Evaluating the Protective Role of Ginseng against Histological and Biochemical Changes Induced by Aflatoxin B1 in the Renal Convoluted Tubules of Adult Male Albino Rats
Received: 19-Jul-2015 / Accepted Date: 02-Sep-2015 / Published Date: 04-Sep-2015 DOI: 10.4172/2161-0681.1000253
Abstract
Background: Aflatoxins are secondary metabolites of the fungi Aspergillus flavus and Aspergillus parasiticus. Chronic aflatoxin exposure is associated with damage to many body organs including the kidneys. Aim of the work: The aim of this study was to investigate the effect of aflatoxin B1 on the renal convoluted tubules of adult male albino rats and to determine the possible protective role of ginseng.
Materials and methods: Thirty six adult male albino rats were divided into three groups; group I: control group, group II (Aflatoxin B1 treated group): Aflatoxin B1 was administered 250 microgram/kg body weight/day, 5 days/ week for 4 weeks by oral gavages. Group III (Aflatoxin B1-Ginseng treated group): received the same dose of Aflatoxin B1 simultaneously with ginseng at a rate of 100 mg/kg body weight, by oral gavage. Blood urea nitrogen, serum creatinine and uric acid were measured. Renal cortex samples from, were processed for light and electron microscope examination.
Results: Examination of Aflatoxin B1 treated group revealed deterioration of renal functions; various degrees of renal tubular affection in the form of vacuolated tubular cells and hyaline casts in the lumen of most tubules. Some tubular cells had small darkly stained nuclei while others had sloughed epithelial cells in their lumina. Weak positive cytoplasmic reaction of Bcl2 was detected in most renal tubular cells. These changes were markedly reduced with administration of ginseng in group III.
Conclusion: Aflatoxin leads to alterations in the histological structure and functions of renal convoluted tubules of albino rats and ginseng supplementation could be protective from these changes.
Keywords: Renal tubules; Aflatoxin B1; Ginseng; Bcl2
323214Introduction
Aflatoxin contamination of foods is a worldwide problem, especially in developing countries. Aflatoxins (AFs) are highly toxic secondary metabolites produced by the species of Aspergillus, especially A. flavus and A. parasiticus . These fungi can grow on a wide variety of foods and feeds under favorable temperature and humidity. Contamination by aflatoxins can take place at any point along the food chain from the field, harvest, handling, shipment and storage [1] four major aflatoxins B1, B2, G1, and G2 (AFB1, AFB2, AFG1and AFG2) are produced from Aspergillus . Aflatoxins are well known to be potent mutagenic, carcinogenic, teratogenic and immunosuppressive agent; also inhibit several metabolic systems, causing liver, kidney and heart damage [2-4]. Acute toxicity demonstrated by AFB1 is the most prevalent and toxic in all species of animals. AFB1 is also known as being one of the most potent genotoxic agents and hepatocarcinogens. Histological changes in the liver have been documented previously in the form of thickening of portal tract with cellular debris and periportal fibrosis. The hepatocytes showed vacuolar degeneration and nuclear pleomorphism [5,6] Aflatoxins (AFS) have been found to contaminate a wide variety of important agricultural products world-wide such as corn, wheat, rice, spices, dried fruits, and nuts. These compounds can enter the food chain mainly by ingestion through the dietary channel of humans and animals [7]. The mechanism of AFB1 toxic effect has been extensively studied. It has been shown that AFB1 is activated by hepatic cytochrome P450 enzyme system to produce a highly reactive intermediate, AFB1-8, 9-epoxide, which subsequently binds to nucleophilic sites. Because of the concerns about the side effects of conventional medicine, the use of natural products as an alternative to conventional treatment in healing and treatment of various diseases has been on the rise in the last few decades. Medicinal plants serve as therapeutic alternatives, safer choices, or in some cases, as the only effective treatment. These plants and their isolated constituents have shown beneficial therapeutic effects, including anti-oxidant, antiinflammatory, anti-cancer, anti-microbial, and immunomodulatory effects [8]. Ginseng is one of the most popular medicinal plants, the roots of which have long been traditionally used for strengthening immunity, providing nutrition and recovering health from fatigue [9]. The pharmaceutical activities of ginseng roots have been proven recently by many investigators, and ginseng has become the famous medicinal plant all over the world. Ginseng roots contain various pharmaceutical components ginsenosides (saponins), polyacetylenes, polyphenolic compounds and acidic polysaccharides, and among the components, ginsenosides are the most pharmaceutically active [10]. Moreover, ginseng intake was associated with a decreased risk for most cancers including carcinomas of the esophagus, stomach, colon, pancreas, lung and liver [11].
In the recent years, many studies have aimed to convert major ginsenosides to the more active minor ginsenosides using heating, treatment, enzymatic and microbial conversion [12]. Ginsenoside has been known to protect kidney from apoptosis and DNA fragmentation caused by chemical drugs and cancer drugs [13]. The aim of the present work was to study the structural and biochemical alterations caused by AFB1 on the rat kidney convoluted tubules and to investigate the possible protective effect of panax ginseng extract on these changes.
Materials and Methods
Chemicals
Aflatoxin B1 preparation: Aflatoxin was purchased from Sigma Chemical Company (St Louis, Missouri, USA). It is a white to faint yellow odorless powder. Aflatoxin B1 was dissolved in olive oil as a vehicle panax ginseng, a product of Pharco Pharmaceuticals, Alexandria, Egypt, was available in the form of capsules with the trade name ‘Ginseng 100’. Each capsule contained 100 mg of the dried roots of panax ginseng.
