Evaluation of Antioxidant, Analgesic and Antidiarrheal Activities of Methanolic Extract of Litsea monopetala (roxb.) Leaves
Received: 01-Jun-2018 / Accepted Date: 20-Aug-2018 / Published Date: 30-Aug-2018 DOI: 10.4172/2167-065X.1000185
Abstract
Antioxidant, analgesic and antidiarrheal activities of Litsea monopetala (LM) leaves extracts were investigated in order to assessment of pharmacological activities. In vitro antioxidant properties, in vivo analgesic and antidiarrheal activities were investigated in this study. Antioxidant plays a remarkable role to protect different kinds of diseases of human by inhibiting free radicals. 1, 1-diphenyl-2- picrylhydrazyl (DPPH) free radical scavenging was considered to evaluate the antioxidant property. Analgesic and antidiarrheal activities were investigated in acetic acid induced writhing and castor oil induced antidiarrheal method by white albino mice, respectively. Methanolic extract of LM leaves has shown antioxidant activity with an IC50 value of 223.22 μg/ml which was compared to the standard ascorbic acid. This extract of LM at a dose of 500 mg/kg showed statistically significantly (p<0.001) and produced 68.75% writhing inhibition of mice while standard diclofenac sodium drug has shown 76.25% inhibition. LM also produced a significant (P<0.01) reduction in the frequency of diarrhea produced by castor oil in mice compared with Loperamide (50 mg/kg) standard. This study clearly supports the medicinal value of LM`s leaves. However, further study is required to find out the active components which are responsible for these activities.
Keywords: Litsea monopetala (LM); Antioxidant; Analgesic; Antidiarrheal
Introduction
Now- a-days plants or phytochemical constituents such as saponins, flavonoids are playing a significant role to treat human related diseases in worldwide from the ancient times [1]. Recently, modern society also started to use the plants extract as an alternative way to protect the human health related diseases. In estimation approximately 20000 medicinal plants species were counted by the World Health Organization (WHO) [2]. In developing countries, approximately 80% people believe to use medicinal plant as a folk medicine [3]. From the ancient time, people generally using different kinds of plant compounds as a primary health care medicine. Recently, it is proposed to isolate and investigate the Phyto chemicals in the modern laboratory for finding the novel compounds [4]. In the world, approximately 200 plants are using as medicine, whereas more than 14000 plants were recognized as medicinal plants [5]. The kind information is that more than 80% medicinal were collected from wild sources, among them 2000 medicinal plants were carried from South Asian Subcontinent [6,7].
Litsea monopetala (LM) is an evergreen medium sized tree. LM is well known as Bara-kukurchita, Mendaphuri, Sukurja, Uruijja (Chittagong), Akorma, Akarma, Lalkhori, Huoria (Sylhet) in Bangladesh (Family: Lauraceae) [8], the genus includes 136 accepted species in tropical and subtropical areas of both hemispheres. It is a small tree up to 18 m tall, up to 60 cm in diameter, leaves are 7.5 to 23 cm long, usually rounded at both ends [9]. In Bangladesh LM is widely distributed in the forests of Noakhali, Sylhet, Chittagong, Chittagong Hill Tractsand Sal forests of Gazipur, Madhupur, Dinajpur also found in Bangladeshi villages. The plant is mainly distributed in Nepal. But outside Nepal it found in Yucatan, Southern Mexico, West Indies, the Bahamas, Bermuda, the Florida Keys, the southern part of the Florida mianland, Philippine, Sri-Lanka, Palestine, South and Central America, China, Burma, West Malaysia, Thailand and Myanmar [10].
The LM leaves are used for the treatment of analgesic, diarrhea, oxidation, stomachic, nerve tonic and arthritis. Powder of leaves, bark and root are used externally against bruises and pains. LM plants also applied in animal fracture [11]. Leaves are reported to exhibit antioxidant [12], antihyperglycemic [13]and analgesic [14,15].
