Research Article Open Access
Evaluation of Aeromonas Spp. In Microbial Degradation and Decolorization of Reactive Black in Microaerophilic – Aerobic Condition
| Maulin Shah* | |
| Industrial Waste Water Research Laboratory, Division of Applied & Environmental Microbiology, Enviro Technology Limited, India | |
| Corresponding Author : | M. Shah Industrial Waste Water Research Laboratory Division of Applied & Environmental Microbiology Enviro Technology Limited, India Tel: 91-9099965504 Fax: +91-2646-250707 E-mail: shahmp@uniphos.com |
| Received August 11, 2014; Accepted September 20, 2014; Published September 22, 2014 | |
| Citation: Shah M (2014) Evaluation of Aeromonas Spp. In Microbial Degradation and Decolorization of Reactive Black in Microaerophilic – Aerobic Condition. J Bioremed Biodeg 5:246. doi:10.4172/2155-6199.1000246 | |
| Copyright: © 2014 Shah M . This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Azo dyes are a widespread class of poorly biodegradable industrial pollutants. In anaerobic environments, azo bonds are reductively cleaved yielding carcinogenic aromatic amines, many of which are assumed to resist further metabolism by anaerobes bacteria. The latter compounds generally require aerobic conditions for their degradation. Reactive Black was found to be degraded using Aeromonas spp. to α-ketoglutaric acid with transient accumulation of 4-aminobenzenesulphonic acid (sulphanilic acid), 4-amino, 3-hydronapthalenesulphonic acid and 4-amino, 5-hydronapthalene 2,7 disulphonic acid as a degradation intermediate in anaerobic facultative batch culture. Colour and Total Organic Carbon (TOC) was successfully removed more than 95% and up to 50% respectively. There is no significant correlation between pH and oxygen depletion since there is slightly change in pH was observed (pH from 7.21 to 7.25) though the anaerobiosis was found developed throughout the experiment (redox potential from 0.7 to 1.6 mV). The anaerobic metabolism of glucose as co-metabolite also shown to provide the electrons required for the initial reductive cleavage of the azo group. This finding suggest that it is possible to mineralize the azo dye in the environment; thereby, avoiding accumulation of toxic intermediates in the water. The results provide evidence that the successive microaerophilic/aerobic stages, using Aeromonas spp. in the same bioreactor, were able to form aromatic amines by the reductive break down of the azo bond and to oxidize them into non-toxic metabolites.
| Keywords |
| Aeromonas; Azo dye; Total organic carbon; Reactive black |
| Introduction |
| Rapid industrialization has necessitated the manufacture and use of different chemicals in day to day life [1,2]. The textile industry is one of them which extensively use synthetic chemicals as dyes. Wastewaters from textile industries pose a threat to the environment, as large amount of chemically different dyes are used. A significant proportion of these dyes enter the environment via wastewater [1]. Approximately 10,000 different dyes and pigments are used industrially and over 0.7 million tons of synthetic dyes are produced annually, worldwide [3]. Pollution due to textile industry effluent has increased during recent years. Moreover, it is very difficult to treat textile industry effluents because of their high BOD, COD, heat, color, pH and the presence of metal ions [4]. The textile finishing generates a large amount of waste water containing dyes and represents one of the largest causes of water pollution [5], as 10-15% of dyes are lost in the effluent during the dyeing process [6]. The new closed-loop technologies such as the reuse of microbial or enzymatic treatment of dyeing effluents could help reducing this enormous water pollution [6]. Azo dyes have been used increasingly in industries because of their ease and cost effectiveness in synthesis compared to natural dyes. However, most azo dyes are toxic, carcinogenic and mutagenic [6]. Azo bonds present in these compounds are resistant to breakdown, with the potential for the persistence and accumulation in the environment [1,2]. However, they can be degraded by bacteria under aerobic and anaerobic conditions [3,4]. Several physico-chemical techniques have been proposed for treatment of colored textile effluents. These include adsorption on different materials, oxidation and precipitation by Fenton’s reagent, bleaching