Animal treatment: Thirty six adult healthy male albino rats with average body weight 150-170 gm were used in this study. They were obtained from the Animal House of the Faculty of Medicine, Zagazig University. All animals were conditioned at room temperature for 1 week before the start of the experiment. A commercial balanced diet and tap water ad libitum were provided. Animals were divided into three groups (12 animals each)
Group I: Control group
Subgroup Ia: Included rats that were allowed water ad libitum and were fed a standard diet (negative control).
Subgroup Ib: Included rats that received olive oil orally as a vehicle /day by means of oral gavage [14].
Subgroup IC: panax ginseng group, which included rats that received ginseng at a rate of 100 mg/kg body weight, dissolved in 100 ml of distilled water and administered by oral gavage [15].
Group II: Aflatoxin treated group
The rats received 250 mg/kg body weight/day of aflatoxin B1 Aflatoxin B1 was dissolved in olive oil as a vehicle and given orally by means of a oral gavage 5 days/week for 4 weeks.
Group III: Aflatoxin-ginseng treated group
Rats received aflatoxin as in previous group and simultaneously supplemented with ginseng at a dose of 100 mg/kg body weight, dissolved in 100 ml of distilled water and administered by oral gavage 5 days/week for 4 weeks [15] . At the end of experiment, the rats were anesthetized with 50 mg/kg. bw sodium pentobarbital intraperitoneally [16].
Blood samples were obtained directly from the heart by cardiac puncture
Blood samples were collected into tubes containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 3000 g for 10 min. Plasma was separated and then stored at 20°C until analyzed. Then intra-cardiac perfusion was done by 2% glutaraldehyde for fixation.
Histological study
The cortex of both kidneys of each animal was dissected, excised and cut into smaller pieces and processed for light (1 cm3) and electron microscope (1 mm3) study. To prepare paraffin blocks, specimens were immediately placed in 10% buffered formalin. After 10 min, when the tissue was hardened, specimens were fixed in 10% buffered formalin for 24 h and processed to prepare 5 μm sections stained with haematoxylin and eosin (H and E) and PAS stains [17]. The immunohistochemical staining for localization of the Bcl2 was carried out by means of the avidin biotin-peroxidase complex method [18] following the manufacturer’s instructions (Dako Company, Wiesentheid/Bavaria, Germany, Biotin Blocking System, and Code 0590X).
Specimens for electron microscope examination were immediately fixed in 2.5% glutaraldehyde buffered with 0.1M phosphate buffer at pH 7.4 for 2 hours at 4°C, Post fixed in 1% osmium tetroxide in the same buffer for one hour at 4°C. The specimens were processed and embedded in Embded-812 resin in BEEM capsules at 60°C for 24 hours. Ultra-thin sections were obtained using lecia ultracut UCT and stained with uranyl acetate and lead citrate15 [17] and examined with JEOL-JEM 1010 electron microscope in Histology Department, Faculty of Medicine, Zagazig University.
Image analysis and histomorphometric study
Sections stained with immunohistochemical reaction were morphometrically analyzed using image analyzer computer system to measure the mean optical density of Bcl2 expression. The data were obtained using Leica Qwin 500 Image Analyzer Computer System (England) at Pathology Department, Faculty of Dentistry, and Cairo University. The image analyzer consisted of a colored video camera, colored monitor, hard disc of IBM personal computer connected to the Olympus microscope (CX 41) and controlled by Leica Qwin 500 software.
Results
Light microscopic results
Histological examination of all control subgroups IA, IB and IC showed nearly similar structure. Figures for subgroup IA were used to differentiate with other groups.
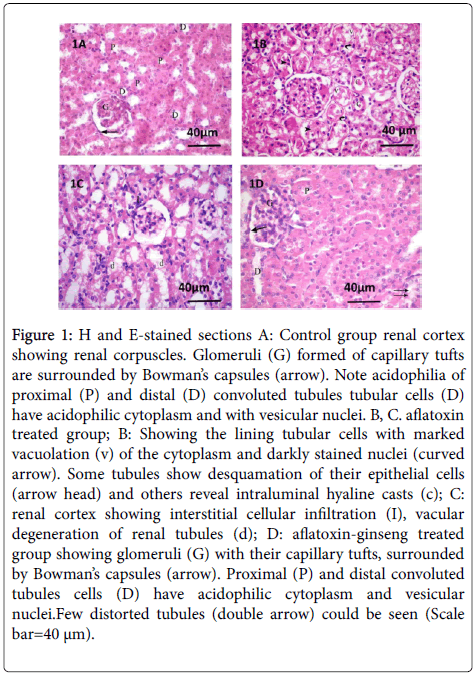
The H and E-stained sections of the renal cortex of group I (control group) showed normal renal structure. Each renal corpuscle was formed of a glomerular tuft of capillaries surrounded by Bowman’s capsule. Proximal and distal convoluted tubules cells showed acidophilic cytoplasm and vesicular nuclei (Figure 1).
