| Phutsadee Pudchakan1, Krittika Tanyasaensook1, Pongparadee Chaudakshetrin2, Penkae Ketumarn2 and Chuthamanee Suthisisang3* | |
| 1Department of pharmacy, Siriraj Hospital, Mahidol University, Bangkok, Thailand | |
| 2Department of Anesthesiology, Siriraj Hospital, Mahidol University, Bangkok, Thailand | |
| 3Department of Pharmacology, Mahidol University, Bangkok, Thailand | |
| *Corresponding Author : | Chuthamanee Suthisisang Department of Pharmacology, Faculty of Pharmacy Mahidol University, Bangkok 10700, Thailand Tel: 662-644-8677 Fax: 662-354-4315 E-mail: chuthamanee.sut@mahidol.ac.th |
| Received: February 08, 2016 Accepted: April 15, 2016 Published: April 18, 2016 | |
| Citation: Pudchakan P, Tanyasaensook K, Chaudakshetrin P, Ketumarn P, Suthisisang C (2016) Evaluation of a Methadone Protocol for Severe Chronic Pain Management in Thai Patients: A Prospective Study. J Pain Relief 5:243. doi:10.4172/2167-0846.1000243 | |
| Copyright: © 2016 Pudchakan P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Background: Methadone is a synthetic opioid that is clinically effective in chronic pain management. However, the use of methadone is very limited in Thailand because physicians are not familiar with its dosing and are concerned about risks relating to drug accumulation and cardiac arrhythmias. Objective: The purpose of this study was to develop and implement a methadone protocol for patients with severe chronic pain in order to assure efficacy and safety of methadone. Methods: The protocol was developed based on published clinical studies and guidelines. The validated protocol was implemented in 34 patients at the Pain Clinic, Siriraj Hospital, Bangkok, Thailand. During the study period, pain score, pain interference score, neuropathic pain score, severity of adverse effects, and QTc intervals were investigated over a 3 month period. Results: The results obtained from 21 patients that completed the study showed a significant reduction in median pain intensity (p < 0.001) and other chronic pain interferences based on BPI-T (p < 0.001), excepted for the emotional score (p < 0.004) using methadone doses ranging from 2-30 mg daily. Neuropathic pain was also significantly reduced (p < 0.001). Common adverse effects were drowsiness (55.88%), constipation (35.29%), and nausea and/or vomiting (11.76%). Regarding ECG monitoring, seven patients without QTc prolongation at baseline developed QTc prolongation after methadone initiation. However, QTc interval greater than 500 msec or presentation of Torsades de Pointes were not found. No significant change in the mean QTc interval was observed after initiating methadone (p=0.951). Conclusion: Administration of methadone according to the protocol described in this study was found to be effective and safe for severe chronic pain management. ECG monitoring and drug interaction screening in patient treatment plan is advised when prescribing methadone.
