per territory and non-reliance on agrarian compost, pesticides, farmable area, and freshwater [8,9]. The major benefits provided by algal biomass over agriculture biomass are (I) biomass production rate per unit area is higher for algal mass, (II) algal mass do not compete with agricultural plants for land, (III) they do not require agricultural input such as fertilizer, pesticides and water, and (IV) they can be easily depolymerization as it does not contain lignin in their cell wall [10]. U. lactuca (chlorophyceae - green macroalgae) has been considered as a potential marine energy crop due to its high gear growth rates and easily composing high carbohydrates content [11]. The carbohydrates of U. lactuca are predominantly in the form of the complex hydrocolloid ulvan, a sulphated glucuronoxylorhamnan, which together with cellulose are structural components of the cell wall [12] and starch, for intracellular energy storage. However, preliminary fermentation test of U. lactuca for bioethanol production indicate relatively poor yields [13]. On the other side, the most consequential microorganisms applied for ethanol production are the yeast. Saccharomyces cerevisiae is classified as major decomposer that is involved the fermentation process. It can only survive and function up to an ethanol concentration of around 10% after that it is degrade and the fermentation process stop [14]. Fermentation process is being carried out by Saccharomyces cerevisiae through two enzymes. The first, invertase enzyme, which catalyst and helps conversation of disaccharide into glucose and fructose (both C6H12O6) , and the second, zymase enzyme which ferments glucose and fructose (both C6H12O6) to produce ethanol [15]. The Plackett-Burman experimental design is well suited for detecting the significant factors in a fermentation process [16-18]. The Plackett-Burman experimental design is experiments are performed at various combinations of high and low values of the process variables and analyzed for their effect on the process [16-18]. The Plackett– Burman design analyzes the input data and presents a rank ordering of the variables with magnitude of effect and designates signs to the effects to indicate whether an increase in factor values is advantageous or not [16-18]. Using the Plackett-Burman design has shown improvement in controlling of fermentation process, and an ability to omen the presence of mixtures and to select main factors for complex medium ingredients [19]. The most paramount design parameters pertaining to an immobilized microbial system are operational stability, internal mass convey efficiencies, catalyst backing, density and reactor contact efficiency [20]. The current study focused on the green alga; Ulva lactuca as a potential feedstock for bioethanol production. The algal polysaccharides were saccharified by Pseudoalteromonas pecicidea, into simple sugars (glucose and galactose) for the bioethanol production. Optimizing the bioethanol production from U. lactuca reducing sugars by local commercial Beaker's yeast (S. cerevisiae) was achieved using the Plackett-Burman design then evaluated by immobilized techniques.
Materials and Methods
Collection of U. lactuca
The green alga (U. lactuca) was collected from the coast of Hurghada, Red Sea, Egypt. The algal samples washed with tap water to remove debris and salts and dried to a constant weight at 50ºC in hot air oven. After drying, the algal samples were powdered using grinder for further experiments
Chemical composition of U. lactuca
The chemical composition of U. lactuca was determined using standard methods [21]. The protein, carbohydrate and lipid contents were estimated by the method of Lowry [22], Dubois [23] and Bligh and Dyer [24], respectively.
Chemical pretreatment of U. lactuca
The concentrations of diluted sulfuric acid and sodium hydroxide prepared by range (0.4- 2N) and autoclaved at 121°C, 1.5 pa for 30 min to enhance the hydrolysis of U. lactuca using P. piscicida. The enzyme activity and reducing sugar concentration were evaluated [25,26].
Preparation of sugar solution
The sugar solution was obtained from saccharificationprocess by P. pecicidea. The container containing the reaction mixture was centrifuged and filtered then the sugar solution was concentrated using a rotary evaporator to achieve concentration about 50 g/l. The reducing sugar concentration was estimated by dinitrosalicylic acid (DNSA) method [25]. The concentrations of monosaccharides were measured by HPLC. The Shimpack SPR-Ca column (Shimadzu, Japan) at 80°C with IR-detector was used. The mobile phase was distilled water at a flow rate of 0.5 ml/min. The samples were filtered with 0.45 μm of cellulose acetate filter and 10 μl of injection volume was added. The measurement was carried out by Regional Center for Mycology and Biotechnology, Al Azhar University, Cairo, Egypt.
Inoculum preparation of S. cerevisiae
The commercial live bakers' yeast, S. cerevisiae was obtained in the forms of active dry yeast from the Egyptian Company for Advanced Foodstuff Industries. Active dry yeast (10 g) was dispersed in 99 ml of 0.1% sterile peptone water pre-warmed at 34°C for 20 min. The yeast solution contained 2×107 viable cells per ml and used as inoculum for fermentation medium [27].
Bioethanol production and estimation
The ethanol production was carried out in 500 ml Erlenmeyer flasks using 100 ml of medium containing 10 g/l yeast extract and 20 g/l peptone and sugar solution (40 g/l) obtained from the saccharification process. The pH values were adjusted to 5.5. One hundred millimeters from the bioethanol production media sterilized by autoclave at 121°C for 10 min before inoculation with 3 ml of S. cerevisiae. After tightly closing, the flask fermented at 35°C for 48 hr. on the other side, the quantitative estimation of ethanol was carried out by potassium dichromate method [28].