Figure 1: H and E-stained sections A: Control group renal cortex showing renal corpuscles. Glomeruli (G) formed of capillary tufts are surrounded by Bowman’s capsules (arrow). Note acidophilia of proximal (P) and distal (D) convoluted tubules tubular cells (D) have acidophilic cytoplasm and with vesicular nuclei. B, C. aflatoxin treated group; B: Showing the lining tubular cells with marked vacuolation (v) of the cytoplasm and darkly stained nuclei (curved arrow). Some tubules show desquamation of their epithelial cells (arrow head) and others reveal intraluminal hyaline casts (c); C: renal cortex showing interstitial cellular infiltration (I), vacular degeneration of renal tubules (d); D: aflatoxin-ginseng treated group showing glomeruli (G) with their capillary tufts, surrounded by Bowman’s capsules (arrow). Proximal (P) and distal convoluted tubules cells (D) have acidophilic cytoplasm and vesicular nuclei.Few distorted tubules (double arrow) could be seen (Scale bar=40 μm). Examination
Examination of group II (aflatoxin treated group) revealed various degrees of renal tubular affection in the form of vacuolated cells and hyaline casts in the lumen of most tubules. Some tubular cells had small darkly stained nuclei while others had sloughed epithelial cells in their lumina (Figure 1).
Examination of group III (aflatoxin-ginseng treated group) revealed that renal corpuscles consisted of glomerular capillaries surrounded by visceral and parietal layers of Bowman's capsule which were separated by Bowman's space. Some renal tubular cells still had vacuolated cytoplasm (Figure 1).
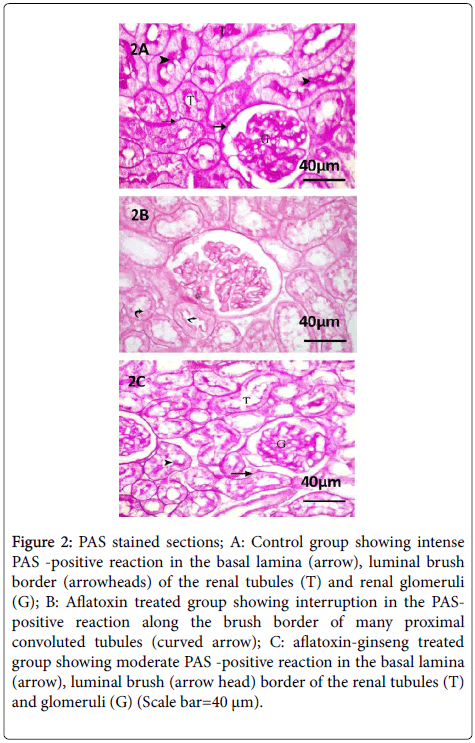
Examination of PAS stained sections of group I revealed strong positive reaction in the basement membranes of glomerular capillaries, renal tubules and Bowman's capsules. Strong PAS positive reaction in the brush borders of proximal convoluted tubules was also seen.
Sections of the renal cortex of group II showed noticeable interruption in the PAS-positive reaction along the brush border of many proximal convoluted tubules.
In the renal cortex of group III, the tubular epithelial cells appeared to be resting on their moderately PAS-positive basement membrane and a moderate PAS-positive reaction was also noticed along the brush border of most proximal convoluted tubules (Figure 2).
Figure 2: PAS stained sections; A: Control group showing intense PAS -positive reaction in the basal lamina (arrow), luminal brush border (arrowheads) of the renal tubules (T) and renal glomeruli (G); B: Aflatoxin treated group showing interruption in the PASpositive reaction along the brush border of many proximal convoluted tubules (curved arrow); C: aflatoxin-ginseng treated group showing moderate PAS -positive reaction in the basal lamina (arrow), luminal brush (arrow head) border of the renal tubules (T) and glomeruli (G) (Scale bar=40 μm).
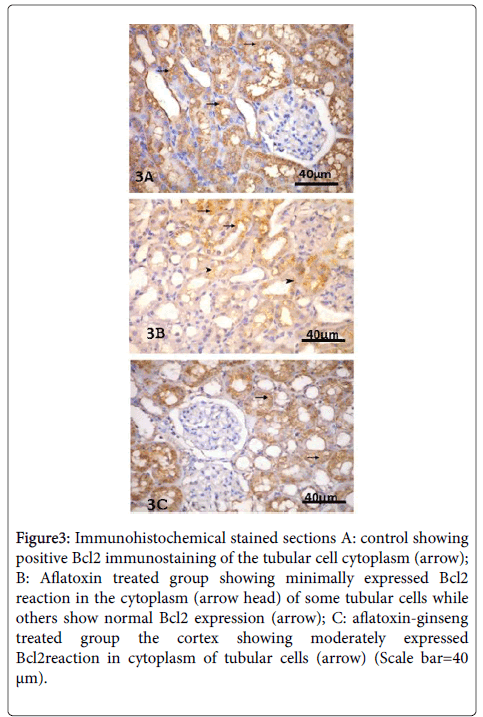
Immunohistochemical examination showed the renal tubular cells of the group I with strong positive Bcl2 immune reaction.
In group II, sections showed weak Bcl2 positive cytoplasmic reaction of most renal tubular cells.
In group III, Bcl2 was moderately expressed in the cytoplasm of most tubular cells (Figure 3).