Genus of Litsea are a rich source of biologically-active compounds, such as butanolides (leaves of Litsea acutivena ) [16], flavonoids (leaves of Litsea coreana and Litsea japonica ) [17], sesquiterpenese (leaves and twigs of Litsea verticillata ) [18], and essential oils (leaves of Litsea cubeba, fruits, flowers and bark of LM, fruits of Litsea glutinosa [19-21].
In Bangladesh LM is generally called Medea sak. In India and Pakistan leaves and bark of LM are used as nerves and bone tonic, stomach ache, stimulant, analgesic, diarrhea and antiseptic. The leaves of this tree are used by the local people as fodder for cattle since they are claimed to enhance milk production. The main objective of this study was to evaluate the antioxidant, analgesic, anti-diarrheal activities of methanolic extract of LM leaves.
Materials And Methods
Chemicals
Analytical graded chemicals were used in this experiment and purchased from Active Fine Chemicals Ltd., Bangladesh.
Plant collection and identification extraction
Leaves of LM were used for this study and plants leaves were purchased from rural area of Noakhali, Bangladesh. The plant was identified by Bangladesh National Herbarium, Mirpur-1, Dhaka, Bangladesh (accession number 45413).
Drying and grinding of plant materials
At first leaves of LM were cut into small pieces and kept under the sun about 15 days for proper drying then dried in mechanical dryer at 50°C–60°C. After complete drying, the LM leaves were pulverized into crush powder by a grinding and blending machine.
Extraction of plant materials
The powder of LM leaves (240 gm) was extracted with 2000 ml methanol (95%) in a flat bottom container, thorough shaking and stirring. After two weeks the extract was the filtered through the cotton at first and then through Whatman filters paper. The filtrate (methanolic extract) was evaporated using rotary evaporation machine [22]. Finally, we got methanolic extract and transferred to an airtight 10 ml vial for use and protection.
Antioxidant activity
Reaction with 1-1 –Diphenyl-2-PicrylHydrazyl (DPPH): An antioxidant can donate an electron to DPPH and changed in absorbency at 517 nm against methanol as blank by UV spetrophotometer then the purple color is typical to free DPPH radical decays. This test shows the ability of a compound to donate a hydrogen atom, and on the mechanism of antioxidant action [23]. Hydrogen donates to free radicals by antioxidants and formulation of a complex between the lipid radical and antioxidant radical (free radical acceptor).
DPPH free radical scavenging activity: According to Brand- Williams method, DPPH was used to find out in vitro free radical scavenging activity of methanolic extract of LM leaves [24]. Solution of DPPH was prepared by adding 3 mg of DPPH in 150 ml of methanol to make the concentration 20 μg/ml. 3 ml of DPPH solution (20 μg/ml) was taken and then mixed at different concentration (500, 250, 125, 62.5, 31.25, 16.625, 7.813, 3.906, 1.953 and 0.997 μg/ml) of 2 ml sample solution of plant extract (10 μg/ml). For control same amount of methanol and DPPH was used. For proper mixing these mixtures were shaken and absorbance was taken at 517 nm against methanol as blank by UV spetrophotometer after 30 minutes reaction period at room temperature in dark place and free radical scavenging activity percentage was calculated by the formula below.
% radical scavenging activity (RSA)
(I%) = (1-Absorbance of test sample/Absorbance of control) × 100 [25].
Similar procedure was repeated for the standard Ascorbic acid instead of sample solution (plant extract) to obtain the percentage inhibition.
IC50 value was calculated from the obtained RSA, which shows scavenging compound concentration that produced 50% neutralization. The extracts of LM were studied for free radical scavenging activity.
Analgesic activity
Against acetic acid induced writhing: To find out analgesic activity of methanolic extract of LM leaves was used acetic acid induced writhing in mice model [26]. Acetic acid induced writhing is an analgesic characteristic observation method where injecting the 0.7% acetic acid solution in mice and then observing the mice for specific contraction of body is called writhing that represents a noxious stimulation in mice [27].Writhing were compared with standard drug Diclofenac and plant extract (test sample) is given orally after 30 minutes of acetic acid injection. The acetic acid given intra-peritoneal [28]. Acetic acid is used to represents writhing, causes algesia release by endogenous substances, which stimulates the pain nerve [29]. If the plant extract sample represents analgesic activity, the mice after taking sample will show less writhing than the control and the extract having analgesic activity will inhibit writhing.