Optimization of bioethanol production by Plackett-Burman design
Seven independent factors were distributed in nine combinations arrange according to the Plackett-Burman design matrix. For each factor, a high level (+1) and low level (-1) were tested [16,29]. The factors tested were: sugar solution concentration (g/l), incubation period (h), peptone (g/l), yeast extract (g/l), inoculum size (%), pH level and temperature (°C). The symbols, levels and distribution of the factors listed in Table 1. The main effect of each factor was evaluated according to the following equation:
Exi = (ΣMi+ - ΣMi-) / N
| Factor | Symbol | Level | ||
|---|---|---|---|---|
| -1 | 0 | +1 | ||
| Sugar solution (g/l) | SS | 30 | 40 | 50 |
| Incubation period (hr) | IP | 24 | 48 | 72 |
| Peptone (g/l) | Pep | 10 | 20 | 30 |
| Yeast extract (g/l) | YE | 5 | 10 | 15 |
| Inoculum size (%) | IS | 1 | 3 | 5 |
| pH | pH | 5 | 5.5 | 6 |
| Temperature (°C) | Temp | 30 | 35 | 40 |
(-1) = low level, (0) = basal conditions and (+1) = high level
Table 1: Factors examined as independent variables affecting the bioethanol production by S. cerevisiae and its levels in the Plackett-Burman experiment.
Where: Exi is the factor main effect, Mi+ and Mi- are the response percentage in trials, in which the independent factor (xi) was present in high and low concentrations, respectively, N is the half number of trials. Nine experiments were generated for the seven factors that can affect the bioethanol production. Using Microsoft Excel, statistical t-values for equal unpaired samples were calculated for the determination of factor significance. In order to validate the results and to evaluate the accuracy of the applied Plackett-Burman statistical design, a verification experiment was carried out in triplicates. According to the main effect results, near optimum and far from optimum levels of the independent factors were examined and compared to the basal condition settings. The bioethanol concentrations were then estimated as described before.
Evaluation of bioethanol production by immobilization technique
The immobilization of yeast cells was investigated by adsorption on different supported solid materials (luffa pulp, synthetic sponge, pumice stone and charcoal). The adsorption process was carried out as follows: Concentrated cell suspension was prepared by centrifugation process of the activated cells from culture medium after 48 hr. The cell pellet was washed and re-suspended in a sterile saline solution. The obtained cell suspension (100 ml) was added to each 500 ml Erlenmeyer flasks which contained about 1g in weight from (10-20) pieces of the tested solid supporter. The flasks were shaken slowly at about 50 rpm. Cell adsorption was monitored in each flask at short time intervals by measuring the decrease in turbidity (OD) of the cell suspension at λ550 nm. The solution was removed from the flasks leaving the supports and their absorbed yeast cells. The bioethanol production medium was added and cultivation was carried out under static condition. Scanning electron micrographs for the adsorbed yeast cells on different solid supports were prepared in the Electron Microscope Center, Faculty of Science; Alexandria University; Egypt. The concentration of bioethanol obtained from predicted near optimum conditions with immobilization techniques were measured by a HPLC. The Shimpack SPR-Ca column (Shimadzu, Japan) at 80°C with IR-detector was used. The mobile phase was distilled water at a flow rate of 0.5 ml/min. The samples were filtered with 0.45 μm of cellulose acetate filter and 10 μl of injection volume was added. This study was carried out by Regional Center for Mycology and Biotechnology, Al Azhar University, Cairo, Egypt.
Results
Chemical composition of U. lactuca
The chemical compositions of U. lactuca were analyzed. The results are as follow to be found carbohydrates (44 ± 2.5%), protein (16 ± 3.3%) and lipid (5 ± 1.1%) contents of dry weight.
Chemical pretreatment of U. lactuca
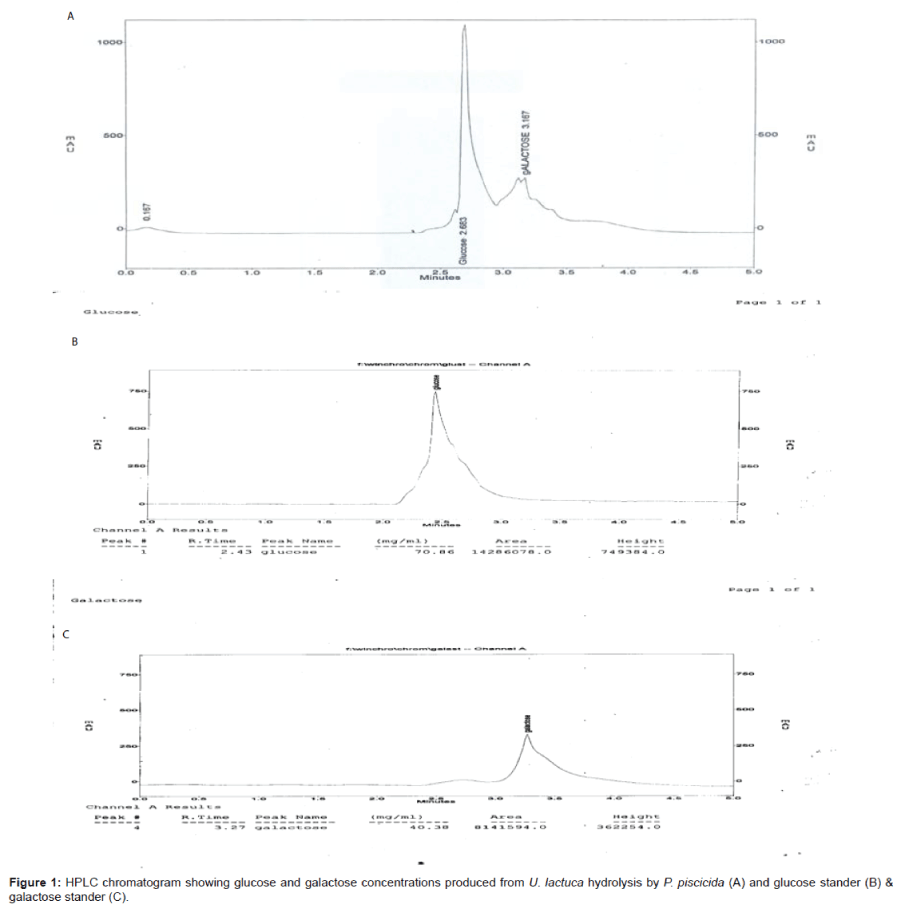
Chemical pretreatment of U. lactuca was investigated. Occasionally, it was observed that the most effective pretreatment conditions for the production of the highest reducing sugar were; bacterial isolate P.piscicida upon the green algae (U. lactuca) pretreatment with 1N H2SO4 with treatment ratio (1:1) yielding reducing sugar concentration 0.079 g/g, the concentration was further enhanced by Plackett-Burman experimental design to reach to 0.158 g/g reported previously in 2014 [1] illustrated in Figure 1.