with chloride or ozone photo degradation or membrane filtration [6]. All these physical or chemical methods are very expensive and result in the production of large amounts of sludge, which creates the secondary level of land pollution. Therefore, economic and safe removal of the polluting dyes is still an important issue. Bioremediation through microorganisms has been identified as a cost effective and environment friendly alternative for disposal of textile effluent [3,4]. In recent years a number of studies have focused on some microorganisms capable of degrading and absorbing dyes from wastewater. A wide variety of microorganisms are reported to be capable of decolonization of dyes [3,7-18]. Reductive azo dye decolourization by microorganisms usually starts with the cleavage reduction of the azo bond under anaerobic or microaerophilic conditions, and leads to the accumulation of toxic aromatic amines [19]. To overcome this problem, recent studies included combinations of anaerobic and aerobic steps in an attempt to achieve not only dye decolourization but also degradation of the aromatic amines [20-22]. However, very few studies have been performed using sequential microaerophilic/ aerobic conditions with the same microorganism, preferring the use of consortia or different microorganisms, used separately under anaerobic, microaerophilic and aerobic conditions [23-26]. In this study, degradation of Reactive Black dyes was carried out under microaerophilic conditions (O2 limited environments) until no colour was observed using a facultative Aeromonas spp. The medium was then aerated by stirring to promote oxidation of the aromatic amines formed by reductive break down of the azo bond, into non-toxic metabolites. The degradation products were characterized by FT-IR and UV–vis techniques and their toxicity and Total Organic Carbon (TOC) measured. Thus, the main achievement of this work was to prove that the degradation of azo dyes in a successive microaerophilic/aerobic process using, exclusively, a facultative Aeromonas sp. bacterium isolated from activated sludge of common effluent treatment plant was possible not only to decolourize the dyes but also to achieve a good degree of mineralization and low toxicity with low running and maintenance costs. |
| Materials and Methods |
| Chemicals, culture medium and isolation process |
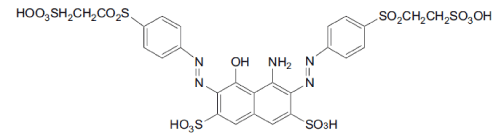
| The azo dyes Reactive Black was kindly provided as a gift by local textile industry (Figure 1). All other reagents were analytical grade and purchased from Sigma and used without further purification. Mineral salts medium (MM), pH 7 was prepared as previously described [25,26]. To evaluate the effect of different carbon sources on dye decolorization MM was supplemented with the indicated amounts of glucose, sodium pyruvate and/or yeast extract, and 100 mg/L of dye. The highest degree and rate of decolorization occurred using MM supplemented with 3 g/l glucose and 1 g/l pyruvate, and this medium was used for all subsequent biodegradation experiments and was designated MM rich mineral medium (MMR). Strain isolation and characterization Aeromonas spp. was isolated from activated sludge obtained from the common effluent treatment plant of Ankleshwar, India. Serial dilutions (10-1 to 10-6) of the sludge were inoculated by the spread plate technique onto Nutrient Agar plates containing azo dyes (100 mg L-1) and incubated under low oxygen conditions. Aeromonas spp. was chosen for further evaluation based the production of a large decolorization zone in the azo dye containing plates. The strain was maintained on slants of Nutrient Agar. The identification of Aeromonas spp. was based on standard morphological and biochemical methods as described by Benson [27], and 16S rRNA gene sequence analysis. Genomic DNA was obtained according to Ausubel et al. [28]. The 16S rRNA gene was amplified by PCR using the bacteria specific primers, 27f and 1401r [29]. |
| Identification |