Experimental animal
4-5 weeks aged Young Swiss-albino mice, mean weight 20-25 gm were used for this experiment. They were purchased from the Animal Research lab of Jahangirnagar University (JU), Bangladesh. For adjustment they were kept in normal environment at Manarat International University research lab for one week and fed JU formulated food and water.
Preparation of sample
The test sample suspension was prepared at the dose 500 mg/kg body weight. 4-5 drops of tween-80 was added due to the trituration property of the plant extract. After appropriate mixing of LM extract and tween-80 then distilled water was added. Finally this suspension was made exact 5.0 ml.
Diclofenac was prepared at the dose of 25 mg/kg-body weight and 12.5 mg of diclofenac was taken to make 5.0 ml suspension.
0.7% acetic acid solution was prepared by adding 0.7 ml glacial acetic acid with distilled water up to 100 ml and mixed them properly.
Methodology
Experimental mice were selected randomly and considered into 3 groups, with consists of 5 mice each group. These 3 groups received a certain treatment i.e. control, standard (Diclofenac Sodium) and the extract. A feeding needle is used for the orally administration of test samples (plant extract) and Diclofenac (standard).To ensure proper absorption of the administered substances after 30 minutes interval acetic acid (writhing inducing chemical) solution (0.7%, 15 ml/kg) was injected intraperitoneally to all experimental mice. After 5 minutes later, writhing number(squirms) of each mice was counted carefully for 15 minutes.
Anti-diarrheal Activity
Castor oil induced diarrhea in mice
Total number of stools or fluid material and latent period (first defecation) of test group are compare with standard group (positive control group). Antidiarrheal agent increase first defecation and diminish total stool count. This experiment was prepared by castor oil model as described by castor oil induced diarrheal in mice [30].
The mice were initially selected only those mice exhibit diarrhea after feeding 0.5 ml of castor oil. Randomly chosen the test animals (mice) and considered into 3 groups, with consists of 5 mice each group. They were kept in 15 individual cages with absorbent paper beneath.
The control (group-I) received distilled water with 1% Tween-80, the standard or positive control (Group-II) received oral suspension of Loperamide (at a dose of 50 mg/kg-body weight) which is standard antimotility drug. The experimental or test group (group III) were received oral suspension of methanolic extract of LM leaves (at a dose of 500 mg/kg-body weight).
Any fluid material or stools number were computed at each consecutive hour during the four hours period and each mice were noted. Also counted the first defecation of individual mice. After 1 hour interval old absorbent papers were replace by new papers.
Results
Antioxidant
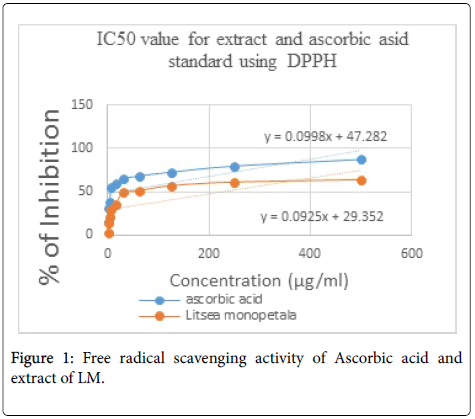
With the antioxidant test performed reacting DPPH with extract, bleaching followed by color change (yellow on purple background) of DPPH was observed. An antioxidant can donate hydrogen to DPPH radical and DPPH radical becomes paired with hydrogen to form DPPH-H complex. DPPH radical scavenging activity is described as IC50. IC50 value was calculated from the obtained RSA. To determine antioxidant property the reaction of 3 ml of DPPH solution (20 μg/ml) and 2 ml sample solution of plant extract (10 μg/ml) mixture were allowed for 30 minutes at room temperature in dark place. Then absorbance was taken at 517 nm by UV spetrophotometer. This quantitative test, the extract showed antioxidant activity with an IC50 of 223.22 μg/ml against DPPH free radical, whereas, standard ascorbic acid produced IC50 at 27.23 μg/ml (Table 1). IC50 was calculated by Y=a * X+b, IC50=(0.5-b)/a formula (Figure 1). Methanolic extract of LM leaves has shown medium antioxidant activity.