Optimization of bioethanol production using Plackett- Burman design
To optimize the bioethanol production by S. cerevisiae, the Plackett-Burman experimental design was applied. Seven factors have been tested as independent factors affecting the production of bioethanol by S. cerevisiae. Data in Table 2 conducted frequently that evaluate optimum bioethanol production that occurred at the trial No.8 (11.4 g/g of sugar/l) with conversion efficiency 44.7%, while the basal condition in trial No.9 yielded (7.5 g/g of sugar) bioethanol with conversion efficiency 36.8%, that increase the bioethanol yield and conversion efficiency to 1.22 folds.
| Trial No. |
Independent variables1 | Sugar solution conc. (g/l) |
Actual bioethanol Yield (g/g of sugar/l) |
Theoretical bioethanol yield (g/g of sugar/l) |
Conversion efficiency (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | IP | Pep | YE | IS | pH | Temp | |||||
| 1 | - | - | - | + | + | + | - | 30 | 2.9 | 15.3 | 18.9 |
| 2 | + | - | - | - | - | + | + | 50 | 7.5 | 25.5 | 33.3 |
| 3 | - | + | - | - | + | - | + | 30 | 5.3 | 15.3 | 34.6 |
| 4 | + | + | - | + | - | - | - | 50 | 8.2 | 25.5 | 32.2 |
| 5 | - | - | + | + | - | - | + | 30 | 3.3 | 15.3 | 21.6 |
| 6 | + | - | + | - | + | - | - | 50 | 9.3 | 25.5 | 36.5 |
| 7 | - | + | + | - | - | + | - | 30 | 2.3 | 15.3 | 15.0 |
| 8 | + | + | + | + | + | + | + | 50 | 11.4 | 25.5 | 44.7 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 7.5 | 20.4 | 36.8 |
1Factor symbols and levels are shown in Table 1
Table 2: The applied Plackett-Burman design for seven cultural variables and the experimental results of bioethanol production by S. cerevisiae.
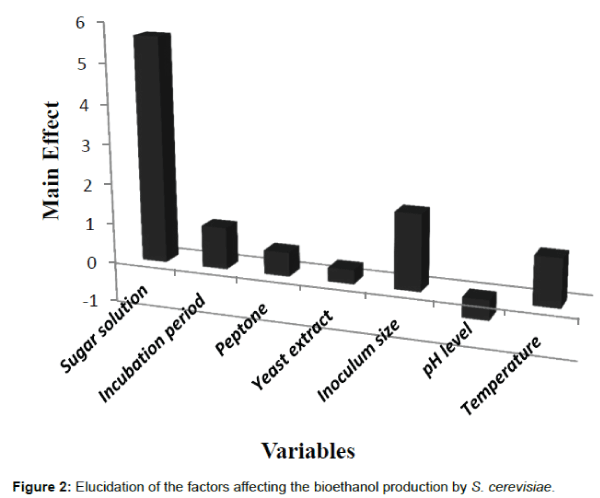
The main effect data presented in Figure 2 suggested that sugar solution and inoculums size were the most effective factors that controlled the bioethanol production via S. cerevisiae. Moreover, the lower pH (5) and the higher sugar solution (50 g/l) were nearer to optimum condition than their opposite levels. Considerable positive effects of the high levels of temperature, incubation period and pH were also noted. On the other hand, variations within the examined levels of yeast extract and peptone recorded slight effects.
In order to validate the obtained results and to evaluate the accuracy of the applied Plackett-Burman statistical design, a verification test was run in triplicates. The predicted near optimum and far from optimum levels of the independent variables were tested and compared to the basal condition. The bioethanol production by S. cerevisiae was shown in Table 3. The applied near optimum condition, resulted in approximately (4.5 g/g of sugar/l) increase in the bioethanol production by S. cerevisiae when compared to the basal medium formulation (7.5 g/g of sugar/l). The fermentation condition predicted to be far from optimum condition recorded approximately (2.8 g/g of sugar/l) decrease in the efficiency of the bioethanol production by S. cerevisiae. These results support importance of Plackett-Burman design application.
| Trail | Basal medium | Near optimum medium1 | Far from optimum medium2 |
|---|---|---|---|
| Bioethanol production (g/g of sugar/l) | 7.5 ± 0.7 | 12 ± 0.5 | 2.8 ± 0.5 |
| Conversion efficiency (%) | 36.8 | 47.1 | 18.3 |
1Near optimum medium formula was predicted according to the results obtained from the Plackett-Burman experiment. 2Far optimum medium formula was predicted according to the results obtained from the Plackett-Burman experiment
Table 3: Verification of the Plackett-Burman experimental results in the bioethanol production (g) and conversion efficiency (%).