| Screening of the strains for dye decolorization was performed by enrichment culture technique using NM9 and DNM9 media as described. After purification by successive single colony isolation on a DNM9 agar plate, Aeromonas spp. was identified by carbon source utilization patterns using Biolog GN2 microplate (Biolog, USA) and the analysis of 16S rDNA sequences. For the 16S rDNA sequence analysis, bacterial genomic DNA was extracted and purified using a Wizard Genomic DNA Prep. Kit (Promega Corp., Madison). Two primers annealing to the 5` and 3` end of the 16S rRNA gene were 5`-GAGTTTGATCCTGGCTCAG-3` (positions 9 to 27 (Escherichia coli 16S rDNA numbering)) and 5`-AGAAAGGAGG TGATCCAGCC-3` (positions 1542 to 1525 (E. coli 16S rDNA numbering)), respectively. Polymerase Chain Reaction (PCR) was performed as follows: predenaturation at 95°C for 5 min, 30 cycles at 95°C for 40 s, 55°C for 40 s and 72°C for 2 min. The PCR product was subcloned into pGEM-T easy vector (Promega, Madison, USA) and its nucleotide sequence was determined by Banglore Genei Ltd. (Banglore, India). The partial rDNA sequences were analyzed using a BLAST search algorithm to estimate the degree of similarity to other rDNA sequences obtained from the NCBI/GenBank. Phylogenetic trees were constructed by the ClustalX program [30]. Physiological characteristics were determined according to the procedures outlined in Bergey`s Manual of Determinative Bacteriology [31]. |
| Decolorization assay |
| Decolourization assays were carried out under static conditions with 350 mL cultures of MMR (pH 7) supplemented with 100 mg L-1 of the dyes. The samples were incubated under microaerophilic conditions at 30°C for 168 h or until no colour was observed. Further aeration was carried out in a shaker at 150 rpm to promote oxidation of the degradation products. Dye decolourization was measured in a UV–visible spectrophotometer for the microaerophilic and aerobic stages, and the percentage of effluent decolourization was calculated. conditions. |
| Detection of aromatic amines |
| The aromatic amines in the solid phase were determined by the modified Marik and Lam [32] method. Samples were taken after incubation under the microaerophilic and aerobic conditions, frozen and freeze-dried. The samples (5 mg) were dissolved in 5 mL of a 0.4% solution of chloranil in dimethylformamide (DMF) and heated to 100°C for 5 min. The absorption was determined in a UV–vis spectrophotometer at 560 nm. A calibration curve was prepared using aniline-2-sulfonic acid as a model product of the reduction of azo dyes, and the sample amine concentration was calculated in millimoles L-1. The control was the MMR medium without the dye, treated with the bacterium under microaerophilic and aerobic conditions. |
| Infra-red analysis |
| The controls and samples were dried and mixed with KBr (1:20; 0.02 g of sample with KBr at a final weight of 0.4 g). The samples were then ground, desorbed at 60°C for 24 h and pressed to obtain IRtransparent pellets. The absorbance FT-IR spectra of the samples were recorded using an FT-IR Spectrum 2000 Perkin–Elmer spectrometer. The spectra were collected within a scanning range of 400–4000 cm- 1. The FT-IR was first calibrated for background signal scanning with a control sample of pure KBr, and then the experimental sample was scanned. The FT-IR spectrum of the control was finally subtracted from the spectra of the non-degraded and degraded dyes. |
| Liquid Chromatography and Mass Spectroscopy analysis (LCMS analysis) |
| About 100 ml of Reactive Black (200 mg/L) containing MSM Media treated with the isolate and the purified enzymes was extracted with equal volume of ethyl acetate at various time intervals (0,24 h). The extract was evaporated in a vacuum evaporator and used for LC-MS analysis. The powdery residue was then dissolved in acetonitrile (HPLC Grade). LC-MS analysis was performed using Finnigan model Mass Spectrometer (Thermo Electron Corporation, USA) using C-18 column from Waters. The cartridges were conditioned with pure acetonitrile, washed with deionized water (0.1% Formic acid) and the elution took place with 70% acetonitrile, containing 0.1% formic acid. The flow rate was 0.8 ml/ min. The ion trap detector with atmospheric pressure electro-spray ionization (API-ESI) source was used for quantification in negative ionization mode. Operating conditions were dry with temperature of 325°C, Capillary voltage 3500 V, Nebulizer 14 psi, dry gas Helium 5.0 l/min. Ion trap full scan analyses were conducted from m/z 200-1400 with an upper full time of 300 minutes. The nebulizer gas flow and the curtain gas flow (Nitrogen gas) were set at 10 and 8 psi. The ion spray, orifice and ring voltage were set at +4800, 40, +70 V respectively. Instrumentation control of data acquisitions were performed with data analysis MS. |