| DPPH free radical scavenging activity of ascorbic acid | DPPH free radical scavenging activity of lm leaves extract | |||||
|---|---|---|---|---|---|---|
| Conc. | Absorbance | % of Inhibition | IC50 (µg/ml) | Absorbance | % of Inhibition | IC50 (µg/ml) |
| (µg/ml) | ||||||
| 500 | 0.018 | 87.3239 | 27.23 | 0.051 | 64.084 | 223.22 |
| 250 | 0.023 | 79.5775 | 0.055 | 61.269 | ||
| 125 | 0.039 | 72.5352 | 0.061 | 57.042 | ||
| 62.5 | 0.045 | 68.3099 | 0.069 | 51.408 | ||
| 31.25 | 0.05 | 64.7887 | 0.072 | 49.295 | ||
| 15.63 | 0.057 | 59.8592 | 0.091 | 35.915 | ||
| 7.813 | 0.064 | 54.9296 | 0.099 | 30.281 | ||
| 3.906 | 0.088 | 38.0282 | 0.112 | 21.126 | ||
| 1.953 | 0.097 | 31.6901 | 0.123 | 13.38 | ||
| 0.977 | 0.12 | 15.493 | 0.139 | 2.112 | ||
Table 1: DPPH Free Radical Scavenging Activity of Ascorbic acid standard and LM Leaves Extract Values are expressed as mean ± standard deviation, n=3.
Analgesic
Analgesic activity of the methanolic extract of LM leaves was measured by counting the writhing in mice, whereas the experiment was prepared with acetic acid. Acetic acid is used to shows writhing, causes algesia release by endogenous substances, which stimulates the pain nerve. The action of phospholipase A2 and other acyl hydrolases influence the release of free arachidonic acid from tissue phospholipid, which is responsible for pain stimulus. In the peritoneal fluid, increased levels of PGE2 and PGF2α could be responsible for pain sensation due to intra- peritoneal administration of acetic acid. Writhing were compared with standard drug Diclofenac and plant extract (test sample). At the dose of 500 mg/kg body weight, the extract of LM represents remarkable reduction in acetic acid induced writhing reflex of mice.
Leaves of LM produced 68.75% writhing inhibition of mice where standard drug Diclofenac sodium having 76.25% of writhing inhibition at the dose of 25 mg/kg body weights of the experimental mice (Table 2) [31].
| Animal group | No. of mice | Total writhing | Mean writhing | % of Writhing | Inhibition of writhing | SD | SEM | T-test (p values) |
|---|---|---|---|---|---|---|---|---|
| Control (Tween 80) | 5 | 160 | 32 | 100 | - | 2.55 | 1.85 | - |
| Diclofenac (25 mg/kg) | 5 | 38 | 7.6 | 23.75 | 76.25 | 0.89 | 1.08 | 11.39 (p<.001) |
| Plant Extract (500mg/kg) | 5 | 50 | 10 | 31.25 | 68.75 | 0.71 | 1.3 | 9.64 (p<.001) |
Table 2: Statistical evaluation of the writhing effect. Values are t-test. (n=5); p<0.001 vs. control, Student’s t-test. Student's t-test and P value less than 0.05 was considered as significant. % of writhing for Standard= (Total writhing of Standard/Total writhing of Control group) *100; % of writhing for Plant Extract= (Total writhing of Plant extract/Total writhing of Control group) *100; Inhibition of writhing= (100- Standard or Plant extract).