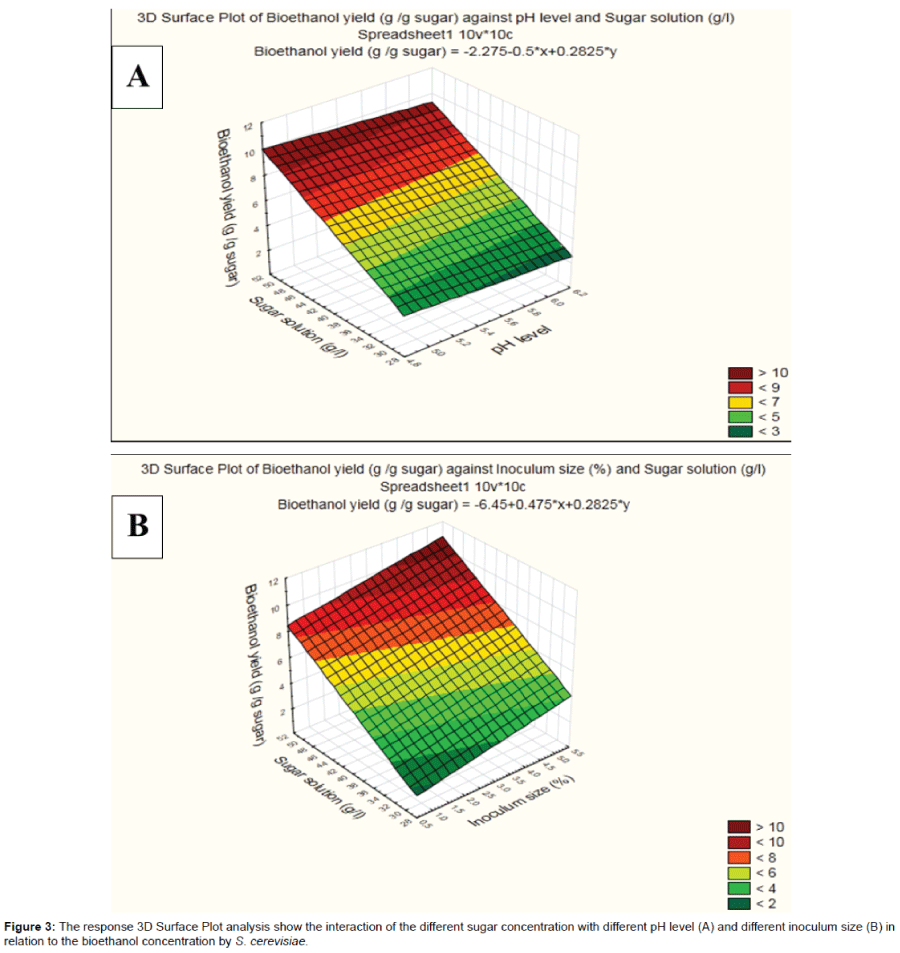
The interacting effects of sugar solution and pH levels as described in three-dimensional graph (Figure 3A), suggest that, within the examined ranges the higher sugar solution accompanied by the lower pH levels would markedly increase the bioethanol expressed by the experimental S. cerevisiae. However, the interaction of sugar solution and inoculum size (Figure 3B) with respect to production of the reducing sugar appeared to be high.
Immobilization technique
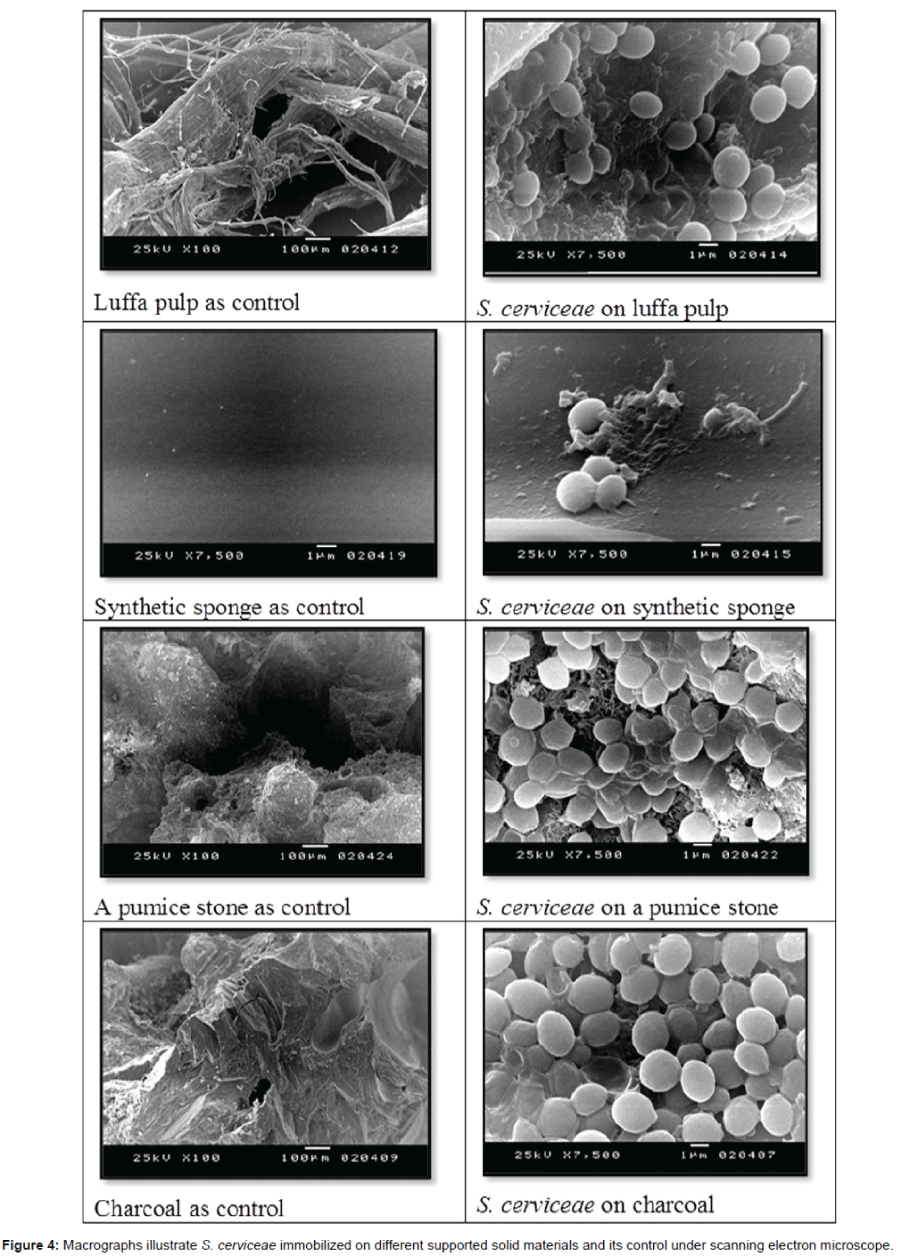
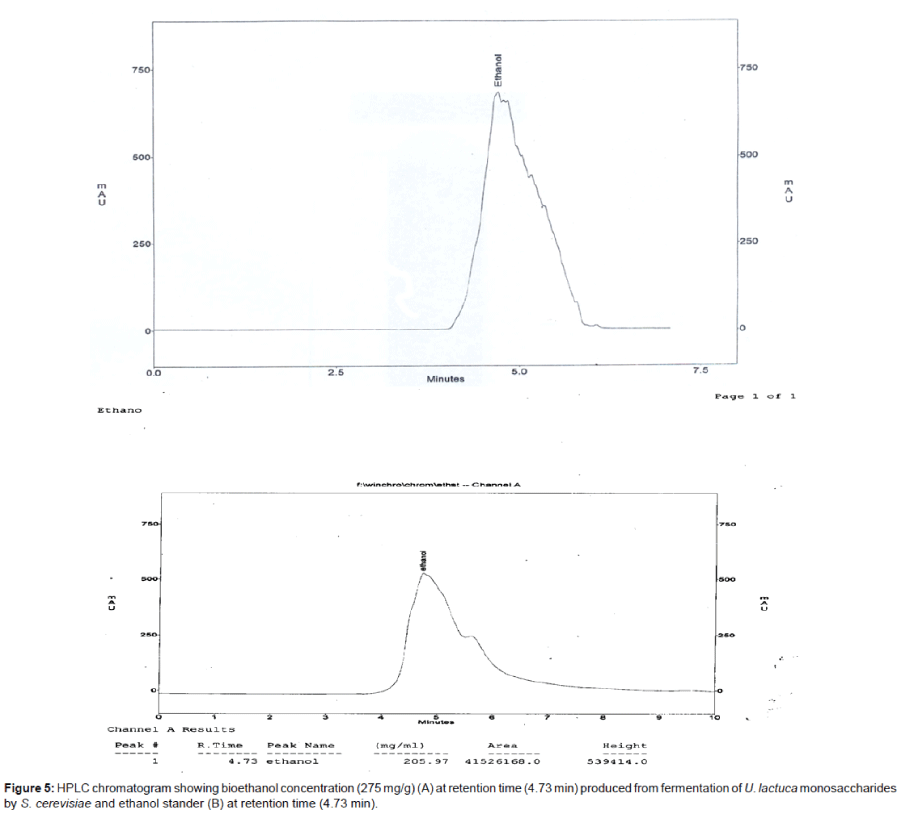
The immobilization technique was applied for evaluating the bioethanol production. The immobilized yeast cell on different supported solid material scanned with scanning electron microscope illustrated at Figure 4. Values of immobilization experiment as shown in Table 4 were compared to that obtained from the free cells of S. cerevisiae. The immobilization cells upon luffa pulp showed the highest bioethanol productivity (13.3 g/g of sugar/l) comparing to that obtained by the other supported materials and free cells (12 g/g of sugar). The supportive luffa pulp was efficiently used and cycled for