| TOC measurement |
| The presence of organic carbon in the samples containing dyes was monitored by measuring the total organic carbon (TOC) under microaerophilic conditions and after agitation, using a TOC analyzer (Shimadzu.Japan) with automatic injection of the samples (500–2000 mL). Samples were centrifuged (20,000 x g for 15 min) and filtered through a 0.45-mm pore size filter. The TOC measurement is based on the combustion of the total carbon (TC) on a sample filled tube with a catalyst and heated to 680°C. The standard deviation is less than 2% for full scale ranging from 2000 ppm to 4000 ppm. All the samples were analysed using a five-point calibration curve. |
| Results |
| Screening |
| Pure bacteria were inoculated (10% v/v) into Chemically Synthetic MgSO4.7H2O (0.1), CaCl2 (0.02), (NH4)2SO4, (1), Glycerol (1), and Reactive Black dye (0.1), adjusted to pH 7 prior to sterilization. Bacteria were grown under facultative anaerobic conditions (1:1 ratio of medium to the total volume of container) for 24 h at 37°C, prior to monitor the decolorization activity. Other parameters were also screened for their removal such as sulfate, phosphate, nitrate and COD. The culture (10 mL) was taken out gradually every 24 hours and reagent was mixed and read by using Shimadzu UV-1800 Spectrophotometer. The decrease of these parameters can increase the quality of the wastewater treated. |
| Methodology for identification |
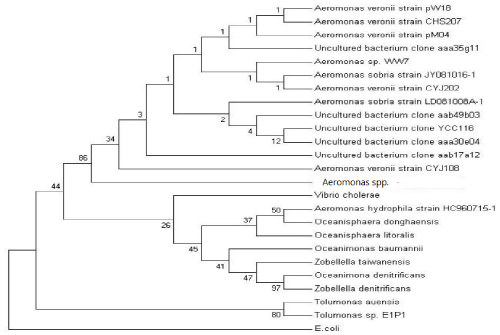
| The total genomic DNA of Aeromonas spp. was isolated using Promega WIZARDR Genomic DNA Purification kit. The forward primers, FD1 (5’-AGAGTT TGATCC TGGCTCAG-3’) and the reverse primer, RD1 (5’-AAGGAGGTGATCCAGCC-3’) were used to amplify the 16S rRNA sequence of Aeromonas spp. PCR conditions included an initial denaturation for 5 min at 95°C followed by 30 cycles of 1 min at 95°C, 1min at 50°C and 2 min at 72°C. The amplicons were purified and sequenced. The 16S rRNA sequence of Aeromonas spp was analyzed using Basic Local Alignment Search Tool (www.ncbi.nlm.nih.gov/ BLAST). The evolutionary history was inferred using the Neighbor- Joining method [33]. The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxonomy analyzed [34]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxonomy clustered together in the bootstrap test (500 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method [35] and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 1420 positions in the final dataset. This tree was rooted with gram negative bacteria Eschericia coli strain K12 MG1655. |
| Determination of aromatic amine concentration: |
| The decolourized dye showed the presence of aromatic amines after the microaerophilic stage. The diazo RB5 showed the amine concentration 0.28 mM. After the aerobic stage a significant reduction in the amine concentrations was observed (Data not shown). The mass balance of the azo dyes (%), was estimated from the amine concentrations after the microaerophilic dye degradation, assuming that 1 mole of RB5 should produce 3 moles of amines [36]. The diazo RB5, which is known as one of the most recalcitrant dyes, achieved only a 40% of mass balance (Figure 2). |
| Dye decolorization |
| The strain Aeromonas was tested to decolourize Reactive Black in a microaerophilic/aerated sequential process. Complete decolourization (>97%, data not shown) of the azo dyes was achieved in the microaerophilic stage, even if the bacteria showed little growth (data not shown), and no significant changes were detected in the following aerobic stage. Aeromonas decolourize the dyes effectively when the medium was supplemented with yeast extract. The decolourization time showed a relationship with the chemical structure of the dyes. The diazo Reactive Black was decolourized after 24. |
| UV-Visible, FT-IR characterization and TOC reduction |
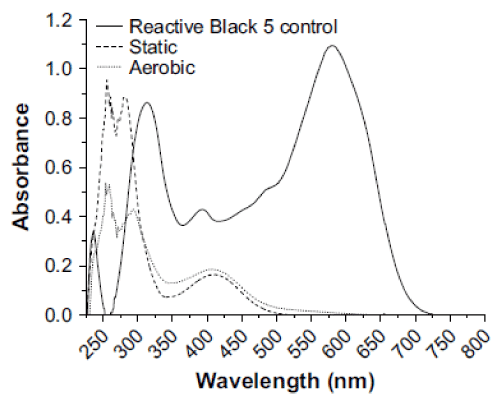
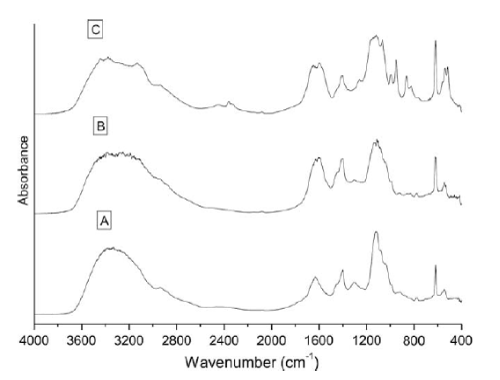