The methanolic extract of LM has shown significant analgesic activity in compare with control animals. However, further study is required for isolate the original active compound from the methanolic extracts of LM which is responsible for analgesic activity.
Anti-diarrhoeal
Anti-diarrhoeal activity was tested by castor oil induced diarrheal model in mice. Castor oil plays an important role to incite diarrhea and it contains 90% ricinoleate that is metabolized to ricinoleic acid. [32]. Ricinoleic acid is responsible for the irritation, inflammation and stimulation of peristalic activity of the intestinal mucosa. Ricinoleic acid is also responsible for altering the electrolytic permeability of the intestinal mucosal membrane. These events in turns excite the release of endogenous prostaglandins that activate the motility and secretion thereby reduced sodium and potassium ion absorption occurs [33]. This experiment was considered into 3 groups. The control group received distilled water with 1% Tween-80, the standard or positive control received oral suspension of Loperamide and the experimental or test group were received oral suspension of methanolic extract of LM leaves Table. Stools number or any fluid material were computed at each consecutive hour during the four hours period and each mice were noted. Also counted the first defecation of individual mice. After 1 hour interval old absorbent papers were replace by new papers.
Total number of stools after latent period at the 4 consecutive hours of study found 6.4, 7.0, 5.0 and 7.2, respectively. Mean latent period for diarrhoeal episode and significant t-test result of LM has shown 0.96 (Table 3) and (P<0.01) (Table 4), respectively. Methanolic extract of LM leaves represents a remarkable anti-diarrheal activity in castor oil induced test in mice at the doses of 500 mg/kg-body weight in comparison with standard antidiarrheal drug loperamide. LM increase in latent period and delayed diarrheal episode and decreased the frequency of stools. We can claim that LM might possess antidiarrhoeal activity.
| Group (Dose) |
NumberOf Mice | Mice weight in gm | Latent period (hr) |
Mean latent period (hr) ±S.E. | Standard Deviation(SD) |
t-test (P-value) |
|---|---|---|---|---|---|---|
| I Control |
1 | 22 | 0.37 | 0.44 ± 0.12 | 0.26 | -- |
| 2 | 21.9 | 0.17 | ||||
| 3 | 20.5 | 0.25 | ||||
| 4 | 21 | 0.75 | ||||
| 5 | 23 | 0.68 | ||||
| II(Positive control/ Standard) Loperamide (50 mg/kg) |
1 | 21 | 2.00 | 2.57 ± 0.17 | 0.39 | 6.9 (p<0. 01) |
| 2 | 23 | 2.76 | ||||
| 3 | 22 | 2.50 | ||||
| 4 | 21.5 | 2.52 | ||||
| 5 | 22 | 3.06 | ||||
| Extract (leaves) 500mg/kg |
1 | 23 | 0.82 | 0.96± 0.16 | 0.37 | 1.0 (p<0. 04) |
| 2 | 22.7 | 0.42 | ||||
| 3 | 23.6 | 1.00 | ||||
| 4 | 21 | 1.33 | ||||
| 5 | 20.5 | 1.26 |
Table 3: Effect of Litsea monopetala on the latent period of castor oil induced Diarrhoeal episode in the experimental mice.
| Treatment Groups | Dose (per kg-body wt.) | Period of study (hr) |
Total no. of stools |
Mean no. of stools |
t-test (p value) |
|---|---|---|---|---|---|
| I Control |
10 | 1 | 13.2 | 10.8 | -- |
| 2 | 14.4 | ||||
| 3 | 9.0 | ||||
| 4 | 6.6 | ||||
| II Positive Control (Loperamide) |
50 | 1 | 3.9 | 3.8 | 22.8 (p<0.01) |
| 2 | 3.4 | ||||
| 3 | 4.1 | ||||
| 4 | 3.8 | ||||
| III Extract of Litsea monopetala 500 mg/kg |
500 | 1 | 6.4 | 6.4 | 16.8 (p<0.01) |
| 2 | 7.0 | ||||
| 3 | 5.0 | ||||
| 4 | 7.2 |
Table 4: Effect of Litsea monopetala (Roxb) on castor oil induced diarrhoea in mice. Values are t-test. (n=5); p<0.01 vs. control, Student’s t-test. Student's t-test and P value less than 0.05 was considered as significant.
Conclusion
The result from our present study allows us to conclude saying that the LM leaves extracts exhibited medium antioxidant activity, meanwhile analgesic and anti-diarrheal shown significant property. The results of the study suggest that future study could be initiative to find or isolate new compound from the LM leaves extracts to develop our medical sector.
Acknowledgement
The author (Md Razowanul Ferdous) gratefully acknowledge the Department of Pharmacy, Manarat International University for providing financial support and extending full laboratory facilities to conduct this research work and heartfelt gratitude, indebtedness, profound appreciation to Sm Faysal Bellah and Md. Shohel Hossain for generous help to complete the research work. Md. Shohel Hossain has collected the plant. Md Razowanul Ferdous has written the manuscript, analyzed data and Sm Faysal Bellah, Md Ashrafudolla have revised the whole manuscript with grammar checking, interpretation of the results and technical design. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Competing Interests
Authors have declared that no competing interests exist.
References
- Rafa ZT, Ashrafudoulla M, Fuad F, Islam R, Hasan M, et al. (2016) Phytochemical and pharmacological investigation of Plumbago indica L. J Medicinal Plants 4: 115-118.
- Penso G (1980) WHO inventory of medicinal plants used in different countries. Geneva. Switzerland, WHO.
- Ashrafudoulla M, Bellah SF, Alam F, Faisal SS, Kafi MAH, et al. (2016) Phytochemical screening of Solanum nigrum L, S. myriacanthus Dunal, Solanum melongena and Averrhoa bilimbi in Bangladesh. J Medicinal Plants 4: 35-38.
- Bellah SF, Islam KMR, Karim MR, Hossain MJ, Ashrafudoulla M, et al. (2016) Phytochemical and pharmacological screening of the fruits of lagerstroemia speciosa (L.) pers. World J Pharm Sci 4: 205-211.
- McChesney JD, Venkataraman SK, Henry JT (2007) Plant natural products: back to the future or into extinction. Phytochemistry 68: 2015-2022.
- Ghani A (1998) Medicinal plants of Bangladesh: Chemical constituents and uses. Asiatic Society of Bangladesh, Dhaka.
- South Asia Enterprise Development Facility (SEDF) & Intercooperation (IC) (2003) Medicinal plants marketing in Bangladesh A market study report. SEDF- Intercooperation, Dhaka.
- Arfan M, Amin H, KosinskaA, Karamac M, Amarowicz R (2008) Antioxidant activity of phenolic fractions of Litsea monopetala (Persimon-leaved Litsea) bark extract. Pol J Food Nutr Sci 58: 229-233.
- Anonymous. Litsea monopetala (Roxb.) Pers. Accessed 10 December (2015) Available: http://www.mpbd.info/plants/litse a-monopetala.php.
- Hasan MF, Iqbal MA, Uddin MS (2016) Antibacterial and antifungal activity of litsea monopetala leaves on selected pathogenic strains. European J Med Plants 12: 1-8.
- Ferdousi A, Rahman MO, Hassan MA (2014) Seed germination behaviour of six medicinal plants from Bangladesh. Bangladesh J Plant Taxonomy 21: 71-76.
- Choudhury D, Ghosal M, Das AP, Mandal P (2013) In vitro antioxidant activity of methanolic leaves and barks extracts of four Litsea plants. Asian J Pl Sci Res 3: 99-107.
- Hasan H, Azad MS L, Islam MZ, Rahman SM, Islam MR, et al. (2014) Antihyperglycemic activity of methanolic extract of Litsea monopetala (Roxb.) Pers. leaves. Adv Nat Appl Sci 8: 51-55.
- Ghosh M, Sinha BN (2010) GC-MS studies on the bark extracts of Litsea polyantha Juss. Middle-East J Sci Res 5: 441-444.