three times in the bioethanol production. The efficacy decreased logically upon the cycles applied to get total bioethanol yields (24.7 g/g of sugar/l) after three cycles represented in Table 5. HPLC chromatogram showing bioethanol concentration is given in Figure 5.
| Technique | Bioethanol (g/g of sugar/l) | Conversion efficiency (%) |
|---|---|---|
| Free cells | 12 ± 0.5 | 47.1 |
| Immobilized cells on luffa pulp | 13.3 ± 0.8 | 52.0 |
| Immobilized cells on syntheticsponge | 9.9 ± 0.5 | 38.8 |
| Immobilized cells on a pumice stone | 12.4 ± 0.5 | 48.4 |
| Immobilized cells on charcoal | 10.3 ± 0.5 | 40.2 |
Table 4: Immobilized and free cells techniques for the bioethanol production (g) and corresponding conversion efficiency (%).
| Time (Days) |
Immobilization Cycle |
Bioethanol (g/g of sugar/l) |
Conversion efficiency (%) | Production rate (g/g of sugar/l /day) |
|---|---|---|---|---|
| 3 | 1st Cycle | 13.3 ± 0.8 | 52.0 | 4.4 |
| 3 | 2nd Cycle | 6.1 ± 0.5 | 23.9 | 3.3 |
| 3 | 3rd Cycle | 5.3 ± 0.5 | 20.8 | 1.8 |
| 9 | - | Total = 24.7 | Ave. = 32.3 | - |
Table 5: Immobilization cycles in the bioethanol production (g) and conversion efficiency (%) on the luffa pulp.