Figure 3: Immunohistochemical stained sections A: control showing positive Bcl2 immunostaining of the tubular cell cytoplasm (arrow); B: Aflatoxin treated group showing minimally expressed Bcl2 reaction in the cytoplasm (arrow head) of some tubular cells while others show normal Bcl2 expression (arrow); C: aflatoxin-ginseng treated group the cortex showing moderately expressed Bcl2reaction in cytoplasm of tubular cells (arrow) (Scale bar=40 μm).
Electron microscope results
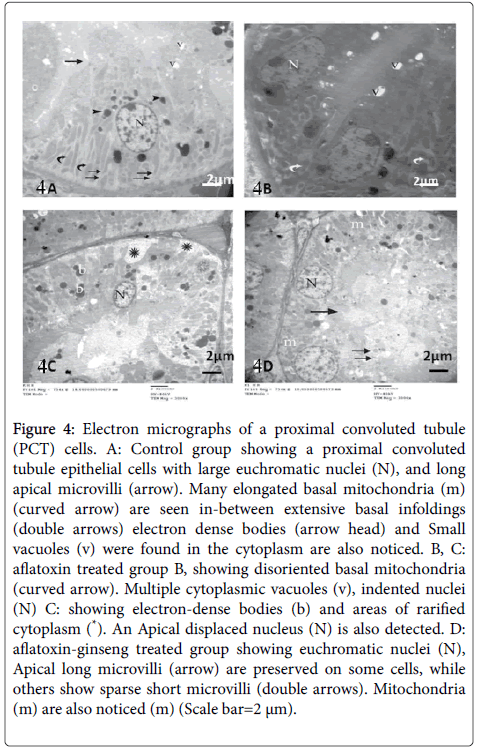
Ultrastructural results of the renal cortex of group I, the proximal convoluted tubular cells had euchromatic rounded nuclei, apical pinocytotic vesicles, electron dense bodies and luminal closely-packed microvilli. Numerous mitochondria appeared elongated and lodged within regular basal infoldings (Figure 4).
Figure 4: Electron micrographs of a proximal convoluted tubule (PCT) cells. A: Control group showing a proximal convoluted tubule epithelial cells with large euchromatic nuclei (N), and long apical microvilli (arrow). Many elongated basal mitochondria (m) (curved arrow) are seen in-between extensive basal infoldings (double arrows) electron dense bodies (arrow head) and Small vacuoles (v) were found in the cytoplasm are also noticed. B, C: aflatoxin treated group B, showing disoriented basal mitochondria (curved arrow). Multiple cytoplasmic vacuoles (v), indented nuclei (N) C: showing electron-dense bodies (b) and areas of rarified cytoplasm (*). An Apical displaced nucleus (N) is also detected. D: aflatoxin-ginseng treated group showing euchromatic nuclei (N), Apical long microvilli (arrow) are preserved on some cells, while others show sparse short microvilli (double arrows). Mitochondria (m) are also noticed (m) (Scale bar=2 μm).
Examination of proximal convoluted tubules (PCTs) of group II showed some cells of the proximal convoluted tubules with indented nuclei and others with apically displaced nuclei. Disoriented basal mitochondria and vacuolation of the cytoplasm were also noticed. The cytoplasm showed areas of rarefaction and electron dense bodies (Figure 4). In group III, PCT cells showed intact apical long microvilli and others appeared with sparse short microvilli. Basal infoldings containing numerous mitochondria, and euchromatic nuclei were also detected (Figure 4).
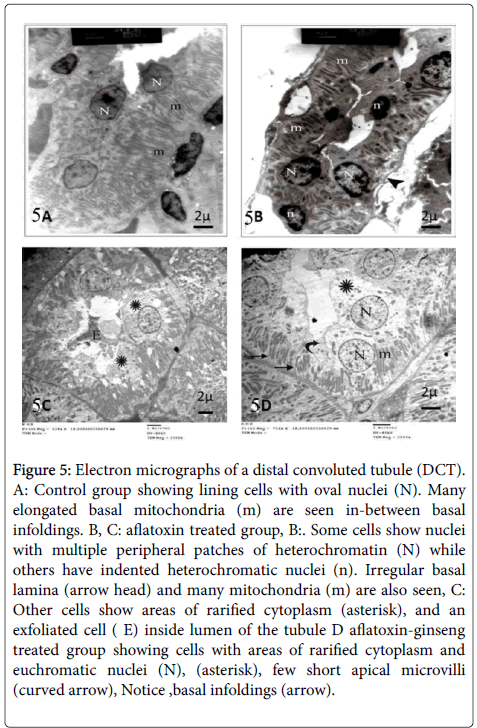
Examination of distal convoluted tubules (DCTs) of group I revealed oval euchromatic nuclei, numerous elongated mitochondria arranged along the long axis of the cell, and basal membrane infoldings (Figure 5).
In group II, cells of the distal convoluted tubules revealed indented nuclei while other nuclei appeared with peripheral clumps of heterochromatin. Some cells showed irregular basal lamina and many mitochondria. Also, there were areas of cytoplasmic rarefaction and exfoliated cells (Figure 5).
