Research Article Open Access
Evaluation of Antimicrobial Activity and Antioxidant Activity by Electrochemical Method of Ethanolic Extract of Pterocarpus marsupium Roxb Bark
Deepa R1, Manjunatha H1*, Krishna V1 and Kumara Swamy BE21Department of PG Studies and Research in Biotechnology and Bioinformatics, Jnanasahyadri, Kuvempu University, Karnataka, India
2Department of PG Studies and Research in Industrial Chemistry, Jnanasahyadri, Kuvempu University, Karnataka, India
- Corresponding Author:
- Manjunatha H
Department of PG Studies and Research in Biotechnology and Bioinformatics
Jnanasahyadri, Kuvempu University
Shankaraghatta, Karnataka, India, 577451
Tel: +91 8282 256198
E-mail: manjunatha75@gmail.com
Received date: September 17, 2014; Accepted date: October 31, 2014; Published date: November 15, 2014
Citation: Deepa R, Manjunatha H, Krishna V, Kumara Swamy BE (2014) Evaluation of Antimicrobial Activity and Antioxidant Activity by Electrochemical Method of Ethanolic Extract of Pterocarpus marsupium Roxb Bark. J Biotechnol Biomater 4:166. doi:10.4172/2155-952X.1000166
Copyright: © 2014 Deepa R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The antimicrobial potential of the ethanolic extract of Pterocarpus marsupium Roxb stem bark has been evaluated against some micro organisms like Bacillus polymyxa, Vibrio cholera and Candida albicans. Results indicated that the phytochemical extracts of P. marsupium exhibited significant activity at different varying dosages. However, the present study depicts that the ethanolic extract of Pterocarpus marsupium bark can be used as a potential source of novel antimicrobial agents. The reducing ability of the ethanol extract was determined by cyclic voltammetry. The low anodic current and low anodic peak potential imply the good reducing ability of the molecules resulting in good antioxidant activity of the extract.
Keywords
Pterocarpus marsupium; Roxb; Antimicrobial; Antioxidant; Cyclic voltammetry; Carbon paste electrode
Introduction
Medicinal plants have been used as an exemplary source for centuries as an alternative remedy for treating human diseases because they contain numerous active constituents of therapeutic value. The development of microbial resistance to antibiotics has led the researchers to investigate the alternative sources for the treatment of resistant strains. Currently 80 percent of the world population relies on plant derived medicines and serves as first line of defense in maintaining health and combating many diseases. P. marsupium has long been used as an anti-inflammatory, anti-elephantiasis, anti-leucoderma agent and often used to treat dysentery, cough and diarrhea. Traditionally, the plant material has been used as a cooling external application for inflammations and headache, as antipyretic, anti-helminthic, aphrodisiac, alexeteic and in biliousness, mental aberrations and ulcers [1]. Parts of the Indian Kino (heart wood, leaves and flowers) have long been used for their medicinal properties in Ayurveda. The heart wood is used as an astringent and in the treatment of inflammation. The wood and bark of Pterocarpus are known for their anti-diabetic activity [2,3]. Phytochemical studies on P. marsupium have shown that the plant contains iso-flavonoids, terpenoids and related phenolic compounds, ß-sitosterol, lupenol, epicatechin, and aurone glycosides [4]. Hence, in the present study the ethanolic extracts of stem bark of P. marsupium was chosen to study and its adequacy was tested with respect to inhibition over the growth of pathogenic bacteria and fungi under in vitro conditions.
In Indian system of medicine, several plants have been identified to solve the problems of neurological disorders. Pterocarpus marsupium Roxb. Is one such indigenous medicinal plant traditionally used to relieve headache, mental aberrations, fever and stomach pain. It is a deciduous leguminous tree growing in the forests of the Western Ghats and commonly known as ‘Indian Kino’. In Indian system of medicine ‘Ayurveda’ the medicinal properties of the stem bark and heart wood has been reported as an anti-inflammatory, anti-leucoderma and neurotransmitter drug [5]. Phytochemical studies on the stem bark shown that the plant contains iso-flavonoids and terpenoids like betulin, betulinic acids and lupeol [6]. The pharmacological property of P. marsupium has been reported for hepatoprotective [7]. Among the most important constituents of P marsupium, antioxidants are the most important species. Living cells of man, animals and plants when exposed to external environment leads to the formation of free radicals and tissue damage resulting in diseases such as atherosclerosis, heart failure, neurodegenerative disorders, aging, cancer, diabetes mellitus, hypertension and several other diseases and are becoming increasingly recognized [8]. Reactive oxygen species (ROS), as well as reactive nitrogen species (RNS), are products of normal cellular metabolism and they are well recognized for playing a dual role as both deleterious and beneficial species, since they can be either harmful or beneficial to living systems [9]. Antioxidant supplements or foods rich in medicinal plants may be used to help the human body in reducing oxidative damage by free radicals and active oxygen [10]. The present paper is aimed in studying the electrochemical behavior of the ethanol extract by cyclic voltammetric technique and hence the assessment of its antioxidant activity from anodic peak values.