Discussion and Conclusion
Presently, bioethanol is an attractive alternative fuel because their benefits in reduction particulate emissions in compression-ignition engines and is also free from sulfur and aromatics; therefore it can be considered clean. In addition, it can effectively protect the future generation against the upheaval resulting from global warming [30,31]. Macroalgae are harnessed as renewable source of biomass intended for bioethanol production. Currently there are few studies on biofuel depletion, and intense further research is required in this area for efficiently utilize algal biomass to produce environmentally friendly bioethanol [7]. The carbohydrate contents of algae are in a range of 30-70%. It depends on the species and culture conditions [6,32,33]. The present work, the biochemical contents of U. lactuca has been investigated; the carbohydrate, protein and lipid content were 44 ± 2.5%, 16 ± 3.3% and 5 ± 1.1%, respectively on dry weigh in this study. Other reports illustrated similar biochemical contents in another closely related genera U .lactuca [34].While Kumar et al. [35] in 2011 have shown in their investigation on the nutritional characteristic of different macroalgae provided slightly rising in contents of carbohydrates ranging from 46% to 57% on dry weight in different genera of Ulva. To obtain reducing sugar, the chemical pretreatment of U. lactuca investigated. Acid treatment illustrates high activity in comparison with alkaline treatment due to sulfuric acid take function through hydrolyzing bonds among cell wall composition. Under treatment via 1N of H2SO4 ratio (1:1) and autoclaved at 121°C, 1.5 pa, for 30 min obtain reducing sugar concentration 0.158 g/g. Several reports confirm current case. In 2013 they obtained sugar about 0.205 g/g biomass at hot buffer with cellulase enzyme from Ulva fasciata [36], as well as in 2011 Kim et al. [6] used acid treatment and enzyme to get yield 0.194 g/g biomass from Ulva lactuca. In contrast, other studies obtained reducing sugars as 0.870 g/g biomass from Gracilaria verrucosa by enzyme hydrolysis only [37]. Additionally, in 2011 obtained reducing sugars ratio less than current case as 0.096 g/g biomass from Sargassum fulvellum by acid plus enzyme hydrolysis [6] (Table 6). On other words, pretreatment play vital role for enhancement the process of saccharefication depending on hydrolysis enzymes, their activities and contents of carbohydrates. The fermentation process was planned to achieve the main target of the current study; production of the bioethanol from hydrolysate U. lactuca by P. piscicida. For such purpose the commercial bakery yeast, S. cerevisiae was used. Therefore, in this study the efficiency of fermentation process was found to be comparatively lower than those values reported by many researchers [6,36-41]. The efficiency of converting reducing sugars to ethanol depending on efficiency of the fermentation process to assimilation it with high ethanol production ability. Additionally, many factors, such as pH level, oxygen rates and temperature, can influence the growth rate and inhibition can be caused either by ethanol production or sugar level [42-44]. On the other side, in the current work the efficiency of fermentation process was found to be comparatively higher than some values reported earlier [45-47]. Moreover, there were some researchers obtained the bioethanol lower than that we had (13.3 g/g of sugar/l) that equals approximately (8.3% or 8.3 g/l) such as; Yanagisawa et al. [48] who produced 5.5% bioethanol by S. cerevisiae IAM 4178 from seaweeds; sea lettuce, chigaiso, and agar weed. Similarly, there workers produced bioethanol more or less in the same range. For instance, Cho et al. [33] used Pichia angophorae KCTC 17574 successfully for the bioethanol fermentation of seaweed slurry with high salt concentration. They obtained maximum ethanol concentration of 9.42 g/l from total carbohydrate of U. pinnatifida. The converting efficiencies of reducing sugars of some algal biomass into bioethanol reported are illustrated in Table 6.
Seaweed |
Conditions | Sugar released (g/g) | Ethanol yield (g/g of sugar/l) | Efficiency (%) | References |
|---|---|---|---|---|---|
| Ulvalactuca | Acid + enzyme | 0.113 | 13.3 | 52.15 | Present study |
| Ulvafasciata | Hot buffer + enzyme | 0.205 | 0.450 | 88.2 | (36) |
| Ulvalactuca | Acid + enzyme | 0.194 | Not available | Not available | (6) |
| GracilariaVerrucosa | Enzyme hydrolysis | 0.870 | 0.430 | 84.31 | (37) |
| Kappaphycusalverzii | Acid hydrolysis | Not available | 0.369 | 72.35 | (40) |
| Gelidiumamansii | Acid hydrolysis | 0.422 | 0.380 | 74.50 | (38) |
| Kappaphycusalverzii | Acid hydrolysis | 0.306 | 0.400 | 80.39 | (41) |
| Saccharina japonica | Acid hydrolysis | 0.456 | 0.169 | 33.13 | (47) |
| Laminaria japonica | Acid + enzyme | 0.376 | 0.410 | 80.39 | (6) |
| Sargassumfulvellum | Acid + enzyme | 0.096 | Not available | Not available | (6) |
| Gracilariasalicornia | Acid + enzyme | 16.6 | 0.079 | 15.49 | (46) |
| Sargassumsagamianum | High temp & pressure | Not available | 0.386 | 75.68 | (39) |
| Sargassumsagamianum | Not available | Not available | 0.13–0.23 | 26– 45 | (45) |
Table 6: Comparison of ethanol production reported for different macroalgal biomass with the current study.
The optimization of fermentation process was applied using Plackett-Burman design. The data conducted frequently that the highest concentration of bioethanol by S. cerevisiae was (11.4 g/g of sugar/l) with conversion efficiency 44.7%. The main effect data suggested that the sugar solution and the inoculums size were the most effective variables that controlled the bioethanol production by S. cerevisiae.
Additionally, the temperature and substrate concentration are vital factor affected on the fermentation process,. Some reports conducted that the high sugar concentration annihilate avoid ethanol yield leading to a lower titer due to ruling of glycolytic enzymes [49].
Few studies on the bioethanol optimization process from seaweeds using Plackett-Burman design. Despite of many other researchers applied Plackett–Burman for bioethanol from various carbohydrates wastes rather than seaweeds.
For instance, Pereira et al. [50] selected the vital nutrients according to a Plackett–Burman design, the study added great insights into costeffective nutritional improvements of industrial bioethanol very high gravity fermentations. Balusu et al. [51] proved that the use of Plackett- Burman design not only helped in short listing few key nutrients, but also proved to be useful in increasing the yield of ethanol in a limited number of experiments. Other worker employed successfully Plackett- Burman design for the optimization of the fermentation process for the yeast S. cerevisiae. While some researchers found the hydrolysate that obtained from saccharification of 15 g dry U. fasciata biomass contained 3.2 ± 0.21 g reducing sugar. The fermentation of this hydrolysate by S. cerevisiae for different time intervals ranging from 12 to 48 hr at 12 hr incremental period gave varied ethanol yields [36].
