Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Evaluation of a Multiplex One-Step TaqMan Real-Time Reverse Transcription- PCR Assays for the Detection of H5N1 Avian Influenza Viruses in Clinical Specimens
| Stepaniuk SV1*, Shevchuk VO2 and Spivak MY2 | |
| 1Department of Biotechnology, PJSC "SPC" Diaproph-Med", Kyiv, Ukraine | |
| 2Department of Microbiology, DK Zabolotny Institute of Microbiology and Virology National Academy of Sciences of Ukraine, Kyiv, Ukraine | |
| Corresponding Author : | Stepaniuk SV Department of Biotechnology PJSC "SPC" Diaproph-Med", Kyiv, Ukraine Tel: +38 (044)433-75-82 E-mail: stepaniuk2008@ya.ru |
| Received: June 19, 2015; Accepted: September 14, 2015; Published: September 20, 2015 | |
| Citation: Stepaniuk SV, Shevchuk VO, Spivak MY (2015) Evaluation of a Multiplex One-Step TaqMan Real-Time Reverse Transcription-PCR Assays for the Detection of H5N1 Avian Influenza Viruses in Clinical Specimens. J Infect Dis Ther 3:236. doi:10.4172/2332-0877.1000236 | |
| Copyright: © 2015 Stepaniuk et al .This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
| Introduction |
| The highly pathogenic influenza A virus subtype H5N1 is an emerging avian influenza virus that continues to pose a significant threat to human health. Highly pathogenic avian influenza (HPAI) is very contagious among wild and domestic birds, and can be deadly to them, especially domestic poultry [1-3]. Outbreaks of avian epizootic in the world after first appearing in 2003 have shown that HPAI A (H5N1) could cross the species barrier to infect humans with high mortalities. Since 2003, 650 human infections with HPAI have been reported to the World Health Orgranization (WHO) by 15 countries About 60% of these people died [4,5]. |
| Since October 2005, H5N1 subtype influenza A virus has been circulating in the industrial and domestic poultry population in Crimea Autonomous Republic and other districts of Ukraine. During this outbreak of H5N1 avian epizootic, virus killed or led to the destruction of 238 000 poultry. Thus, HPAI A (H5N1) represents a serious problem to public health. Sensitive and robust surveillance measures are required to detect any evidence that the virus has acquired the ability to transmit between humans and emerge as the next pandemic strain. |
| Diagnosis of avian influenza can be made by complex of methods, including clinical signs, serologic methods and direct virus detection methods. Currently, methods for detection of H5N1 virus include virus isolation, serologic tests and molecular detection of viral nucleic acids [6]. Virus isolation method is time consuming, technically difficult to perform, has a high false-negative rate and therefore it isn’t suitable for routine diagnostics [7]. Commercially available antigen detection tests are based on either immunofluorescence or enzyme immunoassay (EIA) methods and detect all subtypes of influenza A virus. They have low sensitivity for the detection of avian influenza H5N1 in specimens of humans and thereby have limited utility for detection of HPAI A(H5N1) [4]. At the same time serological tests for the detection of virus specific antibodies (hemagglutination inhibition test, EIA, and virus neutralization test) are available. However due to slow seroconversion and technical limitations these assays are impractical for the routine diagnostic testing of clinical samples and are more useful for research studies [4-7]. |
| Molecular diagnostics are based on amplifying nucleic acid to high levels to allow easy identification of the sample. There are several different types of molecular diagnostic tests. Reverse transcriptionpolymerase chain reaction (RT-PCR) and real-time polymerase chain reaction (RRT-PCR) are the most commonly used tests for rapid detection of influenza viral RNA in clinical and laboratory specimens [8]. The analytical sensitivity of the RRT-PCR and the one-step RTPCR, were 10,000-fold and 100-fold greater than the commercial Antigen Capture EIA in detection of H5N1, respectively [9]. |
| The objective of this study was to develop and evaluate rapid TaqMan RRT-PCR for the detection and subtyping of H5N1-specific influenza A viruses. Samples collected during the 2005 H5N1 birds epizootic, H1N12009 human pandemic and experimental H5N1 human infection were used to assess the relative sensitivity and specificity of the designed TaqMan RRT-PCR. |
| Materials and Methods |
| Virus strains and avian specimen |
| Avian influenza A (H5N1) virus isolate (LA-NK-21205) was isolated in Crimea AR in 2005 during epizootic outbreaks among the industrial and domestic poultry. It was kindly provided for our study by National Centre of microbial strains (Kyiv, Ukraine). |