Figure 5: Electron micrographs of a distal convoluted tubule (DCT). A: Control group showing lining cells with oval nuclei (N). Many elongated basal mitochondria (m) are seen in-between basal infoldings. B, C: aflatoxin treated group, B:. Some cells show nuclei with multiple peripheral patches of heterochromatin (N) while others have indented heterochromatic nuclei (n). Irregular basal lamina (arrow head) and many mitochondria (m) are also seen, C: Other cells show areas of rarified cytoplasm (asterisk), and an exfoliated cell ( E) inside lumen of the tubule D aflatoxin-ginseng treated group showing cells with areas of rarified cytoplasm and euchromatic nuclei (N), (asterisk), few short apical microvilli (curved arrow), Notice ,basal infoldings (arrow).
In group III, most distal tubular cells appeared with euchromatic nuclei. Elongated mitochondria are seen distributed in the cytoplasm and within regular basal infoldings. Some areas of rarified cytoplasm, few short apical microvilli are still seen (Figure 5).
Biochemical results
The mean values of blood urea nitrogen (BUN) (mg/dl), serum creatinine (SC) (mg/dl) and uric acid (UA) (mmol/L) for all groups was presented in Table 1. There was no significant difference between the biochemical results of group IA, group I B, and group IC. There was a significant increase in the levels of BUN, SC and uric acid in group II (P value<0.05) when compared with control group. On the other hand, the level of BUN, SC and UA showed a significant decrease in group II in comparison with group III.
| Group | blood urea nitrogen (BUN) mg/dl | serum creatinine (SC) mg/dl | uric acid (UA) mmol/L |
|---|---|---|---|
| Group IA | 24.76 ± 1.02 | 1. 23 ± 0.1 3 | 1.6 1 ± 0. 23 |
| Group IB | 25.83 ±1.14n | 1.26 ± 0.0 2n | 1.5 8 ± 0.22n |
| Group IC | 26.17 ± 2.10n | 0.98 ± 0.033n | 1.6 08 ± 0.21n |
| Group II Aflatoxin-treated |
58. 56 ± 2. 18a | 2. 87 ± 0.0 9a | 3.4 4 ±0.204a |
| Group III Aflatoxin-Ginseng treated |
28. 35 ± 2. 76b | 1.35 ± 0.0 4b | 1.5 3 ± 0.256b |
nGroup IA ,IB non-significant difference with group IA(P>0.05)
aGroup II significant difference with group IA (P<0.05)
bGroup III significant difference with group II(aflatoxin treated)
Table 1: Mean values of blood urea nitrogen, serum creatinine, uric acid in all studied groups.
Morphometric Results
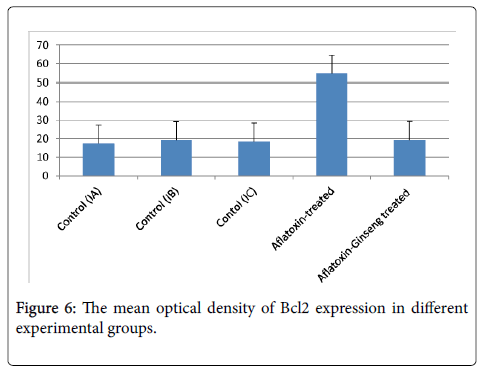
The mean optical density of Bcl2 expression for all groups was presented in (Table 2 and Figure 6): there was a significant decrease in Bcl2 expression in group II as compared with group I (P value<0.05). On the other hand, Bcl2 expression was significantly increased in group III when compared with group II.
| X ± SD | |
|---|---|
| Group IA | 17.5 ± 6.7 |
| Group IB | 19.23n |
| Group IC | 18.54n |
| Group II Aflatoxin-treated | 54.6±18.7a |
| Group III Aflatoxin-Ginseng treated | 19.3±2.7b |
nGroup IA ,IB non-significant difference with group IA(P>0.05)
aGroup II significant difference with group IA (P<0.05)
bGroup III significant difference with group II(aflatoxin treated)
Table 2: Mean values of the optical densityof Bcl2 immumoreaction of the renal cortex in the different studied groups.
A: Control group showing a proximal convoluted tubule epithelial cells lining with large euchromatic nuclei (N), prominent nucleolus and long apical microvilli (arrow). Many elongated basal mitochondria (curved arrow) are seen in-between extensive basal infoldings (double arrows) electron dense bodies (arrow head) and Small vacuoles (v) were found in the cytoplasm are also noticed. B, C: aflatoxin treated group B, showing disoriented basal mitochondria (curved arrow). Multiple cytoplasmic vacuoles (v), indented nuclei (N) C: showing electron-dense bodies (b) and areas of rarified cytoplasm (*). An Apical displaced nucleus (N) is also detected. D: aflatoxin-ginseng treated group showing euchromatic nuclei (N), Apical long microvilli (arrow) are preserved on some cells, while others show sparse short microvilli (double arrows). Mitochondria are also noticed (m) (Scale bar=2 μm).
Discussion
Aflatoxicosis is the poisoning that results from ingestion of aflatoxins, which are the metabolites of Aspergillus flavus and Aspergillus parasiticus . It is one of the most devastating and widespread noninfectious diseases [19]. The individual's sensitivity to aflatoxin is greatly dependent on species, age, sex and nutritional status of animal [20]; young animals show a greater sensitivity to this toxin. The chronic uptake of low levels of this toxin through food may not produce significant clinical signs of toxicosis, rather it may lead to decreased productivity and thus economic loses. Aflatoxin enters the body either directly through consumption of contaminated food or indirectly by animal products [21]. AFB1 has continued to receive major research attention as the most carcinogenic and toxic mycotoxins [22,23]. The toxic metabolites AFB1-8,9-epoxide resulted from biotransformation of AFB1by liver microsomal enzymes and the toxic effects of aflatoxins mostly arise from the binding of this particular epoxide derivative to DNA [24].