Materials and Methods
Collection of plant material
The bark of the tree Pterocarpus marsupium RoB was collected from the hilly regions near Theerthalli, Shimoga district, Karnataka. The bark sample collected was chopped to small pieces and shade dried which was then pulverized mechanically. The powdered bark was defatted using petroleum ether in Soxhlet apparatus and, hot extraction was carried out with defatted material successively with chloroform and ethanol .The extracts were concentrated in vacuum using rotary flash evaporator under reduced pressure. The ethanol extract (PME) was used for further studies.
Microorganisms for test
Gram negative bacteria-Vibrio cholera, Gram positive bacteria– Bacillus polymyxa and fungi-Candida albicans were selected.
Medium; Nutrient agar and broth for bacterial cultures, Czapedox agar and broth for fungi.
Phytochemical screening
Phytochemcial analysis of the extract was carried out as described [11]. By this analysis, the presence of several phytochemicals such as carbohydrates, protein, triterpenoids, flavonoids, saponins, tannins, cardiac glycoside, coumarins, fats and oils were confirmed.
Antimicrobial activity
Agar well diffusion method was employed to determine antimicrobial activity. The medium for growth used here was Nutrient agar and Czapedox. Initially, the stock cultures of bacteria and fungi were revived by inoculating in broth media and grown at 37°C for 18 hrs. The agar plates of the above media were prepared and wells (0.5 cm in diameter) were made in the plate. Each plate was inoculated with 18 hours old cultures (100 μl, 104 cfu) and spread evenly on the plate. After 20 min, the wells were filled with different concentrations of samples (0.1, 0.3, 0.6, 1.25, 2.5, 5 mg). The control wells were filled with Gentamycin and Amphotericin. All the plates were incubated at 37°C for 24 hrs for bacteria, 96 hrs for fungi and the diameter of inhibition zones were noted.
The minimum inhibitory concentration was evaluated by broth dilution method. To begin with, the stock cultures of bacteria and fungi were revived by inoculating in broth media and grown at 30-37°C for 18 hrs. The flasks containing above respective media were prepared, autoclaved and different concentrations of the samples (0.25, 0.5, 0.75, 1.0, 1.25 mg/ml) were added. Each flask was inoculated with 18 hours old cultures (100 μl, 104 cfu). A control flask with inoculums and without any sample was prepared along with a sterile media flask as blank. All the flasks were incubated at 37°C on a shaker with 140 rpm for 24 h, 30°C with 140 rpm for 96 hrs and the growth was measured at 660 nm. The lowest concentration with no visible growth and inhibitory activity more than 90% by absorbance calculation was termed as MIC.
Statistical analysis
The results acquired from the antimicrobial analysis have been expressed as mean ± SEM of triplicates. The data were evaluated by one way analysis of variance (ANOVA) followed by turkey’s multiple pair wise comparison tests to assess the statistical significance. The data were considered at P ± 0.01.
Cyclic voltammetric studies
Cyclic voltammetry is a method for investigating the electrochemical behaviour of a system. Cyclic voltammetry is the most widely used technique for acquiring qualitative information about electrochemical reactions. The power of cyclic voltammetry results from its ability to rapidly provide considerable information on the thermodynamics of redox processes, on the kinetics of heterogeneous electron-transfer reactions, and on coupled chemical reactions or adsorption processes. Cyclic voltammetry is often the first experimental approach performed in an electroanalytical study, since it offers rapid location of redox potentials of the electroactive species and convenient evaluation of the effect of media upon the redox process [12-16].
Apparatus
The electrochemical experiments were carried out using a Electrochemical Workstation CHI 660c. All the experiments were carried out in a conventional three electrochemical cell. The electrode system contained a working electrode was bare CPE and ethanol extract modified CPE (3 mm in diameter), a platinum wire as counter electrode and saturated calomel electrode as reference electrode.