| Keywords |
| Methadone; Chronic pain; Non-cancer pain; Cancer pain; Qtc prolongation |
| Abbreviations |
| BPI-T = The Thai version of the Brief Pain Inventory DN4 = Douleur Neuropathique en 4 questions ECG = Electrocardiogram HERG = Human ether-a-go-go-related gene ITT = Intention-to-treat NE = Norepinephrine QTc intervals = corrected QT interval TdP = Torsades de Pointes 5-HT = Serotonin |
| Introduction |
| Chronic pain is defined as pain that persists beyond normal tissue healing time or a duration longer than 3 months, as defined by the International Association for the Study of Pain (IASP) [1]. Symptoms include either continuous or intermittent pain that may be caused by tumor or multiple other etiologies. Chronic pain is a common problem in countries throughout the world, including Thailand [2-4]. The consequences of uncontrolled chronic pain result in not only physiological symptoms, but also psychiatric disorders that may lead to a severe and debilitating impact on daily life [2,5]. |
| Methadone is a synthetic mu-opioid agonist. It has been proven as a clinically effective in chronic pain management [6,7]. Unlike morphine and fentanyl, methadone inhibits norepinephrine and serotonin reuptake and exhibits non-competitive NMDA receptor blocking activity. These actions make methadone unique and can provide benefits in the management of neuropathic pain, opioid tolerance, and opioidinduced hyperalgesia [6,8,9]. Other positive aspects of methadone include no known active metabolites, long duration of analgesia, and low cost. Methadone, however, is not without associated cautions and concerns; one of which centers on a long and variable elimination halflife, which may lead to accumulation or delayed toxicity. Its metabolism involves cytochrome P450 (particularly CYP3A4, CYP2B6, and CYP2D6), which may results in the potential for drug interactions [10,11]. Moreover, QTc prolongation and/or Torsades de Pointes (TdP) have been reported during methadone therapy [12,13]. |
| In current clinical practice, the use of methadone for pain management in Thailand has been very limited. This is due primarily to physician uncertainty regarding dosing and concerns relating to drug accumulation and cardiac arrhythmias. |
| As such, the aim of this study was to develop an evidence-based methadone protocol for severe chronic pain management and evaluate this protocol in Thai patients. |
| Methods |
| Phase I: Development of a methadone protocol: The development of this methadone protocol was based on an evidence-based review and our clinical experience. Publications relating to methadone dosing were retrieved from MEDLINE, EMBASE, and the Cochrane Database from their respective inceptions to October 2010. For the search, the word “methadone” was combined with the words “AND” or “OR” and then followed individually by each of the following words: “pain”, “chronic pain”, “non-cancer pain”, “cancer pain”, “guideline”, “expert opinion”, “consensus”, and “clinical practice”. These articles were then reviewed. Information considered germane to this study was extracted for use in the development of a methadone protocol. The developed protocol was then validated by pain specialists at Pain Clinic, Siriraj hospital. |
| Phase II: Testing the methadone protocol: An open-label prospective study was conducted at the Pain Clinic, Department of Anesthesiology, Siriraj Hospital, Mahidol University, from June 2011 to December 2013. This trial was approved by the Siriraj Institutional Review Board (SIRB). Calculation of sample size was based on 80% power with two-tailed test and 5% significance level to detect a twopoint reduction in pain intensity using 11-point numerical pain rating scale [14]. Thirty-four out patients, aged 18 years or older, who suffered from severe chronic cancer or non-cancer pain, were eligible for this study. Only the patients who voluntarily signed consent of chronic opioid therapy were enrolled. Outpatients with baseline QTc interval > 500 msec, history of opioid addiction or structural heart diseases, and pregnancy or breastfeeding were excluded from the study. All adjuvant drugs were continued or adjusted by the pain specialist supervisors during the study. |