| Human influenza virus reference strains �/FM1/47 (H1N1), �/ Panama/2007/99 (H3N2), �/New Caledonia/20/99 (H1N1), B/Hong Kong/330/01 were kindly provided for our study by Svitlana L. Rybalko and WHO Collaborating Center for Influenza (CDC, USA). |
| Cloaca swab specimens of birds from Odessa zoo park (eagle, pelican, hawk and peacock) and throat swab samples from died horses were collected during the respiratory disease outbreak in 2005. These samples were kindly provided for our study by the Institute of veterinary medicine UAAN (Kyiv, Ukraine). |
| Human respiratory samples (nasopharyngeal swabs and aspirates, sputum) from viral influenza (VI) patients (n=10) were kindly provided by Irina G. Kostenko (the Main Military Clinical Hospital of Ukraine). The clinical material was validated by Seeplex® Influenza A(H1N1pandemic) RT-PCR assay (Seegene Inc., South Korea) and TaqMan Influenza A (H1N1) Assay Sets [10]. |
| In addition, five respiratory samples (nasopharyngeal swabs and sputum) collected from uninfected patients were contaminated by LANK- 21205 Crimean virus isolate to simulate respiratory clinical H5 samples due to the limited availability of H5 clinical samples from human cases in Ukraine. |
| Viral RNA extraction |
| Viral RNA was extracted from 140 μl of avian specimens or standard virus samples using NucleoSpin RNA Virus Kit (Macherey-Nagel GmbH, Germany) based on spin column extraction technology (according to the manufacturer’s instructions). It was used 5 μl of control RNA (MS2-phage) as Internal Control (IC) during the RNA purification stage. All procedures were carried out under the containment conditions of a biosafety level 3 with additional safety precautions. |
| Nucleotide sequence analysis |
| Reaction mixture contained 13 μl of purified DNA, 1 μl of primer (20 pmol), 4 μl RR mix of BigDye Terminator v3.1 Cycle Sequencing Kit (ABI, Foster City, CA), and 2 μl of 5x sequencing buffer, according to the manufacturer’s instructions. The PCR was performed as follows: denaturation for 1 min at 94°C, followed by 25 cycles of PCR amplification, with each cycle consisting of 10 s of denaturation at 96°C, 5 s of annealing at 50°C, and 4 min of elongation at 60°C. The PCR products were purified with a Sephadex G-50 column and subjected to capillary electrophoresis at 3100-Avant genetic Analyzer (ABI, Foster City, CA). The sequence data were analyzed by Nucleotide BLAST Software [11], using the NCBI database [12]. |
| Primers and probe design |
| Nucleotide sequences (N=30) of the matrix (M1), hemagglutinin (HA) and neuraminidase (NA) genes were taken from the Influenza Virus Resource of the NCBI database. The M1, H5, N1 genes A(H5N1) positiveduring of 2004-2012 period subjected to multiple alignments using the BioEdit Sequence Alignment Editor Software version-7.0- (http://www.mbio.ncsu.edu/ BioEdit/bioedit.html) [13]. The conserved regions were used for design of specific primers and TaqMan fluorescent probes with Primer Express 2.0 Program (Applied Biosystems, Cheshire, United Kingdom). The TaqMan-probes were labeled with the fluorescent reporters FAM, HEX/JOE at the 5′-end and non-fluorescent quencher BHQ1, BHQ2 at the 3′-end. Primers and TaqMan fluorescent probes were synthesized by Invitrogen (Invitrogen, Germany) and Sintol, Evrogen (). The amplified fragments have length of 193 bp, 190 bp and 130 bp. |
| Internal quality control (IC-control) |
| MS2 bacteriophage was used to control nucleic acids extraction, as described by Dreier at al. [14]. Specific primers and TaqMan fluorescent probe were designed for simultaneous detection of MS2 bacteriophage genomic RNA and M-gene RNA of influenza HPAI A (H5N1) viruses. TaqMan-probe was labeled with the fluorescent reporter HEX at the 5′- end and non-fluorescent quencher BHQ1 at the 3′-end. The amplified fragment has length of 57 bp. We used method of limitated titration of internal control (MS2-phage) dilution to evaluate the effectiveness of virus RNA isolation from different clinical materials. |
| Engineering of positive control plasmids for HPAI A(H5N1) detection |