In this study, AFB1 -treated group showed higher levels of blood urea nitrogen, creatinine and uric acid than controls. This finding is keeping in line with the report of previous researchers [25].
On the other hand, histological examination of kidney tissues of the same group showed degenerative and necrotic changes in the convoluted tubules. These changes could explain the kidney dysfunction shown by biochemical tests. Souza et al. [26] demonstrated that oxidative stress is the principle mechanism of AFB1-induced toxicity which could be mitigated by antioxidants. In view of oxidative stress, previous results indicated that aflatoxins induced marked increase in superoxide dismutase and Malondialdehyde while Catalase activity and glutathione were reduced. A significant increase in Malondialdehyde level demonstrated in aflatoxin groups in comparison with the control group were also investigated by Eraslan et al. [27] and Eraslan et al. [28]. Malondialdehyde is considered the most significant indicator of membrane lipid peroxidation, arising from the interaction of reactive oxygen species (ROS) with cell membranes. The induced increase in lipid peroxidation after aflatoxins ingestion may be due to the fact that onset of lipid peroxidation in susceptible sperm leads to the progressive accumulation of lipid hydroperoxides in sperm plasma membranes, which then decomposes to form MDA under stress and toxic conditions [29]. In group II, tubular damage appeared in the form of desquamation of some tubular epithelial cells while others show marked cellular disorganization. Dilated tubules with flattened epithelium were detected. Many inflammatory cells and congested blood vessels were also noticed. Tubular cells had many multiple cytoplasmic vacuoles, areas of rarified cytoplasm electron-dense bodies and luminal casts were detected. These findings were in accordance with the work performed by Kamel [30].
In this study, interstitial cellular infiltrations were detected in between renal tubules in the aflatoxin-received group. These observations were in accordance with the results obtained by other researchers [31] who found that aflatoxin ingestion leads to reactive oxygen species (ROS) production, which indirectly regulates chemokine receptor expression and some function of polymorphonuclear leukocytes (PMNLs). It also facilitates the recruitment and localization of polymorphonuclear leukocyte to the site of infection and inflammation. Previous studies also detected that AFB1 induced mononuclear cell infiltration and/or focal lymphoid cell accumulation in the intertubular areas of the testes and epidedymis; degeneration and desquamation in the epithelium [32]. Ultrastructural study of the present work revealed vacuolation of some epithelial cells lining the tubules. Few abnormal mitochondria were detected in some cells.
These results were also detected by previous workers who proved that oral aflatoxins to rats induced damage of both glomeruli and tubules [33]. The cell membrane has been reported to be a target to the toxic effect of aflatoxin. This fungal metabolite could alter the mitochondrial structure through its inhibitory effect on protein and enzyme biosynthesis [34].
Endogenous and exogenous antioxidants may protect cells and tissues from destructive effects of ROS and other free radicals. Previous studies reported that sperm disorders can be improved by exogenous antioxidants/ROS scavengers [15]. Ginseng or its extracts have been reported to exhibit free radical scavenging activities and can prevent lipid peroxidation [35]. Lee and Lau [36] reported that ginseng inhibits different inducers-activated signaling protein kinases and transcription factor nuclear factor-kappaB leading to decreases in the production of cytokines and mediators of inflammation including TFN-α. Our results revealed that ginseng in group III protected against aflatoxin induced impairment of renal function as biochemical results found that ginseng decreased the levels of urea, creatinine and uric acid. These results were in conformity with those reported by Yokozawa et al. [37].
Who demonstrated that ginseng and its active component, saponin, could significantly reduce the blood urea nitrogen and creatinine levels in the blood of nephrectomized rats. Other studies asserted the nephroprotective effect of Korean ginseng saponin against cisplatin nephrotoxicity [38]. Abdel-Wahhab and Ahmed [39] suggested that Korean ginseng saponin reduced cisplatin-induced cytosolic free [Ca2+] ions overload and formation of DNA interstrand cross-link and DNA-protein cross-link.
In addition, examination of group III (aflatoxin-ginseng treated group) revealed improvement of morphological organization of renal cortex. Cells of proximal and distal convoluted tubules appeared nearly normal however few distorted tubules were also detected.
This ameliorative effect of ginseng may be attributed to either its ability to bind to glucocorticoid receptor triggering transcriptional activation of glucocorticoid response elements promoting cell proliferation and enhances the survival rate of new-born cells [40,41], and/or its free radical scavenging, metal ion and hydroxyl radicals chelating abilities [42,43]. Moreover, ginsenoside fractions have been shown to induce the cytosolic antioxidant enzyme superoxide dismutase via enhanced nuclear protein binding to its gene regulatory sequences [44].
Lee et al. [45] stated that ginseng might reduce cell damage induced by toxic substances and stabilize cell membranes by providing protection against toxic agents induced tissue injury. Immunohistochemical examination of the present study revealed weak positive Bcl2 reaction in renal tubular cells. Whereas, in group III, Bcl2 was moderately expressed in the cytoplasm of most tubular cells. Morphometric results confirmed the results by showing a significant increase of the mean optical density of Bcl2 immumoreaction of group III in comparison with group II suggesting that ginseng protects renal cells from undergoing apoptosis with improvement in renal function and reduced inflammation via decreasing oxidative stress [46]. Ginseng has a potent antiapoptotic effect as it increases the expression of the antiapoptotic gene Bcl-2 [47].