| The Thai version of the Brief Pain Inventory (BPI-T) [15] and Douleur Neuropathique en 4 questions (DN4) [16] were used for pain and neuropathic pain assessment, respectively. The intensity of opioidrelated adverse events, including drowsiness (sedation score) and nausea and/or vomiting were assessed by the patient using a scale from 0 to 3 (0 = “not at all” or “awake”, 1 = “slight” or “slightly drowsy”, 2 = “a lot” or “frequently drowsy”, 3 = “awful” or “somnolence”). Constipation symptoms were rated, as follows: 0 = 1-2 days per one passage, 1 = 3-4 days per one passage, 2 ± 4 days per one passage, 3 = rectal measures [17]. Other side effects reported by patients during the study were also recorded. Moreover, a resting 12-lead ECG was used to determine QTc interval, which was corrected by heart rate using Bazett’s formula [18]. QTc prolongation was defined as >430 msec in men and >450 msec in women. If QTc interval is above 500 msec, it is considered to be a clinically significant prolongation [19,20]. Risk factors of QTc prolongation were also identified. |
| Methadone protocol |
| The starting methadone dose for naïve-opioid patients was 2.5- 5 mg every 8-12 hour. In patients who required opioid rotation, the conversion ratios of morphine to methadone were 4:1, 8:1, and 12:1 for patients receiving less than 90 mg of morphine, receiving 90-300 mg of morphine, and receiving more than 300 mg of morphine, respectively. Switching methods was a stop-and-go or rapid switching. Breakthrough pain will be managed by morphine syrup as needed. Calculated rescue dose was estimated to be 10-15% of total daily dose of methadone which therefore, was switched to be morphine syrup in the ratio of 1:4. The upward or downward titration should be 20-30% of initial daily dose of methadone for methadone solution. If methadone tablet was selected to be rescue drug, dose adjustments would be 2.5 mg each time depending on pain intensity and adverse effects. All adjuvant drugs and supportive medications which relieved constipation or nausea/ vomiting had been continued during the study. The developed protocol was shown in Table 1. Moreover, ECG monitoring and drug interactions screening between methadone and currently prescribed drugs, particularly CYP 3A4 and CYP 2D6 inhibitors, were checked before prescribing methadone. |
| Data collection |
| All patients underwent clinical assessment at baseline (W0), 2 weeks (W2), 4 weeks (W4), 8 weeks (W8), and 12 weeks (W12) after methadone initiation. The following data were gathered and recorded: age; gender; underlying disease; duration of pain; primary tumor site; type of pain; pre-switching analgesic doses; daily methadone dose; number of rescue doses in a 24-hour period; pain score; pain interference scores; intensity of adverse effects; QTc interval; and risk factor of QTc prolongation. |
| Statistical analysis |
| Data were analyzed using descriptive and frequency analysis. The Shapiro-Wilk test was used to test normality of interval data. For data normality, a repeated measures analysis of variance (ANOVA) was used to analyze the differences between group means. The Friedman test was used to detect and evaluate data deviations across multiple test attempts. The Wilcoxon signed-rank test was used for comparing two related samples of non-parametric data. Statistical significance was set at p < 0.05, with a two-sided test. The program used for analysis was SPSS version 18.0 (SPSS, Inc., IBM, Armonk, NY, USA). |
| Results |
| In a search of medical databases for developing methadone protocol, 3,629 articles were identified and retrieved. Some articles were excluded because they were either duplicated or they were not translated into English. Of 3,629 articles, 2,641 met the eligibility criteria for screening. Of those, 15 articles [17,21-34] were identified that provide useful information regarding administration of methadone for pain management. The methadone protocol that we developed is shown in Table 1. |
| Patient characteristics |
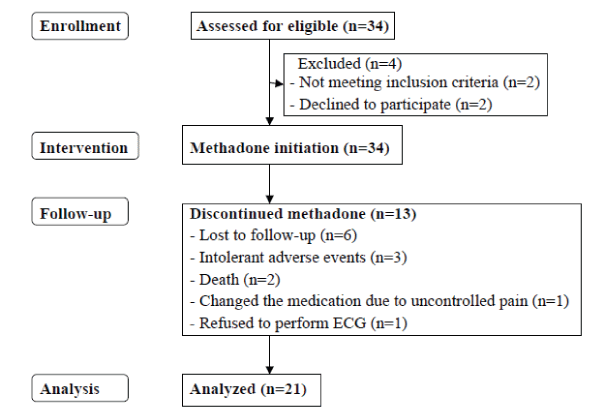
| Thirty four eligible patients were enrolled for protocol testing. A total of 13 patients dropped out of the study for a variety of reasons and at differing time points in the study. At W12, 12 patients with cancer pain and 9 patients with non-cancer pain had completed the study, as shown in Figure 1. |
| Demographic data of the 34 initially recruited patients are summarized in Table 2. Mean duration of pain was 12 months (range: 6-48). In the rotation group, the main reason for switching from oral morphine to oral methadone was poor pain control, despite escalating morphine doses. No participant used oral methadone as an alternative to transdermal fentanyl. |
| Of 23 patients who were diagnosed as cancer, 12 had metastatic stages, 7 had locally advanced cancer or recurrent stages, and 4 had unidentified stages. The top 3 primary tumor sites were: head and neck (43.46%), breast cancer (8%) and lung cancer (8%) |
| Methadone dosing |