To evaluate the bioethanol production, the immobilization of yeast cells upon several supporting solid materials (luffa pulp, charcoal, pumice stone and synthetic spongy) was applied. Obviously, the immobilization cells upon luffa pulp showed higher productivity (13.3 g/g of sugar/l) than conventional fermentations free cells (12 g/g of sugar/l). Therefore we used luffa pulp in current study. Moreover, the supportive luffa pulp was efficiently used for three cycles in the bioethanol production, in the first cycle the productivity rate was (4.4 g/g of sugar/l/d), while in the second cycle was (3.3 g/g of sugar/l/d) and decrease in third cycle to (1.8 g/g of sugar/l).
Immobilization of cells for fermentation has been developed to eliminate inhibition caused by high sugar concentration and also enhance the productivity and yield of ethanol production and minimize the toxicity by end product [52-56].
The main benefits of the immobilization of yeast are the increase of ethanol yield and cellular stability and a decrease of system expenses because of the ability for cell recovery. In addition, the immobilization of whole cells for ethanol production offers several advantages (eliminate cell mass from cultures easily, improve reactor productivity, increase higher efficiency of biocatalyst) [57-59].
Plackett-Burman design is essential tool for evaluated bioethanol production. The current work supported usage of Plackett-Burman design for optimization of fermentation medium. This design predicted that the maximum bioethanol productivity was (12 ± 0.5 g/g of sugar/l), comparing to the basal conditions (7.5 g/g of sugar/l) with increasing the bioethanol yield. Bioethanol production as well as conversion efficiency of fermentation process was improved with yeast cells immobilized upon luffa pulp to (13.3 ± 0.5 g/g of sugar/l), compared to free cells (12 ± 0.5 g/g of sugar/l). Moreover, the immobilized cell system can be recycled for 3 times. The immobilized yeast upon luffa pulp is highly recommended for bioethanol production.
Acknowledgment
I wish to express my deepest thanks and great respect to Prof. Khalid El- Moselhy; Vice-President of the National Institute of Oceanography and Fisheries for his unlimited support and honest advice throughout the period of research.
References
- El-Naggar MM, Abdul-Raouf UM, Ibrahim HAH, El-Sayed WMM (2014) Saccharification of Ulvalactuca via Pseudoalteromonaspiscicida for biofuel production. Journal of Energy and Natural Resources 3: 77-84.
- Singh A, Olsen SI (2011) A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Applied Energy 88: 3548-3555.
- Zverlov V, Berezina O, Velikodvorskaya G, Schwarz W (2006) Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: use of hydrolyzed agricultural waste for biorefinery. ApplMicrobiolBiotechnol 7: 587-597.
- Singh A, Nigam PS, Murphy JD (2011) Mechanism and challenges in commercialisation of algal biofuels. BioresourTechnol 102: 26-34.
- Nguyen TAD, Kim KR, Nguyen MT, Kim MS, Kim D, et al. (2010) Enhancement of fermentative hydrogen production from green algal biomass of Thermotoganeapolitana by various pretreatment methods. International Journal of Hydrogen Energy 3: 13035-13040.
- Kim NJ, Li H, Jung K, Chang HN, Lee PC (2011) Ethanol production from marine algal hydrolysates using Escherichia coli KO11. BioresourTechnol 10: 7466-7469.
- John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. BioresourTechnol 102: 186-193.
- Packer M (2014) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37: 3428-3437.
- Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 78: 53-62.
- Jones CS, Mayfield SP (2012) Algae biofuels: versatility for the future of bioenergy. CurrOpinBiotechnol 23: 346-351.
- Bruhn A, Dahl J, Nielsen HB, Nikolaisen L, Rasmussen MB, et al. (2011) Bioenergy potential of Ulvalactuca: Biomass yield, methane production and combustion. BioresourTechnol 10: 2595-2604.
- Lahaye M, Robic A (2007) Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 8: 1765-1774.
- Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgaeSargassum spp. BioresourTechnol 138: 22-29.
- Yusaf T, Goh S, Borserio J (2011) Potential of renewable energy alternatives in Australia. Renewable and Sustainable Energy Reviews 1: 2214-2221.
- Reed G (1966) Enzymes in Food Processing.Volume 3, Elsevier.
- Plackett RL,Burman JP (1946)The design of optimum multifactorial experiments.Biometrika 33: 305-325.
- Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response surface methodology: process and product optimization using designed experiments.Volume 705, John Wiley & Sons.
- Box GE, Draper NR (1987) Empirical model-building and response surfaces.Volume 424, Wiley, New York.
- Monaghan R,Koupal L (1989) Use of the Plackett&Burman technique in a discovery program for new natural products. Novel Microbial Products for Medicine and Agriculture 2: 94-116.
- Venkatasubramanian K, Harrow L (1979) Design and operation of a commercial immobilized glucose isomerase reactor system. Annals of the New York Academy of Sciences 326: 141-153.
- PáduaMd, Fontoura PSG, Mathias AL (2004) Chemical composition of Ulvariaoxysperma (Kützing) bliding, Ulvalactuca (Linnaeus) and Ulvafascita (Delile). Brazilian Archives of Biology and Technology 47: 49-55.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J biolChem 19: 265-275.
- Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350-356.
- BLIGH EG, DYER WJ (1959) A rapid method of total lipid extraction and purification. Can J BiochemPhysiol 37: 911-917.
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426-428.
- Fritze D, Flossdorf J, Claus D (1990) Taxonomy of alkaliphilic Bacillus strains. Int J SystBacteriol 40: 92-97.
- Sosulski K, Wang S, Ingledew WM, Sosulski FW, Tang J (1997) Preprocessed barley, rye, and triticale as a feedstock for an integrated fuel ethanol-feedlot plant. ApplBiochemBiotechnol 63-65: 59-70.