Our results were in agreement with the findings of other researchers who reported that the protective effect of ginseng was due to its antioxidant property [48]. It also increases the intracellular concentration of glutathione and superoxide anions, thereby inactivating nitric oxide, and increases antioxidant enzymes [49,50].
Conclusion
Aflatoxin leads to alterations in the histological structure and functions of renal cortex of albino rats. Tubular damage predominates. The presence of aflatoxin in food should be avoided.
Ginseng treatment significantly attenuated renal dysfunction, cell apoptosis, and tubular damage. Our results suggest that ginseng had a protective effect against damaging effect of aflatoxcosis on the kidney.
References
- Ashiq S (2015) Natural Occurrence of Mycotoxins in Food and Feed:Pakistan Perspective. Comprehensive Reviews in Food Science and Food Safety 14: 159-175.
- Faisal K, Periasamy VS, Sahabudeen S, Radha A, Anandhi R, et al. (2008) Spermatotoxic effect of aflatoxin B1 in rat: extrusion of outer dense fibres and associated axonemal microtubule doublets of sperm flagellum. Reproduction 135: 303-310.
- Mohamed AM, Metwally NS (2009) Antiaflatoxigenic activities of some plant aqueous extracts against aflatoxin-B1 induced renal and cardiac damage. J Pharmacol Toxicol 4:1-16.
- Kamdem LK, Meineke I, Gödtel-Armbrust U, Brockmöller J, Wojnowski L (2006) Dominant contribution of P450 3A4 to the hepatic carcinogenic activation of aflatoxin B1. Chem Res Toxicol 19: 577-586.
- Miller JD (1995) Fungi and mycotoxins in grain: implications for stored product research. Journal of Stored Products Research 31: 1-16
- Wang JS, Kensler TW, Groopman JD (1998) Toxicants in food: fungal contaminants. In: Ioannides C (ed.) Nutrition and Chemical Toxicity. John Wiley, Chichester, pp. 29-57.
- Khayoon WS, Saad B, Yan CB, Hashim NH, Ali ASM, et al. (2010) Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chemistry 118: 882-886
- Moshafi MH, Sharififar F, Dehghan GHR, Ameri A (2010) Bioassay screening of the essential oil and various extracts of fruits of Heracleumpersicum Desf and rhizomes of Zingiber officinale Rosc. using brine shrimp cytotoxicity assay. Iranian Journal of Pharmaceutical Research 8: 59-63.
- Park HJ, Kim DH, Park SJ, Kim JM, Ryu JH (2012) Ginseng in traditional herbal prescriptions. J Ginseng Res 36: 225-241.
- Christensen LP (2009) Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55: 1-99.
- Yun TK, Choi SY (1995) Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiology Biomarkers and Prevention 4: 401-408.
- Kim MK, Lee JW, Lee KY, Yang DC (2005) Microbial conversion of major ginsenosiderb(1) to pharmaceutically active minor ginsenoside rd. J Microbiol 43: 456-462.
- Hassan AM, Abdel-Wahhab MA (2006) Antioxidant effect of parsley and panax ginseng extract standardized with ginsenosides Rg3 against alteration induced in reproductive functions in male mice. The Egyptian Journal of Hospital Medicine 22: 60-72.
- Tang L, Guan H, Ding X, Wang JS (2007) Modulation of aflatoxin toxicity and biomarkers by lycopene in F344 rats. Toxicology and applied pharmacology 219: 10-17.
- Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T (2008) Therapeutic potential of 20(S)-ginsenosideRg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol 591: 266-272.
- Luque A, Shimizu MH, Andrade L, Sanches TR, Seguro AC (2009) Glomerular filtration is reduced by high tidal volume ventilation in an in vivo healthy rat model. Braz J Med Biol Res 42: 1104-1109.
- Bancroft JD, Gamble M (eds.) (2002) Theory and practice of histological techniques (5thedn.) Churchill Livingstone London, New York, Tokyo.
- Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B, et al. (2008) Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. Journal of veterinary diagnostic investigation 20: 393-413.
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, et al. (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American journal of clinical nutrition 80: 1106-1122.
- Sharlin JS, Howarth B, Thompson FN, Wyatt RD (1981) Decreased reproductive potential and reduced feed consumption in mature white leghorn males fed aflatoxin. PoultSci 60: 2701-2708.
- Kovács M (2004) Nutritional health aspects of mycotoxins. Orv Hetil 145: 1739-1746.
- Binder EM, Tan LM, Chin LJ, Handl J, Richard J (2007) Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal feed science and technology 137: 265-282.
- Kensler TW, Egner PA, Wang JB, Zhu YR, Zhang BC, et al. (2004) Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology 127: S310-318.
- Rawal S, Kim JE, Coulombe R (2010) Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci 89: 325-331.
- Rati ER, Shantha T, Ramesh HP (1991) Effect of long term feeding and withdrawal of aflatoxin B1 and ochratoxin A on kidney cell transformation in albino rats. Indian J ExpBiol 29: 813-817.