| Synthetic positive controls, which represent fragments of M, H5 and/or N1 genes were generated by PCR and cloned into pGEM3Zf(+) vector (Promega). All plasmids were subjected to nucleotide sequencing to ensure the correct target sequences by sequencing with BigDye Terminator v3.1 Cycle Sequencing Kit (ABI, Foster City, CA). Identity of cloned fragments and corresponding virus sequences of target genes were confirmed by multiple alignment using the BioEdit Sequence Alignment Editor Software. Then sequences of engineering fragments M1, H5, N1 genes and corresponding genes A(H5N1) positiveduring (N>100) were analyzed by Nucleotide BLAST Software. The recombinant plasmids were used for amplification as external standards. Standard curve was created with 10-fold serial dilutions of transcribed RNA that contains from 107 to 101 copies of target sequences per reaction. |
| PCR optimization |
| Real-Time RT-PCR was carried out in a 25 μl mixture containing 5 μl RNA, 12.5 μl 2X TaqMan One Step RT PCR Master Mix (Applied Biosystems, USA), 0.75 μl 40X MultiScribe and RNase inhibitor mixture, 0.25 μl forward primer, 0.25 μl reverse primer and 0.125 μl probe using a fluorometric PCR thermocycler (ABI PRIZM 7000). |
| The fixed amount of template in reaction was used to optimize the concentration of primers and probes titration method. The annealing temperature was estimated by the amplification of positive samples. |
| The following criteria were used for determination the optimal amplification conditions: minimal value of threshold cycle (Ct) at maximal value of fluorescence above the background signal (ΔRn). Optimization of temperature regime was carried out to adopt test kit to ABI PRIZM 7000 (Applied Biosystems, USA) and RotorGene 3000/6000 (Corbbett Research, Australia). |
| Results |
| Nucleotide sequence analysis |
| Nucleotide sequences (N=30) of MA, HA, NA genes isolated from HPAI A(H5N1) positive samples during 2004-2006 period downloaded from NCBI database were aligned ( Table 1). |
| We compered human and avian H5N1 HPAI viruses’ nucleotide sequences, which were isolated in different countries: Vietnam, Russia, Iraq, Nigeria, Sudan and three Crimean virus isolates from Ukraine. Conservative regions (99, 8-100% homology) of 130-200 nt in length were selected. These regions were used to design specific primers and probes for detection of HPAI A(H5N1) by multiplex Real-Time RTPCR protocol. BLAST analysis of nucleotide sequences of MA, HA and NA genes of HPAI A(H5N1) strains isolated during 2007-2013 within selected conservative regions revealed 97-99% of nucleotide homology. |
| Multiplex Real-Time RT-PCR protocol |
| In this study we developed a two-stage multiplex RRT-PCR based on the one-step MA, H5 and N1 genes detection. The first stage should distinguish influenza A and B. Then, positive samples were tested by RRT-PCR in one-tube targeting H5- and N1-specific sequences. |
| Design of primers and probes for TaqMan Real-Time RTPCR |
| Using Primer Express V.2 (Applied Biosystems) we designed primers and TaqMan-probes for detection of selected conservative regions of MA, HA and NA genes of HPAI A(H5N1) and for internal control (MS2-fage RNA) detection. Specific primers and probes targeting the M1 gene of influenza A were selected for typing of influenza A virus from influenza B and C and other respiratory viruses. The specific primers and probes targeting the H5 and N1 genes were selected for subtyping of HPAI A(H5N1) from influenza A(H1,H3,H7) viruses. |
| BLAST (Basic Local Alignment Search Tool) analysis of designed primers and probes for M, H5 and N1 genes conferred that they have high homology (>98%) with the corresponding nucleotide sequences of more than 120 genomes of HPAI A(H5N1) strains isolated from birds in Asia, Europe, Russia in 2004 and in Ukraine and Crimea AR in 2005. |
| Optimization of the multiplex assay |
| Primers titration from 100 to 700 nM as well as probes titration from 100 to 300 nM indicated an optimal primers concentration of 500 nM and an optimal probes concentration of 250 nM for all four assays in the multiplex real-time RT-PCR. Application of higher or lower concentrations hadn’t influence on the sensitivity of the multiplex assay significantly (results are’nt shown). The optimized multiplex real-time RRT-PCR assay has a 25 μl PCR reaction volume. |
| The lowest Ct value and highest Δ Rn were observed in the amplification with an annealing temperature of 55°C (at the 1-st step of assay) and of 54°C (at the 2-nd step of assay). Optimization of reaction protocol have been done for Applied Biosystems(ABI PRIZM 7000/7500) and for Corbett Research(Rotor Gene 3000/6000) instruments. |
| Specificity of TaqMan multiplex RRT-PCR assay |
| Specificity of multiplex assay was recently evaluated by crossreactivity with different genotype influenza viruses (H3N8, H7N7) and other avian diseases viruses (Rhinotracheitis virus, Newcastle Disease Virus, Variola avium virus, Chickens Egg Drop Syndrome virus) [15]. In this study we investigated specificity of the Multiplex H5N1 TaqMan RRT-PCR assay by analyzing avian clinical samples from Odessa zoo park, reference CDC human influenza samples and clinical human samples (H1N1) and simulated clinical human samples (H5N1). |