- Capauti A, Veda J, Brown T (1968) Spectrophotometric determination of chronic complex formed during oxidation of alcohol. Journal of Enology and Viticulture 19: 160-165.
- Yu X, Hallett S, Sheppard J, Watson A (1997) Application of the Plackett-Burman experimental design to evaluate nutritional requirements for the production ofColletotrichumcoccodes spores. Applied Microbiology and Biotechnology 47: 301-305.
- Hansen AC, Zhang Q, Lyne PW (2005) Ethanol-diesel fuel blends -- a review. BioresourTechnol 96: 277-285.
- Goh CS, Lee KT (2010) A visionary and conceptual macroalgae-based third-generation bioethanol (TGB) biorefinery in Sabah, Malaysia as an underlay for renewable and sustainable development. Renewable and Sustainable Energy Reviews 1: 842-848.
- Shah YR,Sen DJ (2011)Bioalcoholas Green Energy-A review.Int J Cur Sci Res 1: 57-62.
- Cho Y, Kim H, Kim SK (2013) Bioethanol production from brown seaweed, Undariapinnatifida, using NaClacclimated yeast. Bioprocess BiosystEng 36: 713-719.
- van der Wal H, Sperber BL, Houweling-Tan B, Bakker RR, Brandenburg W, et al. (2013) Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulvalactuca. BioresourTechnol 128: 431-437.
- Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, et al. (2011) Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. Journal of Applied Phycology 2: 797-810.
- Trivedi N, Gupta V, Reddy CR, Jha B (2013) Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga UlvafasciataDelile. BioresourTechnol 150: 106-112.
- Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilariaverrucosa, a red alga, in a biorefinery approach. BioresourTechnol 135: 150-156.
- Park JH, Hong JY, Jang HC, Oh SG, Kim SH, et al. (2012) Use of Gelidiumamansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation.BioresourTechnol 108: 83-88.
- Lee HY, Jung KH, Yeon JH (2011) Repeated-batch operation of surface-aerated fermentor for bioethanol production from the hydrolysateof seaweed Sargassumsagamianum. Journal of Microbiology and Biotechnology 21: 323-331.
- Meinita MDN, Kang JY, Jeong GT, Koo HM, Park SM, et al. (2012) Bioethanol production from the acid hydrolysate of the carrageenophyteKappaphycusalvarezii (cottonii). Journal of Applied Phycology 2: 857-862.
- Khambhaty Y, Mody K, Gandhi MR, Thampy S, Maiti P, et al. (2012) Kappaphycusalvarezii as a source of bioethanol. BioresourTechnol 103: 180-185.
- Aldiguier A, Alfenore S, Cameleyre X, Goma G, Uribelarrea J,et al. (2004) Synergistic temperature and ethanol effect on Saccharomyces cerevisiae dynamic behaviour in ethanol bio-fuel production. Bioprocess and Biosystems Engineering 26: 217-222.
- Kasemets K, Nisamedtinov I, Laht TM, Abner K, Paalme T (2007) Growth characteristics of Saccharomyces cerevisiae S288C in changing environmental conditions: auxo-accelerostat study.Antonie van Leeuwenhoek 9: 109-128.
- Lin Y, Zhang W, Li C, Sakakibara K, Tanaka S, et al, (2012) Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY47.Biomass and Bioenergy 47: 395-401.
- Yeon JH, Seo HB, Oh SH, Choi WS, Kang DH, et al. (2010) Bioethanol production from hydrolysateof seaweed Sargassumsagamianum. KSBB J 2: 283-288.
- Wang X, Liu X, Wang G (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol 53: 246-252.
- Jang JS, Cho Y, Jeong GT, Kim SK (2012) Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess BiosystEng 3: 11-18.
- Yanagisawa M, Nakamura K, Ariga O, Nakasaki K (2011) Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochemistry 46: 2111-2116.
- Bisson LF,Fraenkel D (1984) Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. Journal of Bacteriology 159: 1013-1017.
- Pereira FB, Guimarães PM, Teixeira JA, Domingues L (2010) Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs.BioresourTechnol 10: 7856-7863.
- Balusu R, Paduru RM, Seenayya G, Reddy G (2004) Production of ethanol from cellulosic biomass by Clostridium thermocellum SS19 in submerged fermentation: screening of nutrients using Plackett-Burman design. ApplBiochemBiotechnol 117: 133-141.
- Sakurai A, Nishida Y, Saito H, Sakakibara M (2000) Ethanol production by repeated batch culture using yeast cells immobilized within porous cellulose carriers.J BiosciBioeng90: 526-529.
- Kourkoutas Y, Bekatorou A, Banat IM, Marchant R, Koutinas A (2004) Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiology 21: 377-397.
- Strehaiano P, Ramon-Portugal F, Taillandier P (2006) Yeasts as biocatalysts. In: Querol A, Fleet G(eds.), Yeasts in food and beverages. Springer.
- Baptista C, Cóias J, Oliveira A, Oliveira N, Rocha J, et al. (2006) Natural immobilization of microorganisms for continuous ethanol production. Enzyme and Microbial Technology 40: 127-131.
- Mariam I, Manzoor K, Ali S, Ulhaq I (2009) Enhanced production of ethanol from free and immobilized Saccharomyces cerevisiae under stationary culture. Pak J Bot 41: 821-833.
- Groboillot A, Boadi DK, Poncelet D, Neufeld RJ (1994) Immobilization of cells for application in the food industry. Crit Rev Biotechnol 14: 75-107.
- Selvakumar P, Ashakumary L, Panday A (1994) Microbial fermentations with immobilized cells. J SciInd Res 5: 443-449.
- Ramakrishna S, Prakasham R (1999) Microbial fermentations with immobilized cells.CurrSci 77: 87-100.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 13612
- [From(publication date):
June-2016 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 12411
- PDF downloads : 1201