| The biodegradation of the azo dye Reactive Black was monitored by UV–vis analysis. For untreated dyes, as shown in Figure 3 that Reactive Black presented large peaks at 580 and 314 nm. Two additional peaks with low absorbance were observed at 220 and 390 nm. For treated dye, after biodegradation of the azo dye Reactive Black in the microaerophilic and aerobic treated solutions, the absorbance peaks in the visible region disappeared, indicating complete decolourization. In the UV spectra, the peaks at 285 and 320 nm disappeared and were replaced by new peaks at 245 and 260 nm (Figure 3). The FT-IR spectra obtained from the untreated dye samples showed several peaks in the region where N–H and O–H stretching is normally observed (3300–3500 cm-1). After the microaerophilic and aerobic treatments a significant reduction in absorption was observed in this region. Other bands located in the range from 1610–1630 cm-1 and at 1402 cm-1 disappeared in the microaerophilic stage after reductive treatment. Moreover, in the microaerophilic stage two new bands were observed in the carbonyl region at around 1680–1600 cm-1, attributed to amine groups. These two bands disappeared in the aerobic stage and a new peak around 1680 cm-1 was observed. In the aerated samples a new broad region between 2400 and 2500 cm-1, associated with carboxylic acid and NH3 þ ions and new peaks at 850,950 cm-1 and 1140 cm-1, were observed (Figure 4). |
| The reduction in TOC under microaerophilic conditions was only w15%. Conversely, a significant increase in TOC reduction (up to 88%) was observed in the aerobic stage. |
| LC-MS analyses |
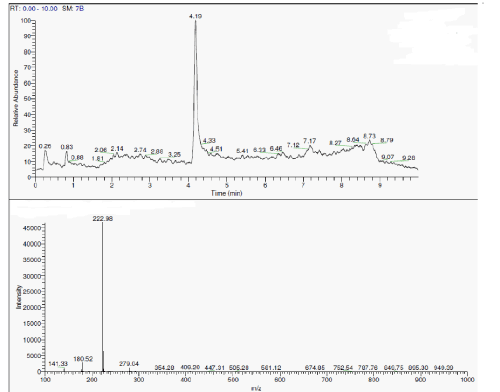
| During the degradation there is asymmetric cleavage of azo bond in RO16 (Figure 5) resulting in formation of 1-amino-1-napthalene sulphonic acid, which was confirmed by the standard NIST library data, this is further, converted to aniline. While the naphthalene part of the dye was further biodegraded with opening of one ring, the formation of aldehyde as one of the intermediate is confirmed from the IR data. |
| Discussion |
| Chen et al. [37] isolated the gene encoding NADPH-flavin azoreductase (Azo1) from the human skin bacterium Staphylococcus aureus ATCC 25923, which confirmed that the enzyme responsible for dye decolourization could be an inducible flavoprotein using both NADH and NADPH as electron donors, as previously reported for other bacterial strains. Azoreductase is the key enzyme responsible for the reductive azo dye degradation in bacterial species. The presence of oxygen normally inhibits the azo bond reduction activity, since aerobic respiration may dominate use of the NADH, thus impeding electron transfer from NADH to the azo bonds [38]. The advantage of the anaerobic reduction of azo dyes is that oxygen depletion is easily accomplished in microaerophilic cultures thus enabling anaerobic, facultative anaerobic and microaerophilic bacteria to reduce azo dyes. The reaction takes place at neutral pH values and is extremely unspecific [39]. However, the precise mechanism of anaerobic azoreduction is still not totally understood. It was recently suggested that microbial anaerobic azoreduction was linked to the electron transport chain, and that dissimilatory azoreduction was a form of microbial anaerobic respiration [40]. In addition, different models for the nonspecific reduction of azo dyes by bacteria, which do not require transport of the azo dyes or reduced flavins through the cell membrane, or that describe the extracellular reduction of azo dyes by anaerobic bacteria, were recently suggested [41]. These results suggested that azo dye reduction was a strain-specific mechanism that could be performed by an azoreductase enzyme or by a more complex metabolic pathway. Thus, due to the lack of information about the metabolism of Staphylococcus arlettae the usual true time dependant kinetic determinations of the azoreductase activity using the azo dye as substrate were not performed, and the azo reduction mechanism in Staphylococcus arlettae will be the subject of a future specific study. In the present work, the strain Aeromonas was tested to decolourize azo dye Reactive Black in a sequential microaerophilic/aerated process in the presence of yeast extract as the source of the electron donors NAD and NADH. It is known that the decolourization rate of azo dyes is increased by using redox mediators such as the water-soluble flavins (FADH2, FMNH2), NADH or NADPH, which speed up the reaction rate by shuttling electrons from the biological oxidation of primary electron donors or from bulk electron donors to the electron-accepting azo dyes [39-41]. Thus, Aeromonas spp. indicated the obligatory requirement of yeast extract as a redox mediator to attain efficient dye decolourization. Yeast extract, a