| As it shown in Table 2, positive results at 1st and 2nd stage were obtained only for specimen of peacock died of avian influenza in Odessa zoo park and for cultural sample of Crimean isolate LANK- 21205 of avian influenza virus � (H5N1), which were used as a positive control. |
| The concentration of cultural sample of Crimean isolate LANK- 21205 was 105 EID50. So, the concentration of cultural avian influenza virus Ð� (H5N1) sample isolated from died peacock in Odessa Zoo Park was the same. Fluorescence increasing was not detectable in the samples of ten non-infected allantoic fluides of embryonated hen’s eggs and clinical avian speciments from Odessa zoo park (eagle, hawk, pelican cloak swabs and sample, isolated from died horse). |
| No cross-detection was observed with CDC human influenza B viruses’ sample and influenza viruses A(H1N1), A(H3N2) by targeting the H5-gene. The data are represented in Table 3. |
| Although the pandemic outbreak of influenza A/H1N1/2009 declared finished, this virus continues to circulate and remains a challenge for patient management. Respiratory human clinical samples (n=10) collected during an H1N1 pandemic influenza in in 2009 were also analyzed. All these samples had previously been demonstrated to contain influenza A H1N1pandem 2009 viral RNA (tested by Seeplex® Influenza A (H1N1pandemic), manufactured by Seegene Inc, South Korea) and then were verified by H1N1pandemic2009 primers and probes, recommended by WHO protocol TaqMan Influenza A (H1N1) Assay Sets [16]. Following designed multiplex H5N1 real-time RT-PCR analysis, no cross-detection were observed with all tested human respiratory samples (influenza A(H1N1) pandemic 2009). |
| Due to the limited availability of H5 clinical samples from human cases in Ukraine, we contaminated five human respiratory samples (nasopharyngeal swabs and sputum) from not infected human by LANK- 21205 Crimean virus isolate. All of five simulated HPAI (H5N1) specimens, which tested by the H5N1 TaqMan RRT-PCR, were shown positive results, contained influenza A H5 viral RNA. |
| Analytical sensitivity (or detection limit) measuring of the assay |
| Ten-fold dilutions of AIV H5N1 recombinant plasmids pIMC-3(Mgene), pIHC-11(H5-gene) и pINC-8(N1-gene) were used for the determination of detection limits and the amplification efficiency of the assay (Table 4). Samples were tested in triplicate for each dilutio |
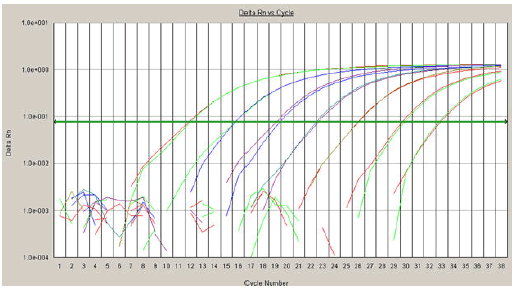
| For determination of the sensitivity of RRT-PCR and creation the standard curve for quantitative analysis and estimation of the linear range, the 10-fold serial dilutions (1-107 copies) of plasmids were used as standards (Figures 1 and 2). |
| The limit of detection was determined as 10 copies in reaction with the Ct value 33 (for H5). The linear range for detection was detected as 10 to 107 copies in reaction. By using the Ct slope method [17,18] and slope values of -3.48 with pIHC-11(H5-gene), -3.45 with pINC-8(N1- gene), and -3.32 for pIMC-3(MA-gene), the amplification efficiency values of the RRT-PCRs were calculated (98, 99, and 100%, respectively). |
| Discussion |
| A number of scientific reports about the use of rapid detection tests for type A influenza virus in poultry have been published [19-25] pointing out the importance of having such tests available. Information about the application of theses assays using human samples or samples collected during an epizootic from naturally infected birds isn’t published. |
| In Ukraine H5N1 infections aren’t endemic, so diagnostic tests for H5N1 avian influenza determination could be performed for detection of influenza virus among tourists returning from H5N1-affected regions and among potentially exposed poultry workers. That’s why, the actual question is the necessity of robust effective HPAI A (H5N1) diagnostics in human and birds populations. |
| The H5N1 RRT-PCR was designed as a two-stage, TaqMan-based multiplex real-time assay to improve efficient processing and reduce the risk of carryover contamination. It proposes to distinguish influenza virus A from influenza virus B and other pathogens at the 1st stage of assay, and to subtype sequence by H5 and N1 assay in one tube at the 2nd stage. Less than 4 h is enough to obtain complete results for the clinical samples. |