| Methadone was initiated and adjusted, according to the developed protocol. In 26 opioid-naïve patients, average initial methadone dose was 7.63 mg daily (range: 4-10 mg). There were 15 patients (57.69%) that did not require a change in their methadone dosing throughout the study. The remaining 11 patients (42.31%) required dose adjustment at least once due to uncontrolled pain and/or intolerant side effects. The most notable intolerant side effect during the follow-up periods was drowsiness. The average dose difference that was increased from baseline was 67.71%. The highest methadone dose for pain control with no associated side effects was 20 mg daily. In addition, some patients who responded well to methadone had an average dose reduction from baseline of 34.71%, with the lowest dose for pain control being 4 mg daily (2 ml of 1 mg/ml methadone solution every 12 hours). |
| Regarding the 8 patients who were switched from oral morphine, the previous median morphine dose was 63 mg daily (range: 20-320 mg). These patients were rotated to an initial median methadone dose of 10 mg daily (range: 5-15 mg). No patients reported withdrawal symptoms after switching to methadone. During the study, 6 patients (75%) required dosage adjustment. The average dose increase from baseline was 61.67%. Dosage was increased up to 30 mg daily with tolerable side effects. The average dose reduction from baseline, which was due to drowsiness, was 43.33%. The lowest dose used for pain control was 2 mg daily (2 ml of 1 mg/ml methadone solution once daily). |
| Overall analgesic efficacy |
| Regarding pain reduction obtained from 21 patients who completed the study, a significant reduction in median overall pain intensity was found throughout the study by both per protocol (p < 0.001) and intention-to-treat (ITT) analysis (p < 0.001). In addition to other chronic pain interferences, median physical score (p < 0.001) and median sleep score (p < 0.001) showed statistically significant improvement. Improvement in median satisfaction score was also found to be statistically significant (p < 0.001). Median emotional score was significantly changed after starting the methadone regimen (p = 0.004), as shown in Table 3. |
| For the subgroup analysis by per protocol approach, a significant reduction in the overall median pain intensity was found in cancer pain patients (p < 0.001), non-cancer pain patients (p = 0.013), naïve-opioid patients (p < 0.001), patients who switched to methadone (p = 0.013), and patients with neuropathic pain (p < 0.001), as shown in Table 4. A comparative analysis of the overall pain score between cancer pain group and non-cancer pain groups demonstrated that there was no difference at W0, W2, W4, W8, but a significant reduction was found at W12 in cancer group (p = 0.041). For naïve-opioid patients and patients who switched to methadone, the overall pain score at W2, W4, W8, and W12 was not significantly different among groups. Focus on 12 patients with cancer pain, there were 7 naïve-opioid patients and 5 patients who switch morphine to methadone. No significant difference in the overall pain score between 2 groups at W2, W4, W8, and W12 was observed. |
| Neuropathic pain assessment |
| In 19 patients with neuropathic pain who completed the study, median neuropathic pain score was 5.67 (range: 3-8), 3.00 (range: 0-7), 2.75 (range: 0-6.5), 1.67 (range: 0-6.5), and 0.75 (range: 0-6.5) for W0, W2, W4, W8, and W12, respectively. A significant reduction in median neuropathic pain score was observed (p < 0.001) at every visit, as shown in Table 3. Intensity of neuropathic pain symptoms, such as electric shocks, needle-liked, tingling and burning were decreased during the study. There were 5 cases who had an improvement of neuropathic pain score with a reduction of the dose of adjuvant analgesic such as gabapentin, oxcarbazepine and Pregabalin [35-37]. |
| Overall adverse effects |
| Common adverse drug reactions found in our study were drowsiness (55.88%), constipation (35.29%), and nausea and/or vomiting (11.76%). Two patients discontinued methadone treatment after developing myoclonus and intolerant constipation, respectively. Frequency of intensity of adverse effects is presented in Table 5. |
| From 20 patients with no drowsiness at baseline, 12 patients reported slight drowsiness and 8 patients reported no drowsiness during the follow-up periods. Only one patient reported slight drowsiness from baseline and for the duration of the study. No patients had nausea and/ or vomiting or received anti-emetics at baseline. During the study, one patient reported nausea and/or vomiting symptoms at W2, but these symptoms resolved and were not seen at W4, W8, and W12. Regarding constipation, 5 patients developed grade-1 constipation despite coadministration with stimulant laxative, as shown in Table 5. |