powder supplement consisting of protein, free amino nitrogen, B vitamins, minerals, nucleotides and other yeast cell components, has been the most commonly used nitrogen source for dye decolourization processes [41]. Many pure cultures like Pseudomonas luteola, Klebsiella pneumoniae and Aeromonas hydrophila have exhibited effective decolourization of different dyes in the presence of yeast extract [42-46]. The chemical structures of the dyes greatly influence their decolourization rates, and the decolourization efficiency is limited to several azo dye structures [47]. Dyes with simple structures and low molecular weights usually exhibit higher rates of colour removal, whereas colour removal is more difficult with highly substituted, high molecular weight dyes [40,48]. For this reason, diazo Reactvie Black showed longer decolourization times (24h) (Data not shown). It has been reported that the turnover rate of monoazo dyes increased with increasing dye concentration, whereas the turnover rate of the diazo and triazo dyes remained constant as the dye concentration increased [49]. Moreover, the azo compounds with a hydroxyl or amino groups were more likely to be degraded than those with methyl, methoxy, sulfo or nitro groups [50]. Usually, the presence of sulfonates in reactive dye structures results in low levels of colour removal. However, this is not applicable to direct dyes (DB71) that usually exhibit high levels of colour removal independent of the number of sulfonate groups in the dye structure, reinforcing the idea that steric hindrance and the number of azo bonds are responsible for the different decolourization times [14]. It has also been reported that a correlation between the enzyme redox potential and its activity towards the substrates could influence the decolourization rate [41,51]. In this context, the decolourization times obtained in the present work were in agreement with those of Kimura et al. [52], who found a linear relationship between the cathodic peak potentials and the time of maximum decolourization for several azo dyes using an ascomycete yeast Issatchenkia occidentalis. Thus the ability of the bio-agents to degrade azo dyes depends on the structural characteristics of the dye, the temperature and the pH of the medium, the presence of intermediates and the difference between the redox potentials of the biocatalyst and the dye. Further studies will be carried out to measure the redox potential of the dyes by cyclic voltametry in order to verify this correlation. The biodegradation of the azo dyes was also monitored by UV–vis and FT-IR analyses (Figures 3 and 4). Although the presence of the typical absorption peak of the hydrogenated azo bond structure (ArdNHdNHdAr0) at 245 nm in the spectra seems to indicate only partial azo bond disruption after biodegradation of the four azo dyes in the microaerophilic and aerobic treated solutions, the absorbance peaks in the visible region disappeared indicating complete decolourization [53]. The presence of high concentrations of aromatic amines in the microaerophilic stage confirmed this statement (Data not shnown). In the UV spectra, the decrease in absorbance of the peaks at 220 and 320 nm corresponding, respectively, to the benzene and naphthalene rings [54,55], and the formation of a new peak at 260 nm, suggested that the reductive destruction of the azo conjugated structure disclosed the narrow multipeaks of aromatic rings in the spectra [35]. In the FT-IR analysis, interference by the yeast extract added to the medium restricted data interpretation, showing very similar spectra. However, some conclusions were attained, and the dye (Reactive Black), used as a model substrate, is shown in Figure 4. The bands located within the range 1610–1630 cm -1 and at 1402 cm-1 were due to azo linkages – N=N– on aromatic structures and of –N=N– stretching in a-substituted compounds, respectively [56]. These peaks decreased during the treatment and in some cases disappeared completely in the spectrum of the microaerophilic and aerobic treated dyes, confirming the previous UV–vis results about azo linkage disruption [57]. In the microaerophilic stage, the reduction of the azo linkage peak was followed by the formation of two bands in the carbonyl region at around 1680–1600 cm-1. Two bands in this region were consistent with an amide derived from ammonia or a primary amine. In