| Primers and TaqMan-probes that fit the criteria for suitable realtime PCR primers were selected from highly conserved regions of the H5, N1 and M- gene of H5N1 HPA influenza viruses. Our investigate of MA, HA and NA genes of HPAI A(H5N1) strains isolated in period of 2007-2012 with selected conservative regions showed 97-99% of nucleotide homology. Therefore, mutations appeared after 2006 year hadn’t affected the scope of the annealed primers and probes. We suggest, that designed diagnostic primers and probes are useful for identification of current A (H5N1) strains. |
| Despite the selection of conserved regions, in order to ensure the amplification of target viral RNA from the HA gene of influenza viruses, two degenerate bases were incorporated into the forward H5 primer and one was incorporated into the H5-probe sequence. |
| The H5N1 assay was designed for use on a TaqMan sequence detection platform (Applied Biosystems); however, the test will be useful in a range of laboratories since the assay is transferable to other real-time platforms, such as Corbett Rotor-Gene. |
| For current WHO recommendations [10] all diagnostics test protocols should be validated to ensure adequate specificity and sensitivity. In order to make sure that designed H5N1 Primers and TaqMan-probes are highly sensitive for the determination of H5N1 infection in different clinical sample types we analyzed human and birds clinical samples. The influenza A H5N1 virus Crimean isolate LA-NK-21205 was selected as the positive control material for the RRT-PCR assay in birds, since this strain is representative of H5N1 HPA strains, circulated in poultry in Crimea AR (2005). It was shown that the Multiplex H5N1 RRT-PCR is specific for the detection of influenza A H5N1 HPA viruses, amplifying viral RNA from cloak swab, allantoic fluids of embryonated hen’s eggs, cultural samples. |
| It wasn’t possible to establish the sensitivity of the Multiplex H5N1 RRT-PCR for human clinical sample types, such as secretions from the upper human respiratory tract (sputum, nasopharyngeal swabs and aspirates) because of the limited availability of H5N1 clinical samples from human cases in Ukraine. In this study we tested five simulated respiratory clinical H5N1 human samples. The study demonstrated that the H5N1 RRT-PCR assay is applicable for human samples testing. |
| Non-reactivity of the H5N1 RRT-PCR assay with other influenza A virus subtypes (seasonal H1N1: �/FM1/47, �/New Caledonia/20/99, H3N2: �/Panama/2007/99), influenza B virus subtype and pandemic 2009 H1N1 was demonstrated on four reference and ten human clinical samples respectively. The results confirmed the high specificity of the assay. |
| We analyzed the effectiveness of viral RNA extraction from different clinical materials by using limitation titration method of internal control (MS2-phage) dilutions (data not shown), which amplified together with M-gene of influenza viruses RNA. Our study demonstrated that viral RNA extraction technology by spin column is suitable for analyzing human samples and birds specimens. Thus, the addition of an internal control (IC) could further improve the assay as a diagnostic tool, as each sample could be tested for the quality of the nucleic acid extraction. |
| The limit of detection of H5N1 RRT-PCR test was determined as 10 copies in reaction and the linear range for detection was detected as 10 to 107 copies in reaction by titration of AIV H5N1 recombinant plasmids methods in this study. This is confirmed by our recent research [26] by titration of cultural sample of Crimean isolate LANK- 21205, when analytical sensitivity was detected as 102 to 103 copies in clinical sample. |
| Conclusion |
| The Multiplex H5N1 real-time RT-PCR is reproducible and costeffective assay because it is rapid, specific, sensitive. Therefore, it is applicable for diagnostics and monitoring of influenza virus infection in patients with respiratory symptoms. Also, the designed test-kit is an effective tool for the epizootic monitoring of avian H5N1 HPA influenza viruses in birds. |
| Acknowledgement |
| We are thankful to Svitlana L. Rybalko for providing us with the virus Standard samples from WHO Collaborating Center for Influenza, CDC, USA. We are thankful to Kucheryavenko A. A. (Institute of veterinary medicine UAAN (Kiev, Ukraine) and German V. V. (National Center of microbiology strains (Kiev, Ukraine) for providing us with Crimean virus isolate LA-NK-21205 HPAI A(H5N1) and clinical material. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14219
- [From(publication date):
October-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9699
- PDF downloads : 4520