| Changes in QTc interval |
| Four patients (19.05%) presented with QTc prolongation at baseline monitoring. However, after long-term follow-up, the QTc interval in these patients showed a decreasing trend. From 17 patients without QTc prolongation at baseline, 7 patients (33.33%) developed QTc prolongation during the study. Yet, no significant change in mean QTc interval was observed after initiating methadone (p = 0.916). QTc interval > 500 msec or conversion to TdP was not found at any point in the study, as shown in Table 6. |
| Many risk factors relating to QTc prolongation were observed in our patients. Unmodifiable risk factors of QTc prolongation were older age, female gender, and history of receiving cardiotoxic chemotherapy. Modifiable risk factors of QTc prolongation included hypokalemia, hypocalcaemia, and QTc prolonging drugs (amitriptyline and nortriptyline), which were detected. Hypokalemia and hypocalcaemia were corrected in all the cases. |
| Discussion |
| Chronic pain is one of many non-communicable diseases commonly found in Thailand. Methadone has several compelling pharmacologic properties, including the effective relief of somatic and neuropathic pain. However, the administration of methadone for pain management has been limited in Thailand due to dose-related uncertainties and concerns relating to risk of QTc prolongation. In response to these concerns, we endeavored to develop an evidence-based methadone protocol for pain management for use by clinicians who’s familiar with its risks and dosing. |
| Although most of the established guidelines prefer to use methadone as an alternative to oral morphine, methadone is a valuable addition to the armamentarium of clinicians treating severe chronic pain, particularly when combined with neuropathic pain [38]. The recent EAPC guideline also suggested that methadone may be used as first- or second-line analgesic for severe cancer pain by a clinician who’s familiar with its use [39]. The Cochrane reviewed 9 RCTs regarding the use of methadone in cancer pain concluded that methadone was similar to morphine in terms of the efficacy and tolerability, but its dose titration should be concerned due to a complex pharmacokinetic profile [40]. For patients with chronic non-cancer pain, Chou et al. [21] and Cannadian practice guideline [41] suggested that opioids may be effective therapy and should be considered. However, Cochrane reviews of methadone in non-cancer pain was controversial [42]. In addition, three guidelines suggested that methadone may be benefit for naïve-opioid and rotation group with careful assessment and monitoring throughout treatment [21,43,44]. In our study, all naïve-opoid patients were enrolled due to severe pain with neuropathic component and expected to gain a benefit from methadone. The subgroup analysis results also demonstrated that the overall pain score in naïve-opioids group was significant improved after starting methadone. After screening many articles relating to methadone dosing, we discovered that many methadone dosage regimens were designed based on physician clinical experience. By way of example, Chou et al. recommended the safe starting dose for naïve opioid is 2.5 mg every 8 hours, however, it also suggested that there is insufficient evidence to recommend specific optimal starting doses. Bruera et al. chosed 7.5 mg every 12 hr of methadone for naïve-opioid therapy. Moreover, we have revised the starting doses of methadone which have been used in Siriraj hospital. It was found that the starting dose in successful clinical naïve-opioid cases is 5 mg 8-12 hourly. Therefore, our recommendation that methadone dose be given 2.5 – 5 mg every 8 or 12 hours was based on clinical research findings and our clinical experience. Moreover, we do not recommend methadone syrup for breakthrough pain (reason: to avoid unintentional accumulated toxicities), even though some studies recommend methadone syrup for breakthrough pain [45,46]. |