Preparation of carbon paste electrode
Carbon paste was prepared by grinding the 70% graphite powder and 30% silicon oil in an agate mortar by hand mixing for about 30 minute to get homogenous bare CPE. The paste was packed into the cavity of homemade carbon paste electrode and smoothened on weighing paper [17-20].
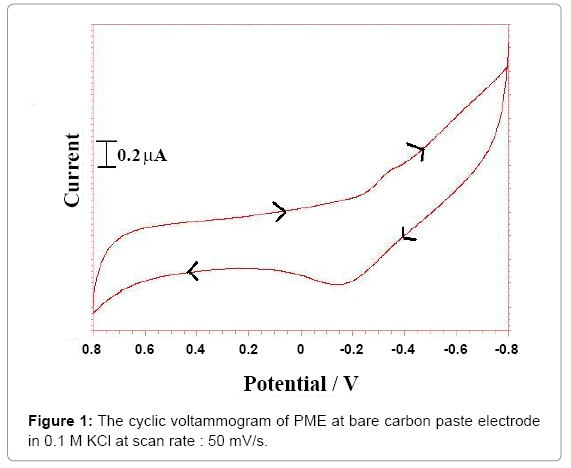
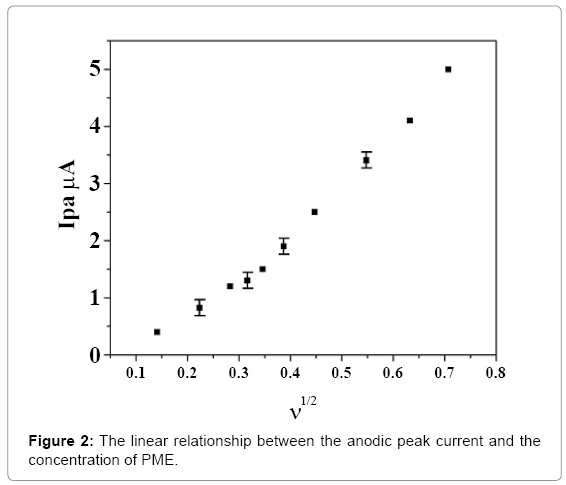
Cyclic voltammetry was applied to characterize the reducing ability of ethanol extract and a good correlation was observed between redox potentials and antioxidant properties. It has been proved that compounds with strong scavenging capabilities, oxidize relatively at low potentials [21]. Cyclic voltammetric behavior of the extract was carried on a carbon paste electrode. To evaluate the effect of different scan rate on the anodic oxidation of the extract, the concentration was kept at 2 mg in 1 M potassium chloride. Cyclic voltammetric behavior of the crude extract was studied by varying the sweep rate from 50 to 300 mV s-1. The effect of scan rate and concentration effects was studied and plots were linear which indicate that the overall electrode process was found to be adsorption controlled as shown in Figures 1 and 2.
Results and Discussion
Qualitative analysis of ethanolic extract of bark revealed the presence of some secondary metabolite alkaloids, glycosides, flavonoids, flavanols, phenols, saponins and terpenoids. The bark extract was found to be containing tannin glycosides, alkaloids, steroids and flavonoids which are biologically active. Among the most of the phytoconstituents which posses potent antibacterial activity, alkaloids also exhibit microbicidal action. Antibacterial and antifungal activity of stem bark extract of P. marsupium against different bacterial pathogens is displayed in table below. The growth of bacteria Bacillus polymyxa, and Vibrio cholera was significantly inhibited by PME and exihibited the inhibition zones 5.0 mm each at 1.25 mg/ml concentration respectively (agar well diffusion method Table 1). The bacterium Bacillus polymyxa and Vibrio cholerae were inhibited at a minimum inhibitory concentration of 1.25 mg/ml while inhibition by fungi Candida albicans was found at 25 mg/ml through broth dilution method (Table 2). The inhibition by negative control was zero while the standard antibiotic Gentamycin inhibited the growth of all the bacterial species effectively at low concentration of 25 μg/ml to a maximum concentration of 800μg/ml with the zone of inhibitions ranging from 13 to 33mm respectively (Table 1).
| Organism | 0.1mg | 0.3mg | 0.6mg | 1.25mg | 2.25mg | 5mg | Gentamycin 25 μg | Amphotericin 100 μg |
|---|---|---|---|---|---|---|---|---|
| V.cholerae | 0 | 0 | 0 | 5 | 10 | 15 | 13 | - |
| B.polymyxa | 0 | 0 | 0 | 5 | 8 | 14 | 15 | - |
| C.albicans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
Table 1: The results are presented in the above table as diameter of inhibition zones in mm along with controls Gentamycin and Amphotericin.