the aerobic stage these two bands disappeared and a new peak around 1680 cm-1 was observed. The presence of this additional group, due to the conjugation of C=C and C=O groups, suggested that this peak could belong to a carbonyl group in a carboxylic acid, ketone, ester or conjugated aldehyde group attached to an aromatic ring [32]. The fact that no new peaks appeared between 3300-3500 cm-1 (attributed to azo bonds and OH groups in position a relative to the azo linkage) and in the region between 1340 and 1250 cm-1 (–NH2) after the aerobic treatment, suggested that the azo linkage could be transformed into N2 or NH3 or incorporated into the biomass [35,58,59]. Moreover, the presence of a new broad region between 2400 and 2500 cm-1 in the aerobically treated samples, could indicate the presence of carboxylic acid and NH3 þ ions (symmetric stretching mode), suggesting partial mineralization. Also the presence of new peaks at 850 and 950 cm-1 (associated with the out-of-plane bending vibration of substituted benzenes) and the peak at 1140 cm-1 that could belong to acetate, formates, propionates, benzoates, etc., suggested that the products were undergoing irreversible chemical changes, probably due to concomitant biodegradation and autoxidation reactions of the products formed during the reductive dye degradation [60]. A large fraction of the aromatic amines from azo dyes are susceptible to autoxidation, producing water-soluble, highly coloured dimers, oligomers and eventually dark-coloured polymers with low solubility [61]. Remarkably, in contrast to the expectation that bio-recalcitrant aromatic amines would tend to autoxidise forming coloured products, in the present experiment no increase in colour in the visible region was observed in the aerobic stage, suggesting that the aromatic amines were effectively biodegraded. However, although in some cases biodegradation of the dye’s cleavage products was demonstrated [52], it is difficult to predict the fate of the aromatic amines during the anaerobic/aerobic treatment of azo dyes, because it is not clear whether their removal was due to biodegradation, adsorption or chemical reactions [59]. The toxicity results for the controls (data not shown), are in agreement with the findings reported by Hunger and Jung [46] that the reactive dyes and hydrolyzed reactive dyes had a low toxic potential towards aquatic organisms as compared to the basic, acid and dispersed dye., when the medium was incubated under microaerophilic conditions, the reduction in TOC was only w15%, even after 7 days of incubation. Conversely, a significant increase in TOC reduction (w70%, data not shown) was observed in the aerobic stage. It was concluded that even if the microorganisms were able to decolourize the dyes under microaerophilic conditions, the aerobic microorganisms needed aeration not only for amine removal but also for TOC stabilization [62]. |
| Conclusion |
| Reactive Black dye was totally and rapidly decolourized under microaerophilic conditions, with some differences in decolourization times depending on the dye structure, as confirmed by the UV–vis analysis. Decolourizationwas strongly dependent on the presence of yeast extract in the medium, indicating the need for additional vitamin and nitrogen sources. The formation of amines in the microaerophilic stage and their disappearance in the aerobic stage was confirmed by the direct measurement and by FT-IR analysis. In the aerobic stage the partial mineralization of the dye degradation products and of the medium metabolites, was confirmed by the FT-IR, toxicity and TOC measurements. Moreover, high decolourization efficiency was attained in the presence of only slight growth of the bacterium, which would result in very low amounts of sludge formation, thus avoiding high disposal costs. This methodology using a single microorganism in a sequential microaerophilic/aerobic process was shown to be very effective in azo dye decolourization. In a single reactor with a single bacterium, only changing the agitation conditions, it was possible not only to decolourize the dyes, but also to achieve a good degree of mineralization and low toxicity, with low running and maintenance costs. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 21701
- [From(publication date):
November-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 17114
- PDF downloads : 4587