| In case of changing from oral morphine to oral methadone, the results obtained from systematic review showed that the conversion ratios widely ranged from 4:1 to 37.5:1 in pain treatment [47]. Some studies revealed that an initail fixed conversion ratio of 10:1 [25] and 5:1 [48] between morphine and methadone was effective and safe in patients with cancer pain. Mercadante et al. suggested that the successful conversion ratio depended on previous daily morphine dose which were 4:1, 8:1, and 12:1 for patients receiving less than 90 mg, 90 to 300 mg, and greater than 300 mg of daily morphine, respectively. The other effective rotation formulas were Ripamonti et al and Ayonrinde et al. Regarding to incomplete cross-tolerance concept, the equi-analgesic dose of methadone is much lower in patients treated previously with very high doses of morphine. Therefore, the different dose ratios were reasonably applied to switch from oral morphine to methadone. In our study, we suggested the ratio of Mercadante et al. because it was proved to be effective in clinical setting. The switching method of Mercadante et al. which was stop-and-go method was similar to our study. While, Ripamonti et al and Ayonrinde et al used a different in switching method. |
| After starting the methadone regimen according to the developed protocol, our findings showed that median overall pain score from the larger group of 21 patients was reduced from baseline by 6 points. In severe chronic non-cancer and cancer pain, a reduction in pain score of 2 points or more by 11-point numerical pain rating scale is considered to be clinically important [14,49]. A significant reduction of overall pain score was found from all subgroup analysis including cancer pain patients, noncancer pain patients, opioid-naïve patients, patients who switched to methadone, and patients with neuropathic pain. Therefore, methadone dosed and delivered according to our protocol was effective in relieving severe chronic pain, in both cancer and noncancer patients. Our data supported that an initial methadone at the protocol-recommended dose was found to effectively control pain. In addition, the calculation of methadone dose based on conversion ratio in this protocol provided effective guidance regarding methadone regimen in patients who switched from morphine to methadone. This methadone protocol was also found to effectively control somatic pain and neuropathic pain with acceptable tolerability. |
| Furthermore, the daily methadone dosages which elicited effective analgesia to improve pain, pain interferences, and neuropathic pain intensity in this study were relatively low (2-30 mg daily). These results were consistent with previous studies that used low doses of methadone (ranged from 2.5-30 mg daily) in management of severe non-cancer, cancer pain, and neuropathic pain [7,45,50]. This may be explained by the several analgesic mechanisms of methadone, including opioid agonist, NMDA antagonist, and monoamine reuptake inhibitor. Notably, stimulation of the NMDA receptor is a key mechanism in the development of chronic pain state, which was characteristic of our patients [51,52]. However, we were not able to rule out the effects of concomitant co-analgesics in pain score reduction, because some patients required increasing co-analgesic doses due to tumor progression. |
| Improvement in physical activities, emotions, sleep, and overall satisfaction score are positive consequences of pain relief. Interestingly, methadone’s ability to antagonize NMDA receptor and inhibit reuptake of monoamine appears to deliver benefit, due to the antidepressantlike effects [53-55]. Previous estimated values of serotonin (5-HT) reuptake inhibitor affinity (Ki) from animal studies showed that Ki values for R-methadone and racemic tramadol were 14.1 and 992 nM, respectively [56]; whereas, Ki values from cloned human receptors for amitriptyline and venlafaxine were 20 and 145 nM, respectively [57,58]. Regarding norepinephrine (NE) reuptake inhibitor affinities, Ki values from animal studies were 702 and 785 nM for R-methadone and racemic tramadol, respectively [56]; while, Ki values from the cloned human receptor of amitriptyline and venlafaxine were 50 and 1,420 nM, respectively [57,58]. For NMDA antagonism, Ki values from MK- 801 binding assays, a binding site of methadone at the NMDA receptor, were 0.53, 0.61, 0.85, and 47 μM for ketamine, dextromethorphan, methadone and pethidine, respectively [59]. These properties also contributed to an improvement in somatic pain, neuropathic pain and emotional score in this study. |