| Sample | Concentration (mg/ml) | V. cholera | B. polymyxa | C. albicans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OD (660 nm) | % inhibition | MIC (mg/ml) | Concentration (mg/ml) | OD (660 nm) | % inhibition | MIC (mg/ml) | Concentration (mg/ml) | OD (660 nm) | % inhibition | MIC (mg/ml) | ||
| PME | Control | 0.78 | 0 | Control | 0.93 | 0 | Control | 0.46 | 0 | |||
| 1.25 | 0 | 100 | 1.25 | 1.25 | 0 | 100 | 1.25 | 30 | 0 | 100 | ||
| 1 | 0.12 | 84.62 | 1 | 0.18 | 80.65 | 25 | 0 | 100 | 25 | |||
| 0.75 | 0.49 | 37.18 | 0.75 | 0.52 | 44.09 | 20 | 0.19 | 58.70 | ||||
| 0.5 | 0.78 | 0.00 | 0.5 | 0.93 | 0 | 15 | 0.38 | 17.39 | ||||
| 0.25 | 0.78 | 0.00 | 0.25 | 0.93 | 0 | 10 | 0.43 | 6.52 | ||||
Table 2: The table shows the minimum inhibitory concentration of the PME against micro organisms. V.cholerae and B. polymyxa shows complete inhibition at PME concentration of 1.25 mg/ml. C. albicans is inhibited at a concentration of 25 mg/ml.
Phenolics and polyphenols present in the plants are known to be toxic to the microorganism. In vitro studies [22] showed that tannins with different structure inhibited the growth of the microorganism. Flavonoids have been reported to have both antibacterial and antifungal activities. The bark extract was found to be containing tannin glycosides, alkaloids, steroids and Flavonoids which are biologically active. The different rates of inhibition observed may be probably due to the quantity of the phytochemicals present in the extract [23-27]. The increase of antibiotic resistance by the pathogenic microorganisms to conventional drugs has necessitated the search for new, efficient and cost effective drugs for the control of infectious diseases.
Antioxidant property by electrochemical method
The shape of the cyclic voltammogram of extract was given in Figure 1. As the concentration of extract was varied from 1 to 6 mg, the cyclic voltammograms were recorded. The anodic peak current shifts Epa in anodic direction with concentration of the compounds has indicated that product of extract was adsorbed over the electrode surface. These observations suggest that the process was diffusioncontrolled [28]. The anodic peak potential Epa was found to shift in the anodic direction with increase in sweep rate indicating the quasireversible nature of the electrode reaction. The anodic peak current (ipa) was found to increase linearly with the square root of sweep rate (ν1/2) and the current function values (ip/1/2) were found to be almost constant. Anodic peak current ipa obtained were found to increase linearly with increase in concentration of the extract. It was also observed that the anodic peak potential (Epa) and half peak potential (Ep/2) were shifted towards more positive values suggesting that the extract could be scavenging oxygen free radicals (Figure 2).
Conclusion
The present study of ethanol extract of stem bark of Pterocarpus marsupium pertaining to antimicrobial activity in the pharmacological point of view portrays that the crude extract has a antimicrobial activity against the organisms mentioned above and further studies for identification and elucidation of active constituents in the plant materials tested is expected to serve as lead in the development of novel bioactive antimicrobial compounds which can be useful in designing of new drugs active against various infectious micro-organisms. The cyclic voltammetric behaviors of ethanol extract show the presence of anodic and cathodic peak illustrate the redox process of extracts. The antioxidant properties of the extracts were assessed by cyclic voltammetric method from its oxidation potential values. Generally there is a relationship between antioxidative and per oxidative activities and oxidative potentials. The lower the antioxidant potential of extract higher would be the antioxidant capacity. Lower the oxidative potential, higher is the ability to donate electron easily to the system generate free radicals.
References
- Sambat-Kumar R, Sivakumar T, Shanmuga-Sundaram R, Sivakumar P, Nethaji R, et al. (2006) Antimicrobial and Antioxidant Activities of Careya arborea Roxb Stem Bark. Iran J. Pharmacol.Therapeut 5: 35-41.
- Ivorra MD, Payá M, Villar A (1989) A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol 27: 243-275.
- Kameswara Rao B, Giri R, Kesavulu MM, Apparao C (2001) Effect of oral administration of bark extracts of Pterocarpus santalinus L. on blood glucose level in experimental animals. J Ethnopharmacol 74: 69-74.