- Souza MF, Tomé AR, Rao VS (1999) Inhibition by the bioflavonoid ternatin of aflatoxin B1-induced lipid peroxidation in rat liver. J Pharm Pharmacol 51: 125-129.
- Eraslan G, Akdogan M, Yarsan E, Essiz D, Sahindokuyucu F, et al. (2004). Effects of aflatoxin and sodium bentonite administered in feed alone or combined on lipid peroxidation in the liver and kidneys of broilers. Bull. Vet. Inst. Pulawy, 48: 301-304.
- Eraslan G, Cam Y, Eren M, Liman CB, Atalay O,Seybek N, (2005) Aspects of using nacetylcysteine in aflatoxicosis and its evaluation regarding some lipid peroxidation parameters in rabbits. Bull Vet Inst Pulawy 49: 243-247.
- Kandeil M, El-Saad AA (2005) Biochemical effects of ascorbic acid on oxidative stress induced by aflatoxinB1 in male albino rats. Fourth International Science Conference.Fac Vet. Med. Mansoura Univ, pp. 265-82.
- Kamel ES (2013) Histological study on the effect of aflatoxin B1 on the renal tubules of adult rats. Egyptian Journal of Histology 36: 246-252.
- Shephard GS(2003)Aflatoxin and food safety: recent African perspectives. J Toxicol Toxin Rev 22: 267-286.
- Ortatatli M, Ciftci MK, Tuzcu M, Kaya A (2002) The effects of aflatoxin on the reproductive system of roosters. Res Vet Sci 72: 29-36.
- Rastogi R, Srivastava AK, Rastogi AK (2001) Long term effect of aflatoxin B(1) on lipid peroxidation in rat liver and kidney: effect of picroliv and silymarin. Phytother Res 15: 307-310.
- Bbosa GS, Lubega A, Kyegombe DB, Kitya D, Ogwal-Okeng J, et al. (2013) Review of the biological and health effects of aflatoxins on body organs and body systems. INTECH Open Access Publisher.
- Zhang HA, Wang M, Zhou J, Yao QY, Ma JM, et al. (2010) Protective effect of ginsenoside against acute renal failure and expression of tyrosine hydroxylase in the locus coeruleus. Physiol Res 59: 61-70.
- Lee DC, Lau AS (2011) Effects of panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules 16: 2802-2816.
- Yokozawa T, Zhou JJ, Hattori M, Inaba S, Okada T, et al. (1994) Effects of ginseng in nephrectomized rats. Biol Pharm Bull 17: 1485-1489.
- Liu SJ, Zhou SW (2000) Panaxnotoginsengsaponins attenuated cisplatin-induced nephrotoxicity. ActaPharmacol Sin 21: 257-260.
- Abdel-Wahhab MA, Ahmed HH (2004) Protective effects of Korean Panax ginseng against chromium VI toxicity and free radical generation in rats. J Ginseng Res 28: 11-17.
- Lee YJ, Chung E, Lee KY, Lee YH, Hur B, et al. (1997) Ginsenoside- Rg1, one of the major active molecules from panax ginseng, is a functional ligand of glucocorticoid receptor. Molecular and Cellular Biochemistry133: 135-140.
- Shen L, Zhang J (2003) Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. NeurosciLett 344: 1-4.
- Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, et al. (1997) Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 28: 191-200.
- Fu Y, Ji LL (2003) Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr 133: 3603-3609.
- Chang MS, Lee SG, Rho HM (1999) Transcriptional activation of Cu/Zn super oxide dismutase and catalase genes by panoxadiolginsen -osides extracted from panax ginseng. Pytother Res 13: 641-644.
- Lee HC, Hwang SG, Lee YG, Sohn HO, Lee DW, et al. (2002) In vivo effects of panax ginseng extracts on the cytochrome P450-dependent monooxygenase system in the liver of 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed guinea pig. Life Sci 71: 759-769.
- Doh KC, Lim SW, Piao SG, Jin L, Heo SB, Zheng YF, Yang CW (2013) Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. American journal of nephrology 37: 421- 433.
- Kim EH, Lee MJ, Kim IH, Pyo S, Choi KT, Rhee DK. (2010). Anti-apoptotic effects of red ginseng on oxidative stress induced by hydrogen peroxide in SK-N-SH. Cells J Ginseng Res, 34:138-144.
- Zhu D, Wu L, Li CR, Wang XW, Ma YJ, et al. (2009) Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem 108: 117-124.
- Zhang HA, Wang M, Zhou J, Yao QY, Ma JM, et al. (2010) Protective effect of ginsenoside against acute renal failure and expression of tyrosine hydroxylase in the locus coeruleus. Physiol Res 59: 61-70.
- Sen S, Chen S, Feng B, Wu Y, Lui E, et al. (2012) Preventive effects of North American ginseng (Panax quinquefolium) on diabetic nephropathy. Phytomedicine 19: 494-505.
Citation: Zidan RA, Elnegris HM, Wahdan RA (2015) Evaluating the Protective Role of Ginseng against Histological and Biochemical Changes Induced by Aflatoxin B1 in the Renal Convoluted Tubules of Adult Male Albino Rats. J Clin Exp Pathol 5:253. DOI: 10.4172/2161-0681.1000253
Copyright: © 2015 Zidan RA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12371
- [From(publication date): 12-2015 - Jun 30, 2025]
- Breakdown by view type
- HTML page views: 11321
- PDF downloads: 1050