- Hasan H, Azad MS L, Islam MZ, Rahman SM, Islam MR, et al. (2014) Antihyperglycemic activity of methanolic extract of Litsea monopetala (Roxb.) Pers. leaves. Adv Nat Appl Sci 8: 51-55.
- Cheng HI, Lin WY, Duh CY, Lee KH, Tsai IL, et al. (2001) New cytotoxic butanolides from Litsea acutivena. J Nat Prod 64: 1502-1505.
- Lee SY, Min BS, Kim JH, Lee J, Ki TJ, et al. (2005) Flavonoids from the leaves of Litsea japonica and their anti-complement activity. Phytother Res 19: 273-276.
- Zhang HJ, Tang GT, Santarsiero BD, Mesecar AD, Hung NV, et al. (2003) New sesquiterpenes from Litsea verticillata. J Nat Prod 66: 609-615.
- Wang F, Yang D, Ren S, Zhang H, Li R (1999) Chemical comÂposition of essential oil from leaves of Litsea cubeba and its antifungal activities. J Chinese Medic Materials 22: 400-402.
- Amer A, Mehlhorn H, (2006) Repellancy effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 99: 478-490.
- Choudhury SN, Singh RS, Ghosh AC, Leclercq PA (1996) Litsea glutinosa (Lour) CB. Rob, a new source of essential oil from northeast India. J Essential Oil Res 8: 553-556.
- Bulbul IJ, Nahar L, Ripa FA, Haque O (2011) Antibacterial, cytotoxic and antioxidant activity of chloroform, n-hexane and ethyl acetate extract of plant amaranthus spinosus. Int J Pharm Tech Res 3: 1675-1680.
- Liang N, Kitts DD (2014) Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 19: 19180-19208.
- Brand-Williams W, Cuvelier ME, Berest C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28: 25-30.
- Ismail A, Hong TS (2002) Antioxidant activity of selected commercial seaweeds. Malays J Nutr 8: 167-177.
- Whittle BA (1964) The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol Chemother 22: 246.
- Ahmed F, Selim MST, Das AK, Choudhuri MSK (2004) Anti-inflammatory and antinociceptive activities of Lippia nodiflora Linn. Pharmazie 59: 329-333.
- Derardt R, Jougney S, Delevalcee F, Falhout M (1980) Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 51: 17-24.
- Taesotikul T, Panthong A, Kanjanapothi D, Verpoorte R, Scheffer JJC (2003) Anti-inflammatory, antipyretic and antinociceptive activities of Tabernaemontana pandacaqui Poir. J Ethnopharmacol 84: 31-35.
- Yegnanarayan R, Shostri DS (1982) Comparison of antidiarrhoeal activity of sons drugs in experimental diarrhea. Indian J Pharmacol 14: 293-299.
- Mohammad AMM, Faysal SB, Khan MR, Md Iqubal HR, Md Mustafizur R, et al. (2013) Phytopharmacological evaluation of ethanolic extract of Feronia limonia leaves. Am J Sci Ind Res 4: 468-472.
- Magaji MG, Yaro AH, Maiha BB, Maje IM, Musa AM (2008) Preliminary gastrointestinal studies on aqueous methanolic stem bark extract of Maerua angolensis (Capparaceae). Nig J Pharm Sci 7: 108-113.
- Dash PR, Nasrin M, Raihan SZ, Ali MS (2014) Study of antidiarrhoeal activity of two medicinal plants of Bangladesh in castor-oil induced diarrhea. Int J Pharmaceut Sci Res 5: 3864-3868.
Citation: Ferdous MR, Ashrafudolla M, Hossain MS, Bellah SF (2018) Evaluation of Antioxidant, Analgesic and Antidiarrheal Activities of Methanolic Extract of Litsea monopetala (roxb.) Leaves. Clin Pharmacol Biopharm 7: 185. DOI: 10.4172/2167-065X.1000185
Copyright: © 2018 Ferdous MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4777
- [From(publication date): 0-2018 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 3984
- PDF downloads: 793