- Narendra Kumar, Seshadri TR (1976) A new triterpene from Pterocarpus santalinus bark. Phytochemistry., 15: 1417-1418.
- Mankani KL, Krishna V, Manjunatha BK, Vidya SM. Singh JSD, et al. (2005) Studies on the sedative effect of Pterocorpus marsupium Roxb. Advanced Pharmacology and Toxicology 7: 19-22.
- Mankani KL, Krishna V, Manjunatha BK, Vidya SM. Singh JSD, et al. (2004) Evaluation of wound healing activity of stem bark of Diospyros cordifolia. Indian Drugs 41: 628-632.
- Mankani KL, Krishna V, Manjunatha BK, Vidya SM. Singh JSD, et al. (2006) Hepatoprotective effects of the triterpeens isolated from the stem bark of Diospyros cardifolia Roxb. J. Natual. Remedies 6: 147 -152.
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266: 37-56.
- Flora SJ (2007) Role of free radicals and antioxidants in health and disease. Cell Mol Biol (Noisy-le-grand) 53: 1-2.
- Shajiselvin CD, Kottai Muthu A (2011) Antioxidant activity of various extracts from whole plant of Bauhinia Purpurea (Linn): An invitro Evaluation. Journal of Advanced Pharmaceutical Research 2: 31-37.
- Harborne JB (1998) Phytochemical methods: A guide to modern techniques of plant analysis. 3rd ed. Chapman & Hall Pub., London, UK.
- Hibbert DB (1993) Introduction to Electrochemistry, Macmillan Press Ltd., London.
- Kaifer AE, Gomez-Kaifer M (1999) Supramolecular Electrochemistry, Willey, VCH, New York.
- Bard AJ, Faulkner LR (1996) Electrochemical Methods: Fundamentals and Applications, John Willey & Sons.
- Davis DG (1978) Physical Chemistry: The porphyrins, Part A, Vol. III. D. Dolphin, Ed Academic Press, New York.
- Brown ER and Large RF (1971) in Physical Methods of Chemistry, Vol-1, Part IIA: Electrochemical Methods, eds. A. Weissberger and B. Rossiter, (Willey-Interscience, New York).
- Niranjana E, Kumara Swamy BE, Raghavendra Naik R, Sherigara BS, Jayadevappa H (2009) Electrochemical investigations of potassium ferricyanide and dopamine by sodium dodecyl sulphate modified carbon paste electrode: A cyclic voltammetric study J. Electroanal. Chem 631: 1–9.
- Ongera Gilbert, Kumara Swamy BE, Umesh Chandra, Sherigara BS (2009) Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study J. Electroanal. Chem 636: 80–85.
- Sathish Reddy, Kumara Swamy BE, Jayadevappa H (2012) CuO nanoparticle sensor for the electrochemical determination of dopamine Electrochim. Acta 61: 78–86.
- Mohan Kumar, Kumara Swamy BE, Sathish Reddy, Sathisha TV, Manjanna J (2013) Synthesis of ZnO and its surfactant based electrode for the simultaneous detection of dopamine and ascorbic acid. Anal. Methods 5: 735-740.
- Simić A, Manojlović D, Segan D, Todorović M (2007) Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 12: 2327-2340.
- Chung KT, Wong TY, Wei CI, Huang YW, Lin Y (1998) Tannins and human health: a review. Crit Rev Food Sci Nutr 38: 421-464.
- Tsuchiya H, Sato M, Miuazaki T, Fujiwara S, Tanigaki S (1996) Comparative study on the antibacterial activity of phytochemical flavonones against methicillin resisitant Staphylococcus aureus. J. Ethnopharmacol 50: 27-34.
- Bojase G, Majinda RR, Gashe BA, Wanjala CC (2002) Antimicrobial flavonoids from Bolusanthus speciosus. Planta Med 68: 615-620.
- Shimada T (2006) Salivary proteins as a defense against dietary tannins. J Chem Ecol 32: 1149-1163.
- Sarkar SD, Muniruzzaman S, Khan SI (1991) Antimicrobial activity of Piper Chaba Hunter (Chui). Bangladesh J. Bot 20: 179–182.
- Adekunle AA, Ikumapayi AM (2006) Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J 55: 219-223.
- Darshan Raj CG, Sarojini BK, Bhanuprakash V, Yogisharadhya R, Kumara Swamy BE, et al. (2011) Studies on radioprotective and antiviral activities of some bischalcone derivatives. Med Chem Res 21: 2671-2679.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15514
- [From(publication date):
December-2014 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10834
- PDF downloads : 4680