| Concerning patient safety, acceptable levels of adverse effects like dizziness, constipation, nausea, and vomiting were reported during this study. These adverse effects were similar to those of other strong opioids accounting for μ receptor agonist [60]. Symptomatic treatments, including anti-emetics, laxatives, and/or methadone dose adjustment, effectively relieved these symptoms. No reduction in laxative requirement after switching from morphine to methadone was found during this study. This finding was inconsistent with a previous study that showed reduction in laxative doses after rotation to methadone [61]. Possible causes for increased constipation severity in our study may include poor intake, anticholinergic side effect of amitriptyline, and/or physical immobility of advanced cancer patients. One patient developed myoclonus while receiving oral methadone 10 mg daily, even though methadone is listed as being free of neuroexcitatory effects due to a lack of neurotoxic metabolites [62]. A possible mechanism for methadone-induced myoclonus may mediate through a non-opioid pathway, which is the serotonin reuptake blocking effect [62-65] revealed three cases of methadone-related myoclonus who received high methadone doses, ranging from 90-432 mg a day. It was reported that one patient developed myoclonus after receiving only 24 mg of methadone per day [66]. Myoclonus that is triggered by variation in methadone dose might be explained by differences in patient metabolic capacity and other aggravating factors, such as concomitant SSRI or SNRI administration and/or hepatic impairment. |
| Regarding cardiac safety, several previous experimental studies revealed that methadone could block the rapid component of the cardiac delayed rectifier potassium current (IKr) encoded by hERG (human ether-a-go-go-related gene), resulting in QTc prolongation [67,68]. From the group of patients without QTc prolongation at baseline, 7 (33.33%) patients developed QTc prolongation during the study. However, clinically significant change in QTc interval or TdP was not found in our study. This may be explained by the use of lowdose methadone, as compared with high methadone doses described in previous reports especially among patients with drug addiction (ranged 45-680 mg of methadone) [12,69]. Some studies have reported methadone related QTc prolongation ranging from 11% to 49.4% with low-dose methadone ranging from 5-80 mg during chronic pain management. There was also no report of methadone induced cardiac death or TdP among these patients [70-72]. |
| Despite the fact that our protocol uses lower doses, we do recommend that a baseline 12-lead ECG is recorded and then repeated when risk factors occur, such as initiating unavoidable CYP 3A4 or 2D6 inhibitors or QTc prolonging drugs. Although not considered or evaluated in this study, genetic predisposition should also be considered as another contributing risk factor. Previous studies also reported that CYP2B6 [73] and CYP2C19 [74] polymorphisms, responsible for methadone metabolism for 12-32% and 2-14%, respectively [75], and hERG potassium channel polymorphisms [76,77] are involved in methadoneinduced QTc prolongation. With respect to CYP2B6 polymorphisms, [73] demonstrated that CYP2B6 *6/*6 carriers had an increased risk of methadone-induced QTc prolongation, as compared with non-carriers (odds ratio = 4.5; 95% confidence interval = 1.2-17.7). This genotype presented in about 7.4%, 17%, and 7% of Caucasian [78], West African [79], and Thai [80] populations, respectively. |
| Our study had a high dropout rate of approximately 38% (13 patients). This high dropout rate reflected the complicated nature of advanced stage cancer patients; patients with poor prognosis and/or severe physical problems that discouraged follow-up. These impacted the power of the study that was decreased to 68%. |
| Conclusion |
| Methadone dosing based on the protocol presented in this study elicited pain management efficacy with acceptable tolerability, according to protocol-directed monitoring. Our findings indicate that low-dose methadone (2-30 mg daily) provides benefits that include improvements in pain, pain interferences, and neuropathic pain. However, monitoring of QTc prolongation and common adverse effects, such as constipation, drowsiness, and nausea and/or vomiting should be performed periodically during methadone usage. |
| Acknowledgements |
| The authors would like to gratefully acknowledge the team members of the Pain Management Unit, Department of Anesthesiology, Faculty of Medicine Siriraj Hospital, Mahidol University for their support of and contribution to this study. The authors also wish to acknowledge and thank Ms. Pimrapat Tengtrakulcharoen for her valuable advice and guidance regarding statistical analysis and data management. Funding support for this study was provided by the Siriraj Routine to Research (R2R) Management Fund. |
| References |
|
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
 |
| Figure 1 |
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals